
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (12): 1357-1364.DOI: 10.15541/jim20200152
Special Issue: 能源材料论文精选(一):锂离子电池(2020)
Previous Articles Next Articles
YAN Yiyuan1( ),JU Jiangwei2,YU Meiyan1,CHEN Shougang1(
),JU Jiangwei2,YU Meiyan1,CHEN Shougang1( ),CUI Guanglei2(
),CUI Guanglei2( )
)
Received:2020-03-23
Revised:2020-05-11
Published:2020-12-20
Online:2020-06-09
About author:YAN Yiyuan(1994–), male, Master candidate. E-mail: yanyiyuan94@163.com
Supported by:CLC Number:
YAN Yiyuan, JU Jiangwei, YU Meiyan, CHEN Shougang, CUI Guanglei. In-situ Polymerization Integrating 3D Ceramic Framework in All Solid-state Lithium Battery[J]. Journal of Inorganic Materials, 2020, 35(12): 1357-1364.
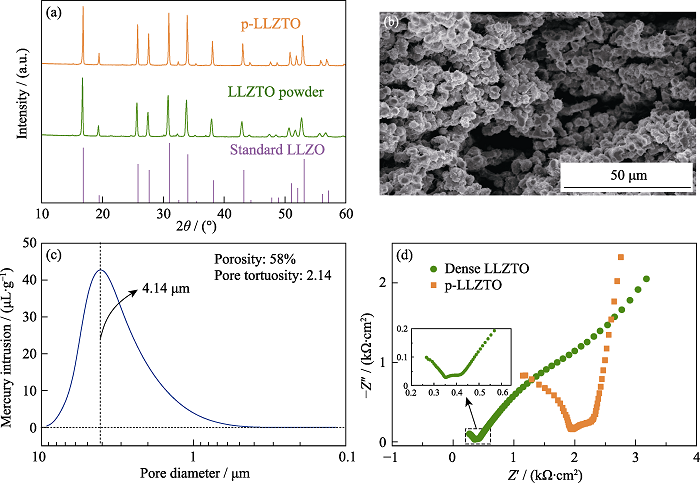
Fig. 2 (a) XRD patterns of standard LLZO, the as-prepared LLZTO powders and p-LLZTO; (b) Cross sectional SEM image of p-LLZTO; (c) Pore size distribution of p-LLZTO; (d) EIS plots of dense LLZTO and p-LLZTO at room temperature with inset showing the partial magnified spectrum of the dense LLZTO
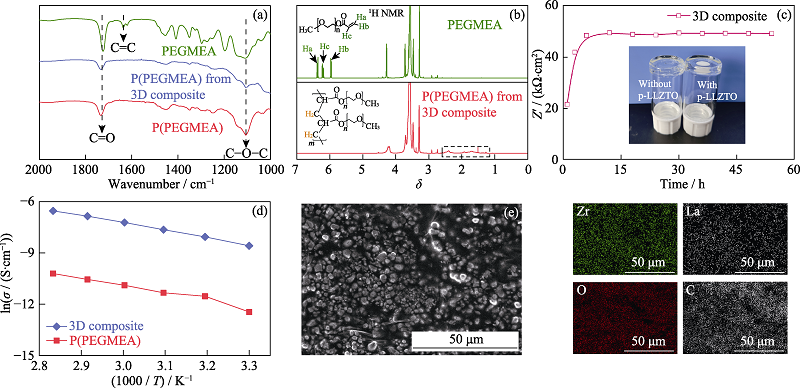
Fig. 3 (a) FT-IR spectra of PEGMEA, P(PEGMEA), and P(PEGMEA) from the 3D composite; (b) 1H NMR spectra of PEGMEA and P(PEGMEA) from the 3D composite(the solvents are deuterated N,N-dimethylformamide) with insets showing the corresponding structural formula of PEGMEA and P(PEGMEA); (c) Thermal evolution of ohmic resistance at 60 ℃ for steel|3D composite|steel symmetrical cell with inset showing the digital image of PEGMEA with/without p-LLZTO after heat-treatment at 60 ℃ for 24 h; (d) Relation between ionic conductivity of electrolyte and temperature for P(PEGMEA) and 3D composite; (e) Cross sectional SEM image and element mapping analysis of the 3D composite
| Electrolyte | Lithium salt | EOa : Li+ | Conductivity of polymer/(S·cm-1) | Conductivity of composite/(S·cm-1) | Promotion factor | Ref. |
|---|---|---|---|---|---|---|
| PEO/LATP particles | LiClO4 | 15 : 1 | 1.3×10-6 | 9.5×10-6 | 7.5 | [ |
| PEO/LLZO fibers | LiTFSIb | - | 2.5×10-6 | 2.7×10-5 | 11 | [ |
| PEO/LATPc fibers | LiTFSI | 8 : 1 | 3.2×10-6 | 4.9×10-5 | 15 | [ |
| PEO/3D LLZO | LiTFSI | 10 : 1 | 1.8×10-6 | 8.5×10-5 | 47 | [ |
| PEO/3D LLTOd | LiTFSI | 10 : 1 | 2.2×10-6 | 8.8×10-5 | 40 | [ |
Table 1 Conductivities $(\sigma_{Li^+})$ of different solid electrolytes at room temperature
| Electrolyte | Lithium salt | EOa : Li+ | Conductivity of polymer/(S·cm-1) | Conductivity of composite/(S·cm-1) | Promotion factor | Ref. |
|---|---|---|---|---|---|---|
| PEO/LATP particles | LiClO4 | 15 : 1 | 1.3×10-6 | 9.5×10-6 | 7.5 | [ |
| PEO/LLZO fibers | LiTFSIb | - | 2.5×10-6 | 2.7×10-5 | 11 | [ |
| PEO/LATPc fibers | LiTFSI | 8 : 1 | 3.2×10-6 | 4.9×10-5 | 15 | [ |
| PEO/3D LLZO | LiTFSI | 10 : 1 | 1.8×10-6 | 8.5×10-5 | 47 | [ |
| PEO/3D LLTOd | LiTFSI | 10 : 1 | 2.2×10-6 | 8.8×10-5 | 40 | [ |
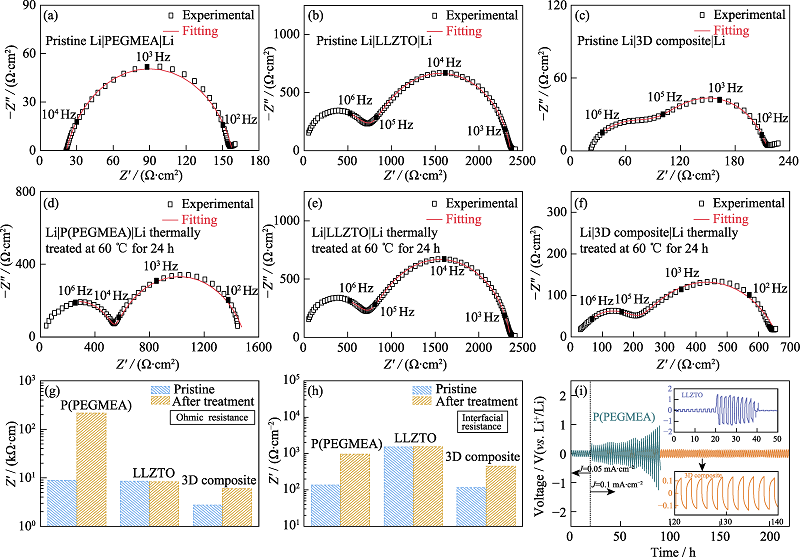
Fig. 4 EIS plots of (a-c) pre- and (d-f) post-treated Li-Li symmetrical batteries based on (a, d) PEGMEA, (b, e) LLZTO, (c, f) 3D composites; (g) Ohmic and (h) interfacial resistance comparison of pre- and post-treated Li-Li symmetrical cells; (i) DC galvanostatic cycle of Li-Li symmetrical batteries based on P(PEGMEA) and the 3D composite under room temperature at 0.1 mA·cm-2 with insets showing D.C. galvanostatic cycle of Li-Li symmetrical battery based on LLZTO(up) and the magnified profile of Li|3D composite|Li(down)
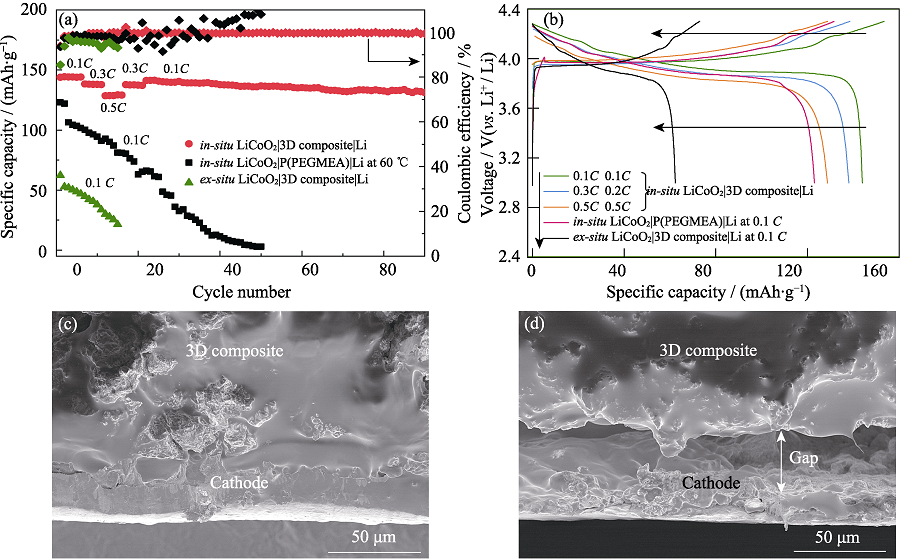
Fig. 5 (a) Cycle performances of in-situ LiCoO2|3D composite|Li, in-situ LiCoO2|P(PEGMEA)|Li, ex-situ LiCoO2|3D composite|Li ASLBs; (b) Charge-discharge curves of in-situ LiCoO2|3D composite|Li, in-situ LiCoO2|P(PEGMEA)|Li, ex-situ LiCoO2|3D composite|Li ASLBs; Cross-sectional SEM images of the LiCoO2/3D composite interface from the disassembled (c) in-situ and (d) ex-situ LiCoO2|3D composite|Li ASLBs
| [1] |
GAO Z, SUN H, FU L, et al. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Advanced Materials, 2018,30(17):e1705702.
DOI URL PMID |
| [2] |
BACHMAN J C, MUY S, GRIMAUD A, et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chemical Reviews, 2016,116(1):140-162.
DOI URL PMID |
| [3] | ZHENG F, KOTOBUKI M, SONG S, et al. Review on solid electrolytes for all-solid-state lithium-ion batteries. Journal of Power Sources, 2018,389:198-213. |
| [4] | ZHANG B, TAN R, YANG L, et al. Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Materials, 2018,10:139-159. |
| [5] | CHEN R, QU W, GUO X, et al. The pursuit of solid-state electrolytes for lithium batteries: from comprehensive insight to emerging horizons. Materials Horizons, 2016,3(6):487-516. |
| [6] | FAN L, WEI S, LI S, et al. Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Advanced Energy Materials, 2018,8(11):1702657. |
| [7] | YUE L, MA J, ZHANG J, et al. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Materials, 2016,5:139-164. |
| [8] | MANTHIRAM A, YU X, WANG S. Lithium battery chemistries enabled by solid-state electrolytes. Nature Reviews Materials, 2017,2(4):16103 |
| [9] | GAO Y, WANG D, LI Y C, et al. Salt-based organic-inorganic nanocomposites: towards a stable lithium metal/Li10GeP2S12 solid electrolyte interface. Angew. Chem. Int. Ed., 2018,57(41):13608-13612. |
| [10] | BUANNIC L, ORAYECH B. Dual substitution strategy to enhance Li+ ionic conductivity in Li7La3Zr2O12 solid electrolyte. Chemistry of Materials, 2017,29(4):1769-1778. |
| [11] | ZHANG Z, SHAO Y, LOTSCH B, et al. New horizons for inorganic solid state ion conductors. Energy & Environmental Science, 2018,11(8):1945-1976. |
| [12] | CHENG X B, ZHAO C Z, YAO Y X, et al. Recent advances in energy chemistry between solid-state electrolyte and safe lithium- metal anodes. Chem, 2019,5(1):74-96. |
| [13] |
ZHA W, CHEN F, YANG D, et al. High-performance Li6.4La3Zr1.4Ta0.6O12/poly(ethylene oxide)/succinonitrile composite electrolyte for solid-state lithium batteries. Journal of Power Sources, 2018,397:87-94.
DOI URL |
| [14] |
ZHU P, YAN C, DIRICAN M, et al. Li0.33La0.557TiO3 ceramic nanofiber- enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries. Journal of Materials Chemistry A, 2018,6(10):4279-4285.
DOI URL |
| [15] | WAN Z, LEI D, YANG W, et al. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder. Advanced Functional Materials, 2019,29(1):1805301. |
| [16] | CHEN L, LI Y, LI S P, et al. PEO/garnet composite electrolytes for solid-state lithium batteries: from “ceramic-in-polymer” to “polymer- in-ceramic”. Nano Energy, 2018,46:176-184. |
| [17] | XIE H, YANG C, FU K K, et al. Flexible, scalable, and highly conductive garnet-polymer solid electrolyte templated by bacterial cellulose. Advanced Energy Materials, 2018,8(18):1703474. |
| [18] | BAE J, LI Y, ZHANG J, et al. A 3D nanostructured hydrogel- framework-derived high-performance composite polymer lithium-ion electrolyte. Angew. Chem. Int. Ed., 2018,57(8):2096-2100. |
| [19] | BAE J, LI Y, ZHAO F, et al. Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries. Energy Storage Materials, 2018,15:46-52. |
| [20] |
LIU Y, SUN Q, ZHAO Y, et al. Stabilizing the interface of NASICON solid electrolyte against Li metal with atomic layer deposition. ACS Applied Materials & Interfaces, 2018,10(37):31240-31248.
DOI URL PMID |
| [21] |
JU J, WANG Y, CHEN B, et al. Integrated interface strategy toward room temperature solid-state lithium batteries. ACS Applied Materials & Interfaces, 2018,10(16):13588-13597.
DOI URL PMID |
| [22] | ZHAO Q, LIU X, STALIN S, et al. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nature Energy, 2019,4(5):365-373. |
| [23] |
DUAN H, YIN Y X, SHI Y, et al. Dendrite-free Li-metal battery enabled by a thin asymmetric solid electrolyte with engineered layers. Journal of the American Chemical Society, 2018,140(1):82-85.
DOI URL PMID |
| [24] | PARANJAPE N, MANDADAPU P C, WU G, et al. Highly- branched cross-linked poly(ethylene oxide) with enhanced ionic conductivity. Polymer, 2017,111:1-8. |
| [25] | BAN X, ZHANG W, CHEN N, et al. A high-performance and durable poly(ethylene oxide)-based composite solid electrolyte for all solid-state lithium battery. The Journal of Physical Chemistry C, 2018,122(18):9852-9858. |
| [26] | GONG Y, FU K, XU S, et al. Lithium-ion conductive ceramic textile: a new architecture for flexible solid-state lithium metal batteries. Materials Today, 2018,21(6):594-601. |
| [27] |
LI D, CHEN L, WANG T, et al. 3D fiber-network-reinforced bicontinuous composite solid electrolyte for dendrite-free lithium metal batteries. ACS Applied Materials & Interfaces, 2018,10(8):7069-7078.
DOI URL PMID |
| [28] |
LI Z, HUANG H M, ZHU J K, et al. Ionic conduction in composite polymer electrolytes: case of PEO:Ga-LLZO composites. ACS Applied Materials & Interfaces, 2019,11(1):784-791.
DOI URL PMID |
| [29] | WANG Q, WEN Z, JIN J, et al. A gel-ceramic multi-layer electrolyte for long-life lithium sulfur batteries. Chem. Commun. (Camb), 2016,52(8):1637-1640. |
| [30] |
HAN X, GONG Y, FU K K, et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nature Materials, 2017,16(5):572-579.
DOI URL PMID |
| [31] | JU J, CHEN F, XIA C. Ionic conductivity of impregnated samaria doped ceria for solid oxide fuel cells. Electrochimica Acta, 2014,136:422-429. |
| [32] | WU B, WANG S, LOCHALA J, et al. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy & Environmental Science, 2018,11(7):1803-1810. |
| [33] |
HU J L, TIAN J, Li C L. Nanostructured carbon nitride polymer- reinforced electrolyte to enable dendrite-suppressed lithium metal batteries. ACS Applied Materials & Interfaces, 2017,9:11615-11625.
DOI URL PMID |
| [34] | HU J L, YAO Z G, CHEN K Y, et al. High-conductivity open framework fluorinated electrolyte bonded by solidified ionic liquid wires for solid-state Li metal batteries. Energy Storage Materials, 2020,28:37-46. |
| [1] | KONG Jianfeng, HUANG Jiecheng, LIU Zhaolin, LIN Cunsheng, WANG Zhiyu. Development of Quasi-solid-state Na-ion Battery Based on DPEPA-derived Gel Polymer Electrolyte [J]. Journal of Inorganic Materials, 2024, 39(12): 1331-1338. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||