
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (5): 547-553.DOI: 10.15541/jim20210311
• RESEARCH ARTICLE • Previous Articles Next Articles
WANG Hongli1( ), WANG Nan1, WANG Liying1, SONG Erhong2(
), WANG Nan1, WANG Liying1, SONG Erhong2( ), ZHAO Zhankui1(
), ZHAO Zhankui1( )
)
Received:2021-05-17
Revised:2021-07-12
Published:2022-05-20
Online:2021-07-12
Contact:
SONG Erhong, associate professor. E-mail: ehsong@mail.sic.ac.cn;ZHAO Zhankui, professor. E-mail: zhaozk@ccut.edu.cn
About author:WANG Hongli (1989-), female, associate professor. E-mail:wanghongli@ccut.edu.cn
Supported by:CLC Number:
WANG Hongli, WANG Nan, WANG Liying, SONG Erhong, ZHAO Zhankui. Hydrogen Generation from Formic Acid Boosted by Functionalized Graphene Supported AuPd Nanocatalysts[J]. Journal of Inorganic Materials, 2022, 37(5): 547-553.
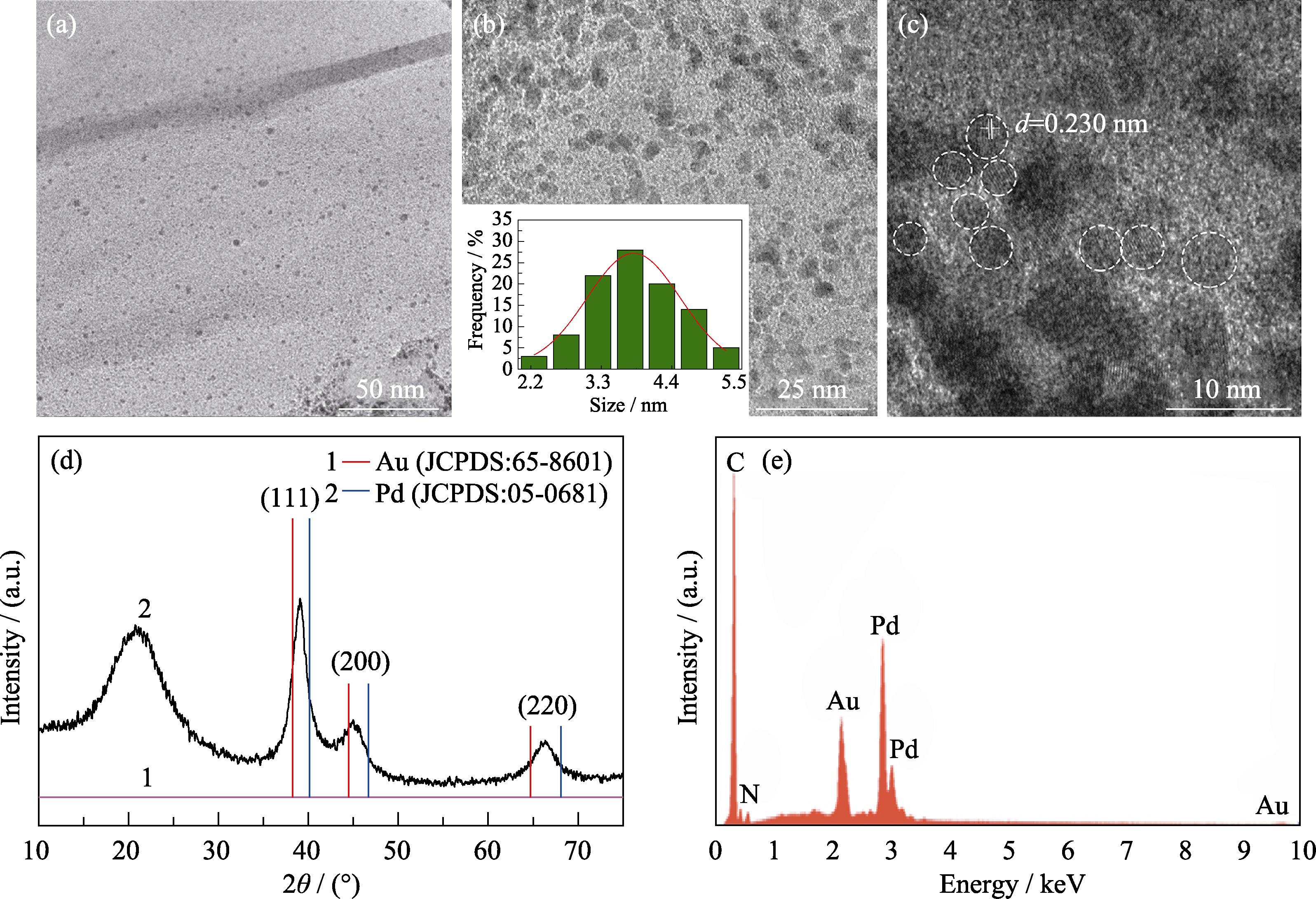
Fig. 2 TEM images, XRD pattern and EDX spectrum of Au0.3Pd0.7/PEI-rGO (a-b) TEM images and (c)HRTEM image for Au0.3Pd0.7/PEI-rGO with inset in (b) showing corresponding histogram of particle size distribution, (d) XRD pattern and (e) EDX pattern for Au0.3Pd0.7/PEI-rGO

Fig. 3 XPS spectra of Au0.3Pd0.7/PEI-rGO and Au0.3Pd0.7/rGO (a) XPS total spectra for (1) Au0.3Pd0.7/rGO and (2) Au0.3Pd0.7/PEI-rGO; (b) High resolution XPS spectra of N1s for Au0.3Pd0.7/PEI-rGO; (c) Au4f, (d) Pd3d XPS spectra for (1) Au0.3Pd0.7/PEI-rGO and (2) Au0.3Pd0.7/rGO
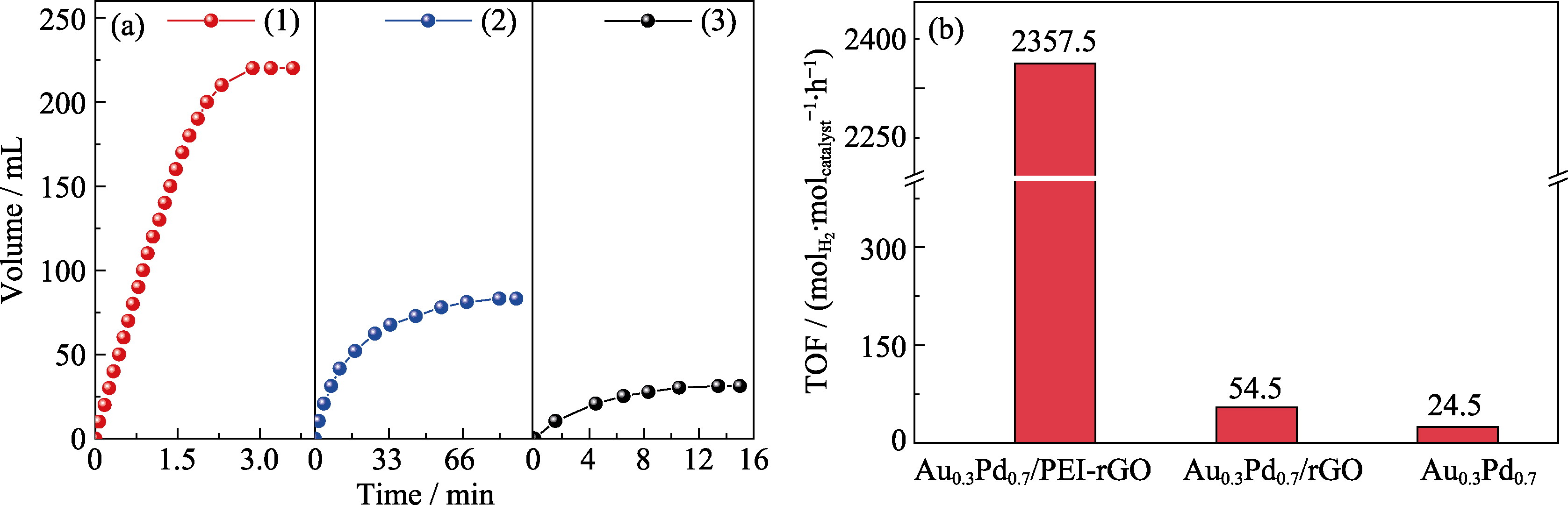
Fig. 4 Comparison of the catalytic performances for hydrogen evolution from FA dehydrogenation reaction of Au0.3Pd0.7/PEI-rGO, Au0.3Pd0.7/rGO and Au0.3Pd0.7 catalysts (a) Volume of gas versus time for the dehydrogenation of FA (1 mol/L, 5 mL) catalyzed by (1) Au0.3Pd0.7/PEI-rGO, (2) Au0.3Pd0.7/rGO and (3) Au0.3Pd0.7; (b) Corresponding TOF values
| Catalyst | Temperature/K | ncatalyst/nFA | TOF/(molH2∙ molcatalyst-1∙h-1) |
|---|---|---|---|
| Pd-NPs@TA-CoP[ | 328 | 0.013 | 233.0a |
| Pd@ED/Cr-MIL-101[ | 328 | 0.003 | 583.0b |
| Ni0.4Pd0.6/NH2-N-rGO[ | 298 | 0.020 | 954.3a |
| Pd0.7Ag0.3/CeOx-NPC[ | 323 | 0.008 | 1101.9a |
| Pd@TB-POP[ | 323 | 0.100 | 1344.0b |
| Pd/ImIP-2[ | 323 | 0.008 | 1593.0b |
| AuPd/n-CNS[ | 333 | 0.020 | 1896.0a |
| PdAg-CeO2[ | 303 | 0.033 | 2272.8a |
| Au0.3Pd0.7/PEI-rGO | 323 | 0.020 | 2357.5a |
| Pd/MSC-30[ | 323 | 0.013 | 2623.0a |
| Pd-Co2P/NPC[ | 323 | 0.026 | 2980.0b |
Table 1 TOF of different catalysts for FA dehydrogenation
| Catalyst | Temperature/K | ncatalyst/nFA | TOF/(molH2∙ molcatalyst-1∙h-1) |
|---|---|---|---|
| Pd-NPs@TA-CoP[ | 328 | 0.013 | 233.0a |
| Pd@ED/Cr-MIL-101[ | 328 | 0.003 | 583.0b |
| Ni0.4Pd0.6/NH2-N-rGO[ | 298 | 0.020 | 954.3a |
| Pd0.7Ag0.3/CeOx-NPC[ | 323 | 0.008 | 1101.9a |
| Pd@TB-POP[ | 323 | 0.100 | 1344.0b |
| Pd/ImIP-2[ | 323 | 0.008 | 1593.0b |
| AuPd/n-CNS[ | 333 | 0.020 | 1896.0a |
| PdAg-CeO2[ | 303 | 0.033 | 2272.8a |
| Au0.3Pd0.7/PEI-rGO | 323 | 0.020 | 2357.5a |
| Pd/MSC-30[ | 323 | 0.013 | 2623.0a |
| Pd-Co2P/NPC[ | 323 | 0.026 | 2980.0b |
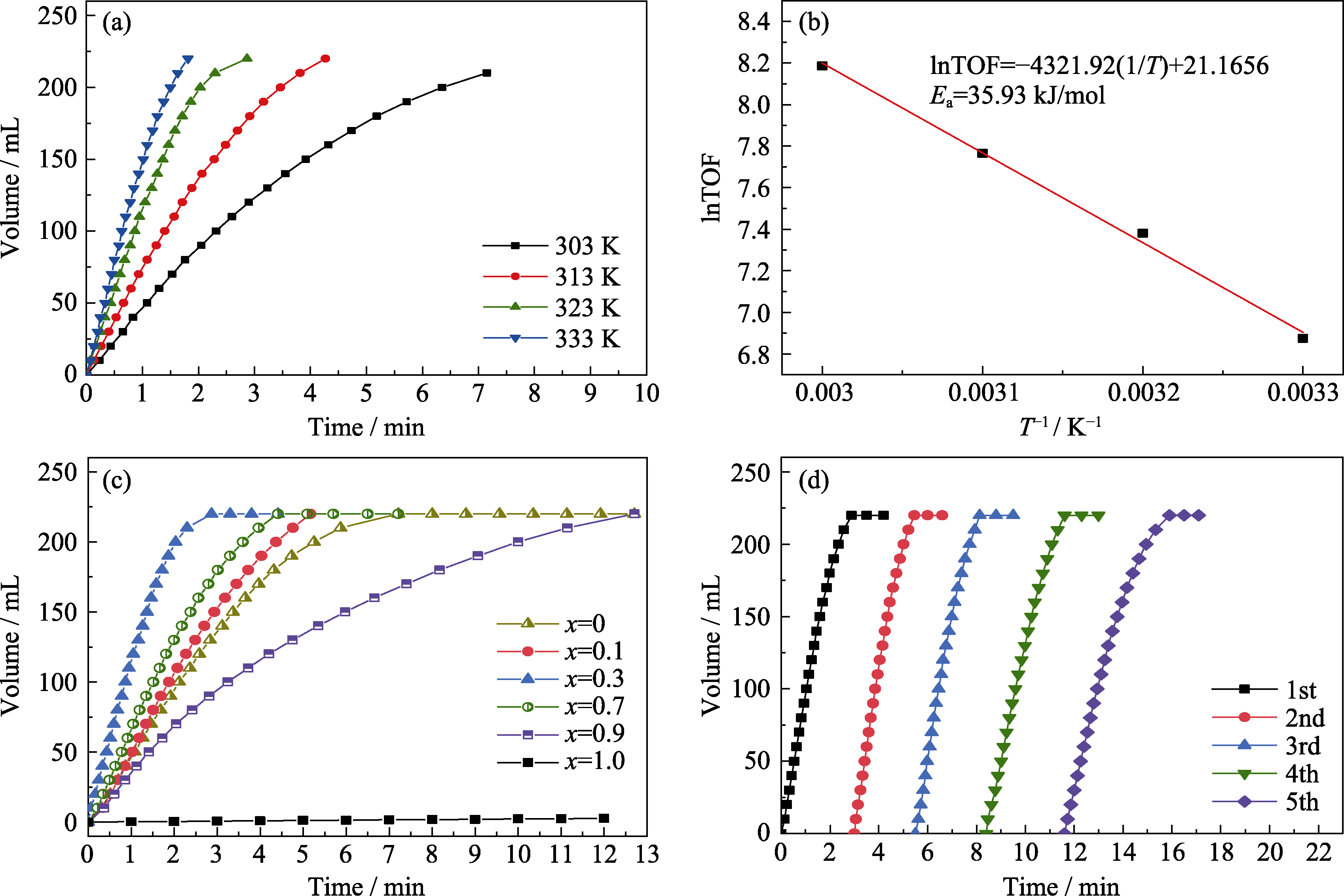
Fig.5 (a) Volume of gas versus time for the dehydrogenation of FA at different temperatures over Au0.3Pd0.7/PEI-rGO catalyst; (b) Arrhenius plot (lnTOF versus 1/T) for Au0.3Pd0.7/PEI-rGO; (c) Volume of gas versus time for the dehydrogenation of FA at different ratios of AuxPd1-x/PEI-rGO (x=0, 0.1, 0.3, 0.7, 0.9, 1.0) at 323 K; (d) Durability tests of Au0.3Pd0.7/PEI-rGO towards the decomposition of FA (1.0 mol/L, 5.0 mL)
| [1] | LI H, ZHOU Y, ZHAO M, et al. Suppressed shuttle via inhibiting the formation of long-chain lithium polysulfides and functional separator for greatly improved lithium-organosulfur batteries performance. Advanced Energy Materials, 2019,10:1902695. |
| [2] | LI H, ZHAO M, JIN B, et al. Mesoporous nitrogen-doped carbon nanospheres as sulfur matrix and a novel chelate-modified separator for high-performance room-temperature Na-S batteries. Small, 2020,16:1907464. |
| [3] | WANG P, LI X Y, SHI Z L, et al. Synergistic effect of Ag and Ag2O on photocatalytic H2-evolution performance of TiO2. Journal of Inorganic Materials, 2020,35(7):781-788. |
| [4] | ZHANG Y Q, ZHANG S J, WAN Z R,et al. RuFe nanoparticles modified sheet-like BiVO4: high-efficient synergistic catalyst for ammonia borane hydrolytic dehydrogenation. Journal of Inorganic Materials, 2020,35(7):809-816. |
| [5] | LI J, CHEN W, ZHAO H, et al. Size-dependent catalytic activity over carbon-supported palladium nanoparticles in dehydrogenation of formic acid. Journal of Catalysis, 2017,352:371-381. |
| [6] | WANG Z L, YAN J M, WANG H L, et al. Au@Pd core-shell nanoclusters growing on nitrogen-doped mildly reduced graphene oxide with enhanced catalytic performance for hydrogen generation from formic acid. Journal of Materials Chemistry A, 2013,1:12721-12725. |
| [7] | CUI C Y, TANG Y J, ZIAEE M A, et al. Highly dispersed ultrafine palladium nanoparticles enabled by functionalized porous organic polymer for additive-free dehydrogenation of formic acid. ChemCatChem, 2018,10:1431-1437. |
| [8] | GUO S L. Ge nanoparticles in MXene sheets: one-step synthesis and highly improved electrochemical property in lithium-ion batteries. Journal of Inorganic Materials, 2020,35(1):105-111. |
| [9] | ZHANG Y Q. Preparation and dehydrogenation property of NH2- UIO-66 supported RuCuMo nanocatalyst. Journal of Inorganic Materials, 2019,34(12):1316-1324. |
| [10] | YAN J M, LI S J, YI S S, et al. Anchoring and upgrading ultrafine NiPd on room-temperature-synthesized bifunctional NH2-N-rGO toward low-cost and highly efficient catalysts for selective formic acid dehydrogenation. Advanced Materials, 2018,30:1703038. |
| [11] | LIN Q M, CUI J G, YAN X, et al. First-principles study on electronic structure and optical properties of single point defect graphene oxide. Journal of Inorganic Materials, 2020,35(10):1117-1122. |
| [12] | ZHANG F. A new polyethylene composite material based on nano silver particels loaded graphene oxide. Journal of Inorganic Materials, 2019,34(6):633-640. |
| [13] | DUAN J M, XIANG Z Q, ZHANG H S, et al. Pd-Co2P nanoparticles supported on N-doped biomass-based carbon microsheet with excellent catalytic performance for hydrogen evolution from formic acid. Applied Surface Science, 2020,530:147191. |
| [14] | RANA S, JONNALAGADDA S B. A facile synthesis of Cu-Ni bimetallic nanoparticle supported organo functionalized graphene oxide as a catalyst for selective hydrogenation of p-nitrophenol and cinnamaldehyde. RSC Advances, 2017,7:2869-2879. |
| [15] | IMANI R, EMAMI S H, FAGHIHI S. Synthesis and characterization of an octaarginine functionalized graphene oxide nano-carrier for gene delivery applications. Physical Chemistry Chemical Physics, 2015,17:6328-6339. |
| [16] | SHU D, FENG F, HAN H L, et al. Prominent adsorption performance of amino-functionalized ultra-light graphene aerogel for methyl orange and amaranth. Chemical Engineering Journal, 2017,324:1-9. |
| [17] | FAN Z J, KAI W, YAN J, et al. Facile synthesis of graphene nanosheets via Fe reduction of exfoliated graphite oxide. ACS Nano, 2011,5(1):191-198. |
| [18] | HOU Y, ZHANG B W, XING R G, et al. One-step synthesis and electrochemical properties of reduced graphene oxide/MnO2 composites. Journal of Inorganic Materials, 2015,30(8):855-860. |
| [19] | KANNANGARA Y Y, RATHNAYAKE U A, SONG J K. Hybrid supercapacitors based on metal organic frameworks using p- phenylenediamine building block. Chemical Engineering Journal, 2019,361:1235-1244. |
| [20] | ZHANG L, ZHANG J W, KUANG Q, et al. Cu2+-assisted synthesis of hexoctahedral Au-Pd alloy nanocrystals with high-index facets. Journal of the American Chemistry Society, 2011,133:17114-17117. |
| [21] | LAI L F, CHEN L W, ZHAN D Z, et al. One-step synthesis of NH2-graphene from in situ graphene-oxide reduction and its improved electrochemical properties. Carbon, 2011,49:3250-3257. |
| [22] | NOURUZI N, DINARI M, MOKHTARI N, et al. Selective catalytic generation of hydrogen over covalent organic polymer supported Pd nanoparticles (CoP-Pd). Molecular Catalysis, 2020,493:111057. |
| [23] | ALAMGHOLILOO H, ROSTAMNIA S, HASSANKHANI A, et al. Formation and stabilization of colloidal ultra-small palladium nanoparticles on diamine-modified Cr-MIL-101: synergic boost to hydrogen production from formic acid. Journal of Colloid and Interface Science, 2020,567:126-135. |
| [24] | YIN B, ZHAO E F, HUA X L, et al. Ultrafine PdAg nanoparticles immobilized on nitrogen-doped carbon/cerium oxide for superior dehydrogenation of formic acid. New Journal of Chemistry, 2020,44:2011-2015. |
| [25] | ZIAEE M A, ZHONG H, CUI C Y, et al. Additive-free hydrogen generation from formic acid boosted by amine-functionalized imidazolium-based ionic polymers. ACS Sustainable Chemistry & Engineering, 2018,6:10421-10428. |
| [26] | JIANG Y Q, FAN X L, CHEN M, et al. AuPd nanoparticles anchored on nitrogen-decorated carbon nanosheets with highly efficient and selective catalysis for the dehydrogenation of formic acid. The Journal of Physical Chemistry C, 2018,122:4792-4801. |
| [27] | ZHANG Z J, LUO Y X, LIU S W, et al. A PdAg-CeO2 nanocomposite anchored on mesoporous carbon: a highly efficient catalyst for hydrogen production from formic acid at room temperature. Journal of Materials Chemistry A, 2019,7:21438-21446. |
| [28] | LI Z P, XU Q. Metal-nanoparticle-catalyzed hydrogen generation from formic acid. Accounts of Chemical Research, 2017,50:1449-1458. |
| [29] | BI Q Y, LIN J D, LIU Y M, et al. Dehydrogenation of formic acid at room temperature: boosting palladium nanoparticle efficiency by coupling with pyridinic-nitrogen-doped carbon. Angewandte Chemie International Edition, 2016,55:11849-11853. |
| [30] | LI S J, ZHOU Y T, KANG X, et al. A simple and effective principle for a rational design of heterogeneous catalysts for dehydrogenation of formic acid. Advanced Materials, 2019,31:1806781. |
| [31] | MORI K, NAKA K, MASUDA S, et al. Palladium copper chromium ternary nanoparticles constructed inβsitu within a basic resin: enhanced activity in the dehydrogenation of formic acid. ChemCatChem, 2017,9:3456-3462. |
| [1] | GUO Lina, HE Xuebing, LYU Lin, WU Dan, YUAN Hong. Modulation of CuO Surface Properties for Selective Electrocatalytic Reduction of CO2 to HCOOH [J]. Journal of Inorganic Materials, 2022, 37(1): 29-37. |
| [2] | HUANG Xiao-Ling, LIN Zhou, LIAN Xing-Yi, ZHANG Xiao-Feng, LIN Shen. Preparation and Electrocatalytic Properties of Pd/PMo12-GN Composite towards Formic Acid Oxidation [J]. Journal of Inorganic Materials, 2014, 29(7): 722-728. |
| [3] | JIANG Hong,FENG Lan-Ying,ZHU Hong,GUO Zhi-Jun,ZHANG Xin-Wei. Effect of Adding Fe on the Performances of Pd/C Catalyst [J]. Journal of Inorganic Materials, 2008, 23(4): 847-850. |
| [4] | ZHANG Yao-Jun,ZHANG,Li. Synthesis of Composite Material CdS/Al-HMS and Hydrogen Production by Photocatalytic Pollutant Degradation under Visible Light Irradiation [J]. Journal of Inorganic Materials, 2008, 23(1): 66-70. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||