
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (7): 805-810.DOI: 10.15541/jim20170610
Special Issue: 光催化材料与技术
• Orginal Article • Previous Articles
LI Jian1, ZHANG Gang-Hua2, FAN Li-Kun2, HUANG Guo-Quan1, GAO Zhi-Peng3, ZENG Tao1,2
Received:2017-12-28
Published:2018-07-10
Online:2018-06-19
About author:LI Jian(1990-), male, candidate of Master degree. E-mail: 18521099679@163.com
Supported by:CLC Number:
LI Jian, ZHANG Gang-Hua, FAN Li-Kun, HUANG Guo-Quan, GAO Zhi-Peng, ZENG Tao. Enhanced Visible-light-driven Photocatalytic Activity of Multiferroic KBiFe2O5 by Adjusting pH Value[J]. Journal of Inorganic Materials, 2018, 33(7): 805-810.
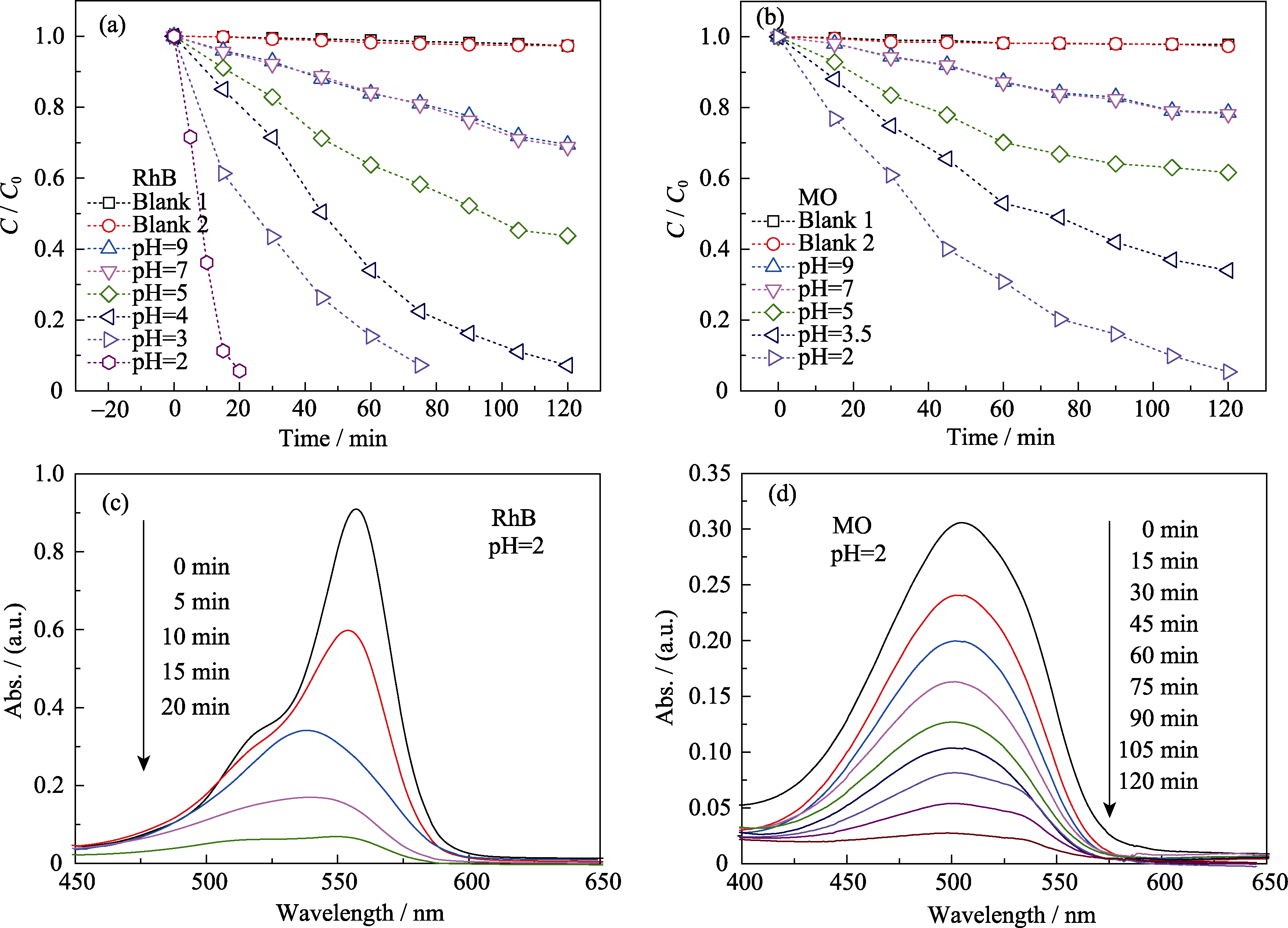
Fig. 3 Degradation rates of RhB (a) and MO (b) using KBiFe2O5 at different pH values, and absorption changes of RhB (c) and MO (d) solution in photocatalytic process at pH 2
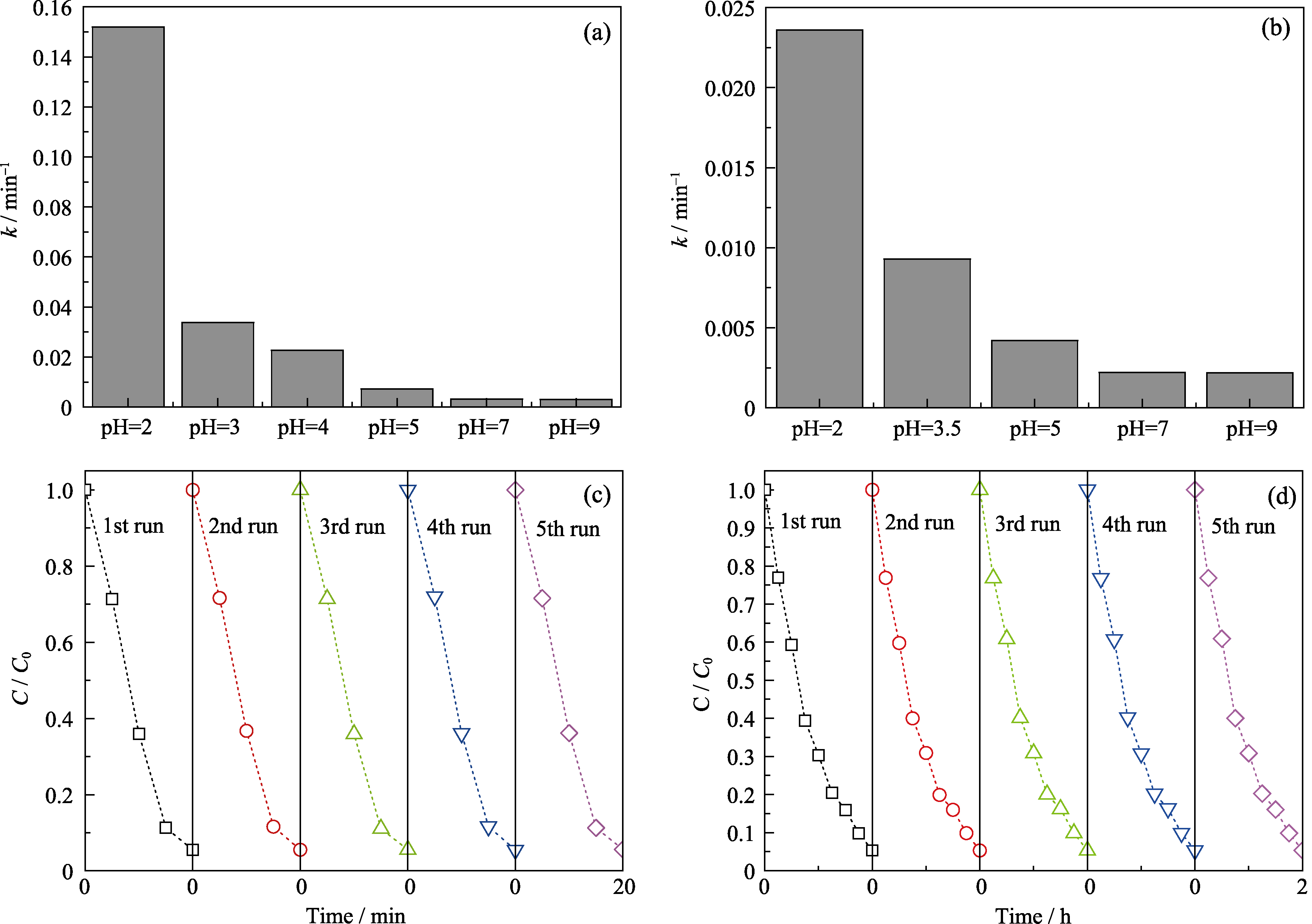
Fig. 4 Corresponding reaction rate constant k for the photocatalytic degradation of RhB (a) and MO (b) by KBiFe2O5 at various pH value, and circulating runs in the photocatalytic degradation of RhB (c) and MO (d) at pH 2
| Sample | pH=2 | pH=3.5 | pH=5 | pH=7 |
|---|---|---|---|---|
| D50/μm | 0.341 | 0.654 | 1.576 | 2.796 |
Table 1 Average particle size of KBiFe2O5 in suspensions at various pH values
| Sample | pH=2 | pH=3.5 | pH=5 | pH=7 |
|---|---|---|---|---|
| D50/μm | 0.341 | 0.654 | 1.576 | 2.796 |
| [1] | CHOI H, SHIN D, YEO B C,et al.Simultaneously controllable doping sites and the activity of a W-N codoped TiO2 photocatalyst.ACS Catal., 2016, 6(5): 2745-2753. |
| [2] | CHOI H, SOFRANKO A C, DIONYSIOU D D.Nanocrystalline TiO2 photocatalytic membranes with a hierarchical mesoporous multilayer structure: synthesis, characterization, and multifunction.Adv. Funct. Mater., 2006, 16: 1067-1074. |
| [3] | LIN JING-CHENG, TANG XIAO, CHU WAN-YI. Synthesis and photocatalysis property of ultra-small TiO2 nanoclusters in aqueous media. J. Inorg. Mater., 2017, 32(8): 863-869. |
| [4] | NG K H, CHENG C K. Photo-polishing of POME into CH4-lean biogas over the UV-responsive ZnO photocatalyst. Chem. Eng. J., 2016, 300: 127-138. |
| [5] | AKHAVAN O. Graphene nanomesh by ZnO nanorod photocatalysts. ACS nano, 2010, 4(7): 4174-4180. |
| [6] | HSU MU-HSIANG, CHANG CHI-JUNG, WENG HAU-TING.Efficient H2 production using Ag2S-coupled ZnO@ZnS core-shell nanorods decorated metal wire mesh as an immobilized hierarchical photocatalyst.ACS Sustain. Chem. Eng., 2016, 4: 1381-1391. |
| [7] | XUE CHAO, AN HUA, YAN XIAO-QING, ,et al. Spatial charge separation. Spatial charge separation and transfer in ultrathin CdIn2S4/rGO nanosheet arrays decorated by ZnS quantum dots for efficient visible-light-driven hydrogen evolution. Nano Energy, 2017, 39: 513-523. |
| [8] | CHEN SHAN-SHAN, TAKATA T, DOMEN K. Particulate photocatalysts for overall water splitting. Nature Rev. Matal., 2017, 2(10): 17050-1-17. |
| [9] | FAN YING-YING, MA WEI-GUANG, HAN DONG-XUE,et al.Convenient recycling of 3D AgX/ graphene aerogels (X = Br, Cl) for efficient photocatalytic degradation of water pollutants.Adv. Mater., 2015, 27: 3767-3773. |
| [10] | ZHANG NING, CHEN DA, NIU FENG, ,et al. . Enhanced visible light photocatalytic activity of Gd doped BiFeO3 nanoparticles and mechanism insight. Sci.Rep., 2016, 6: 26467-1-11. |
| [11] | LI SHUN, LIN YUAN-HUA, ZHANG BO-PING,et al.Controlled fabrication of BiFeO3 uniform microcrystals and their magnetic and photocatalytic behaviors.J. Phys. Chem. C, 2010, 114: 2903-2908. |
| [12] | ZHANG GANG-HUA, LIU FENG-LIANG, GU TING-TING, ,et al. Enhanced ferroelectric. Enhanced ferroelectric and visible-light photoelectric properties in multiferroic KBiFe2O5 via pressure-induced phase transition. Adv. Electron. Mater., 2017, 3(3): 1600498-1-8. |
| [13] | RAMIREZ F, JR E M, SOUZA J A, et al.Comprehensive theoretical and experimental study of electrical transport mechanism on BiFeO3 multiferroic nanoparticles. J. Alloy and Compd., 2017, 720: 47-53. |
| [14] | ZHANG PENG, TENG XIAO-XU, FENG XIANG-HUA, ,et al.. Preparation of Bi2WO6 photocatalyst by high-energy ball milled Bi2O3-WO3 mixture. Ceram. Int., 2016, 42: 16749-16757. |
| [15] | ZENG TAO, LOU QI-WEI, BAI YANG, ,et al. . Recent progress on the photocatalysis of ferroelectric matrrial. J. Inorg. Mater., 2014, 23(28): 220-226. |
| [16] | SU R, SHEN Y, LI L, et al. . Silver-modified nanosized ferroelectrics as a novel photocatalyst. Small, 2015, 11(2): 202-207. |
| [17] | MOHAN S, SUBRAMANIAN B, BHAUMIK I,et al. Nanostructured Bi1-xGdxFeO3-α multiferroic photocatalyst on its sunlight driven photocatalytic activity. RSC Adv., 2014, 4: 16871-16878. |
| [18] | GUO JIA, ZHU YI, ZHANG YUAN-MING, ,et al. . Hydrothermal synthesis and visible-light photocatalytic properties of BiVO4 with different structures and morphologies. J. Inorg.Mater., 2012, 27(1): 26-32. |
| [19] | WÜRFEL P, WÜRFEL U. Physics of Solar Cells. Weinheim : Wiley, 2009. |
| [20] | ZHANG GANG-HUA, WU HUI, LI GUO-BAO. New high Tc multiferroics KBiFe2O5 with narrow band gap and promising photovoltaic effect. Sci. Rep., 2013, 3: 1265-1-8. |
| [21] | GRINBERG I, WEST D V, TORRES M, ,et al. . Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials.Nature, 2013, 503(7477): 509-512. |
| [22] | RANJBARI A, MOKHTARANI N. Post treatment of composting leachate using ZnO nanoparticles immobilized on moving media. Appl. Catal. B: Environ., 2017, 220: 211-221. |
| [23] | WANG XIONG, LIN YING, DING XI-FENG, ,et al.. Enhanced visible-light-response photocatalytic activity of bismuth ferrite nanoparticles. J. Alloy and Compd., 2011, 509: 6585-6588. |
| [24] | ZHOU YING, ZHANG XIAO-JING, ZHAO ZI-YAN, ,et al. . Effects of pH on the visible-light induced photocatalytic and photoelectrochemical performances of hierarchical Bi2WO6 microspheres. Superlattice Microst., 2014, 72: 238-244. |
| [25] | YAO WEI-FENG, XU XIAO-HONG, WANG HONG,et al.Photocatalytic property of perovskite bismuth titanate.Appl. Catal. B: Environ., 2004, 52: 109-116. |
| [26] | SUN YUE, LIU JIA-WEN, LI ZHONG-HUA,et al.Design of highly ordered Ag-SrTiO3 nanotube arrays for photocatalytic degradation of methyl orange. J. Solid State Chem., 2011, 184: 1924- 1930. |
| [27] | GUO REN-QING, FANG LIANG, DONG WEN,et al.Enhanced photocatalytic activity and ferromagnetism in Gd doped BiFeO3 nanoparticles.J. Phys. Chem. C, 2010, 114: 21390-21396. |
| [28] | ZENG TAO, BAI YANG, LI HAO,et al.Fabrication of barium titanate nanophotocatalysts with gridding structures and photocatalytic activities.J. Inorg. Mater., 2015, 30(12): 1334-1338. |
| [1] | CAI Miao, CHEN Zihang, ZENG Shi, DU Jianghui, XIONG Juan. CuS Nanosheet Decorated Bi5O7I Composite for the Enhanced Photocatalytic Reduction Activity of Aqueous Cr(VI) [J]. Journal of Inorganic Materials, 2021, 36(6): 665-672. |
| [2] | LIU Cai, LIU Fang, HUANG Fang, WANG Xiaojuan. Preparation and Photocatalytic Properties of Alga-based CDs-Cu-TiO2 Composite Material [J]. Journal of Inorganic Materials, 2021, 36(11): 1154-1162. |
| [3] | XU Shichao,ZHU Tianzhe,QIAO Yang,BAI Xuejian,TANG Nan,ZHENG Chunming. Fabrication of Z-scheme BiVO4/GO/g-C3N4 Photocatalyst with Efficient Visble-light Photocatalytic Performance [J]. Journal of Inorganic Materials, 2020, 35(7): 839-846. |
| [4] | ZHU Enquan,MA Yuhua,AINIWA· Munire,SU Zhi. Adsorption-enrichment and Localized-photodegradation of Bentonite-supported Red Phosphorus Composites [J]. Journal of Inorganic Materials, 2020, 35(7): 803-808. |
| [5] | WANG Dan-Jun, WANG Chan, ZHAO Qiang, GUO Li, YANG Xiao, WU Jiao, FU Feng. Au Nanoparticles (NPs) Surface Plasmon Resonance Enhanced Photocatalytic Activities of Au/Bi2WO6 Heterogeneous Nanostructures [J]. Journal of Inorganic Materials, 2018, 33(6): 659-666. |
| [6] | TONG Qin, DONG Ya-Mei, YAN Liang, HE Dan-Nong. High-efficient Synthesis and Photocatalytic Properties of Ag/AgBr/TiO2 Monolithic Photocatalysts Using Sodium Alginate as Substrate [J]. Journal of Inorganic Materials, 2017, 32(6): 637-642. |
| [7] | YANG Min, JIA Xiao-Peng, LI Bing-Ke, DENG Guo-Wei, WANG Qi-Hui, LIU Xiao-Yang. One-pot Synthesis and Photocatalytic Hydrogen Evolution Properties of Zn2GeO4 Microspheres [J]. Journal of Inorganic Materials, 2017, 32(2): 141-147. |
| [8] | SUN Tong, CHEN Yang, MA Xiao-Qing, LI Zhong, LI Hui, CUI Xiao-Li. Facile Synthesis of Visible Light Activated Carbon-incorporated Mn Doped TiO2 Microspheres via Flame Thermal Method [J]. Journal of Inorganic Materials, 2015, 30(9): 1002-1008. |
| [9] | QU Ting, HUANG Qiang, ZHAO Zhen-Bo. Preparation and Visible Light Responsive Photocatalytic Activity of Bi2MoO6/Ni-Fe LDH Composites [J]. Journal of Inorganic Materials, 2015, 30(8): 825-832. |
| [10] | YU Zhong-Xiong, XIANG Lei, ZHONG Fang-Long, LI Yan-Wen, MO Ce-Hui, CAI Quan-Ying, HUANG Xian-Pei, WU Xiao-Lian, ZHAO Hai-Ming. Effects of Solution pH on Photocatalytic Degradation of Rhodamine B Using Bismuth Tungstate Prepared by Low-temperature Combustion Method [J]. Journal of Inorganic Materials, 2015, 30(5): 535-541. |
| [11] | ZHOU Yu, ZHANG Zhi-Jie, XU Jia-Yue, CHU Yao-Qing, YOU Ming-Jiang. Synthesis and Enhanced Photocatalytic Activity of Er3+-doped ZnWO4 [J]. Journal of Inorganic Materials, 2015, 30(5): 549-554. |
| [12] | LIU Shi-Xin, LI Xiao-Song, DENG Xiao-Qing, SUN Zhi-Guang, ZHU Ai-Min. Influence of Calcination Temperature on Nano-TiO2 Photocatalyst Synthesized by Gliding Arc Plasma [J]. Journal of Inorganic Materials, 2015, 30(2): 189-194. |
| [13] | ZENG Tao, BAI Yang, LI Hao, MAO Chao-Liang, DONG Xian-Lin, GUI Shu-Xiang. Fabrication of Barium Strontium Titanate Nanophotocatalysts with Gridding Structures and Their Photocatalytic Activities [J]. Journal of Inorganic Materials, 2015, 30(12): 1334-1338. |
| [14] | HAN Cheng, LEI Yong-Peng, WANG Ying-De. Recent Progress on Nano-heterostructure Photocatalysts for Solar Fuels Generation [J]. Journal of Inorganic Materials, 2015, 30(11): 1121-1130. |
| [15] | ZHU Kai-Ping, WANG De-Ping, FAN Hong-Yuan, WANG Hui, YAO Ai-Hua, YE Song. In-situ Transformation of Borate Glass and Its Effect on pH of Soaking-liquid [J]. Journal of Inorganic Materials, 2015, 30(10): 1069-1074. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||