
Journal of Inorganic Materials ›› 2019, Vol. 34 ›› Issue (8): 817-826.DOI: 10.15541/jim20180487
Previous Articles Next Articles
ZHU Meng-Meng,LI Guo-Hua( ),ZHANG Xue-Ming,ZHAI Jia-Xin,GAN Si-Ping,SONG Xiao
),ZHANG Xue-Ming,ZHAI Jia-Xin,GAN Si-Ping,SONG Xiao
Received:2018-10-16
Revised:2019-01-07
Published:2019-08-20
Online:2019-05-13
CLC Number:
ZHU Meng-Meng, LI Guo-Hua, ZHANG Xue-Ming, ZHAI Jia-Xin, GAN Si-Ping, SONG Xiao. Boron Nitride Nanosheets Supported Cu2O Nanoparticles: Synthesis and Catalytic Reduction for 4-nitrophenol[J]. Journal of Inorganic Materials, 2019, 34(8): 817-826.
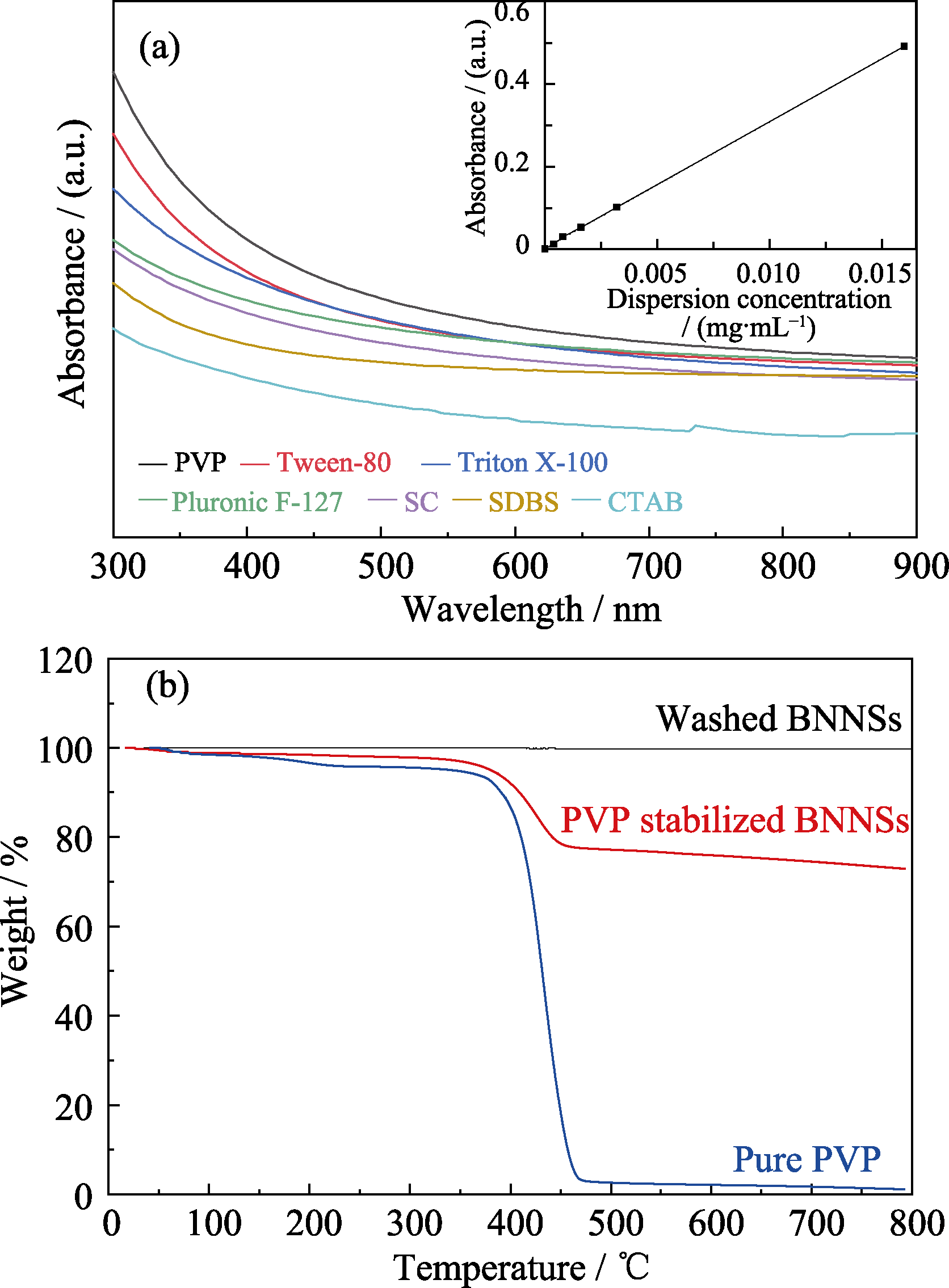
Fig. 2 Absorption spectra for h-BN dispersions stabilized with various surfactants (a), TGA curves of washed BNNSs, PVP stabilized BNNSs, and pure PVP (b)
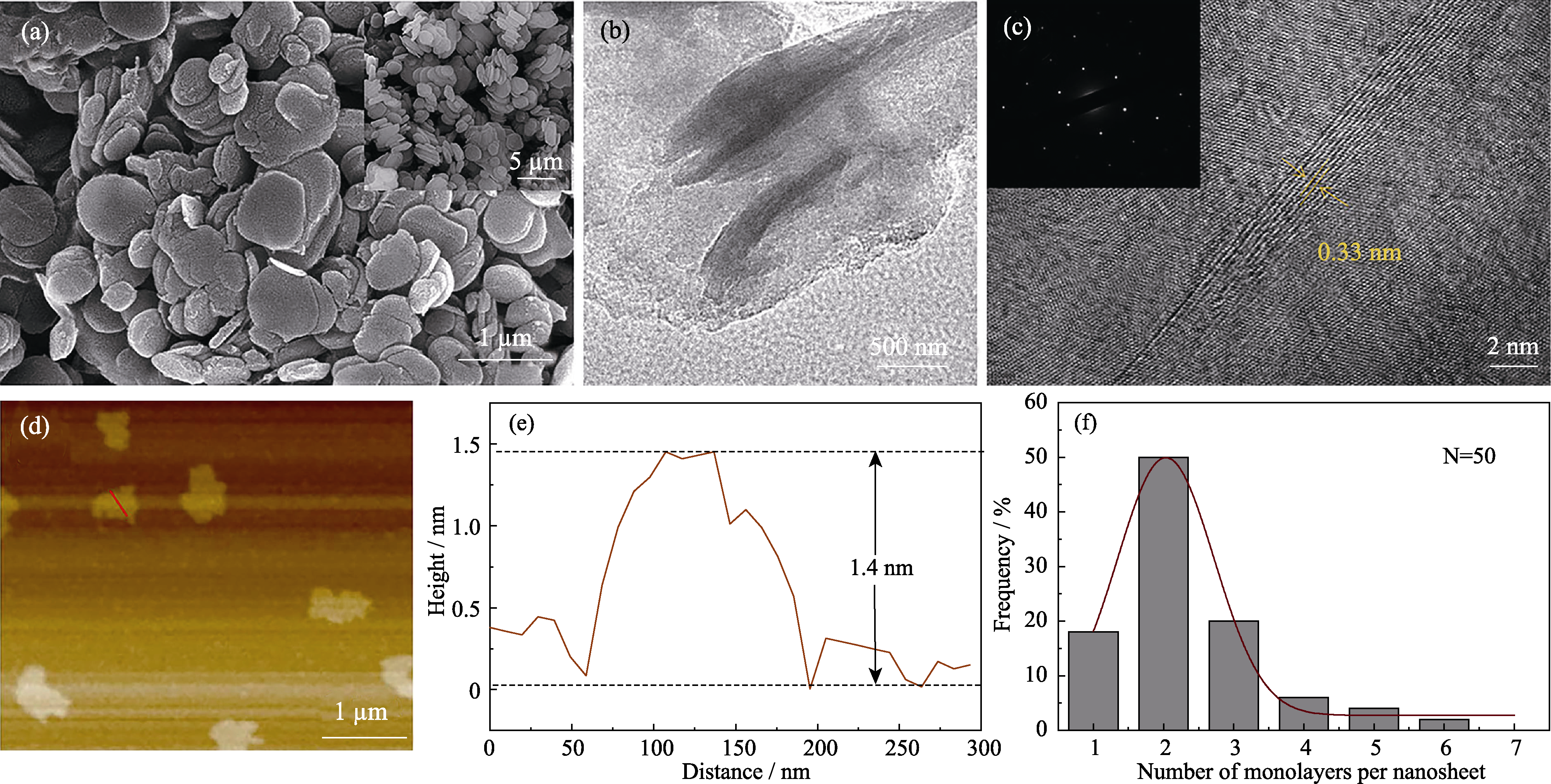
Fig. 3 SEM image (a) with inset showing bulk h-BN, TEM image (b), HRTEM image (c) with inset showing corresponding SAED pattern, AFM image (d), and the corresponding height profile of random nanosheet along the red trace (e) and statistical analyse on the number of monolayers per sheet (f) of BNNSs
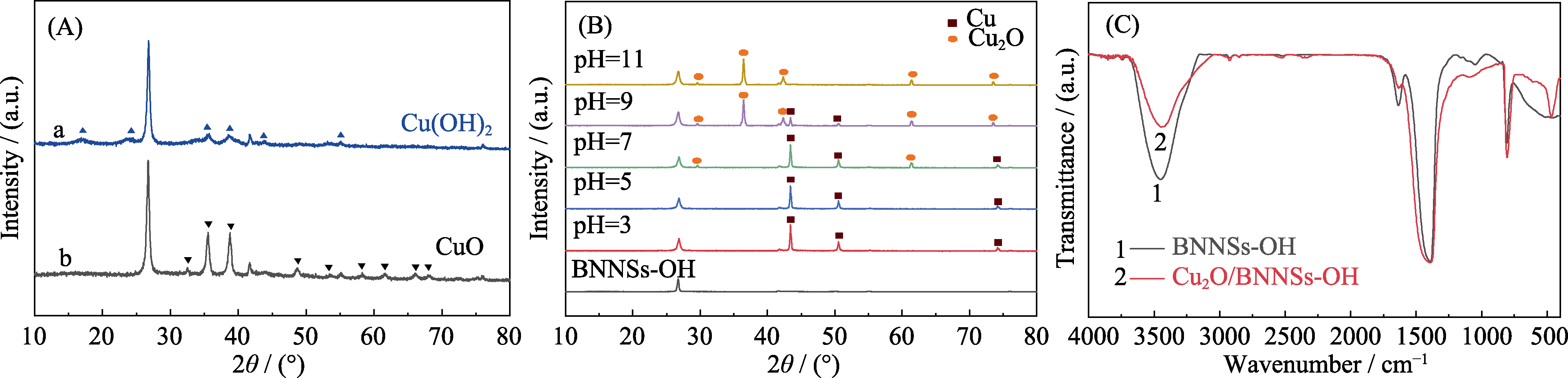
Fig. 5 XRD patterns of precursors (A) before adding VC at pH 5-7 (a) and pH 9-11 (b), specimens (B) in ascorbic acid solution reduction system at different pH, and FT-IR spectra of the as-obtained samples at pH 11 (C)
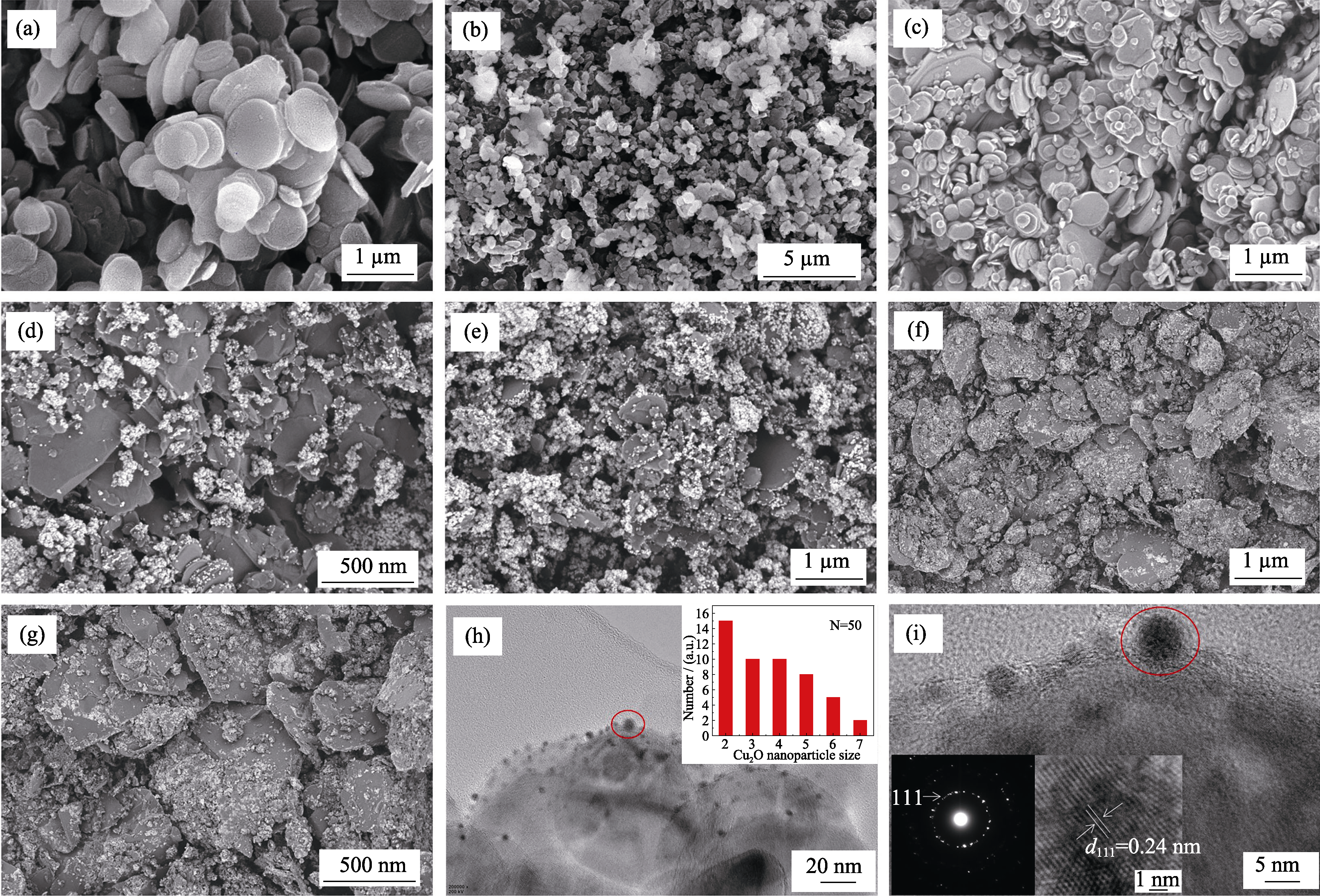
Fig. 7 SEM images of BNNSs-OH (a), CuO/BNNSs-OH (b), products prepared in ascorbic acid solution reduction system at different pH((c) pH 3, (d) pH 5, (e) pH 7, (f) pH 9, (g) pH 11), HRTEM images of Cu2O/BNNSs-OH (h-i) with inset in (h) showing the corresponding size distributions of Cu2O NPs with inset in (i) showing the corresponding selected SAED pattern and lattice fringe pattern at pH 11
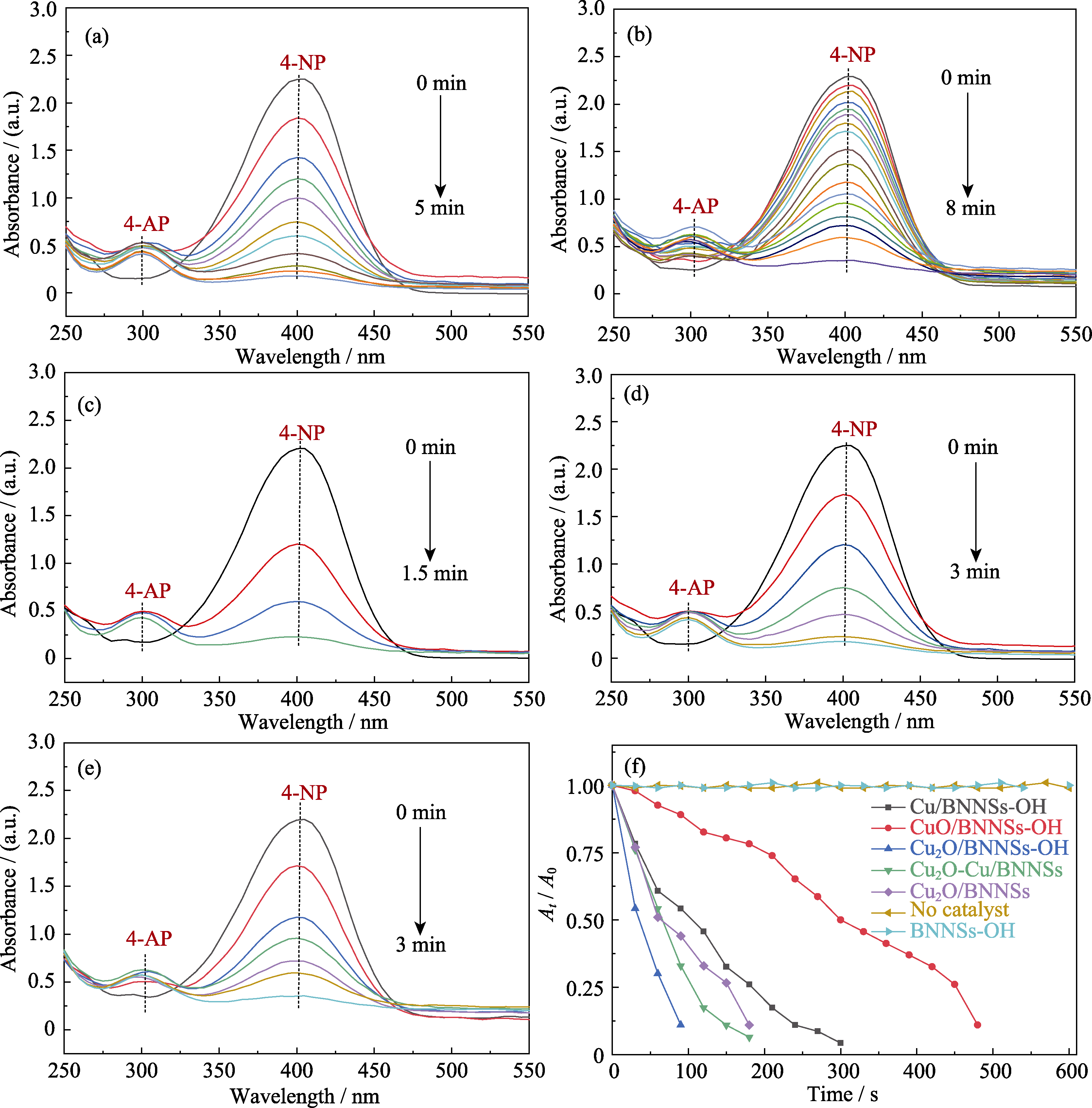
Fig. 9 UV-Vis absorption spectra of Cu/BNNSs-OH (a), CuO/BNNSs-OH (b), Cu2O/BNNSs-OH (c), Cu2O-Cu/BNNSs-OH (d), and Cu2O/BNNSs (e) in contrast to the reduction of 4-NP as a function of reaction time with excess amount of NaBH4 over various catalysts (f)
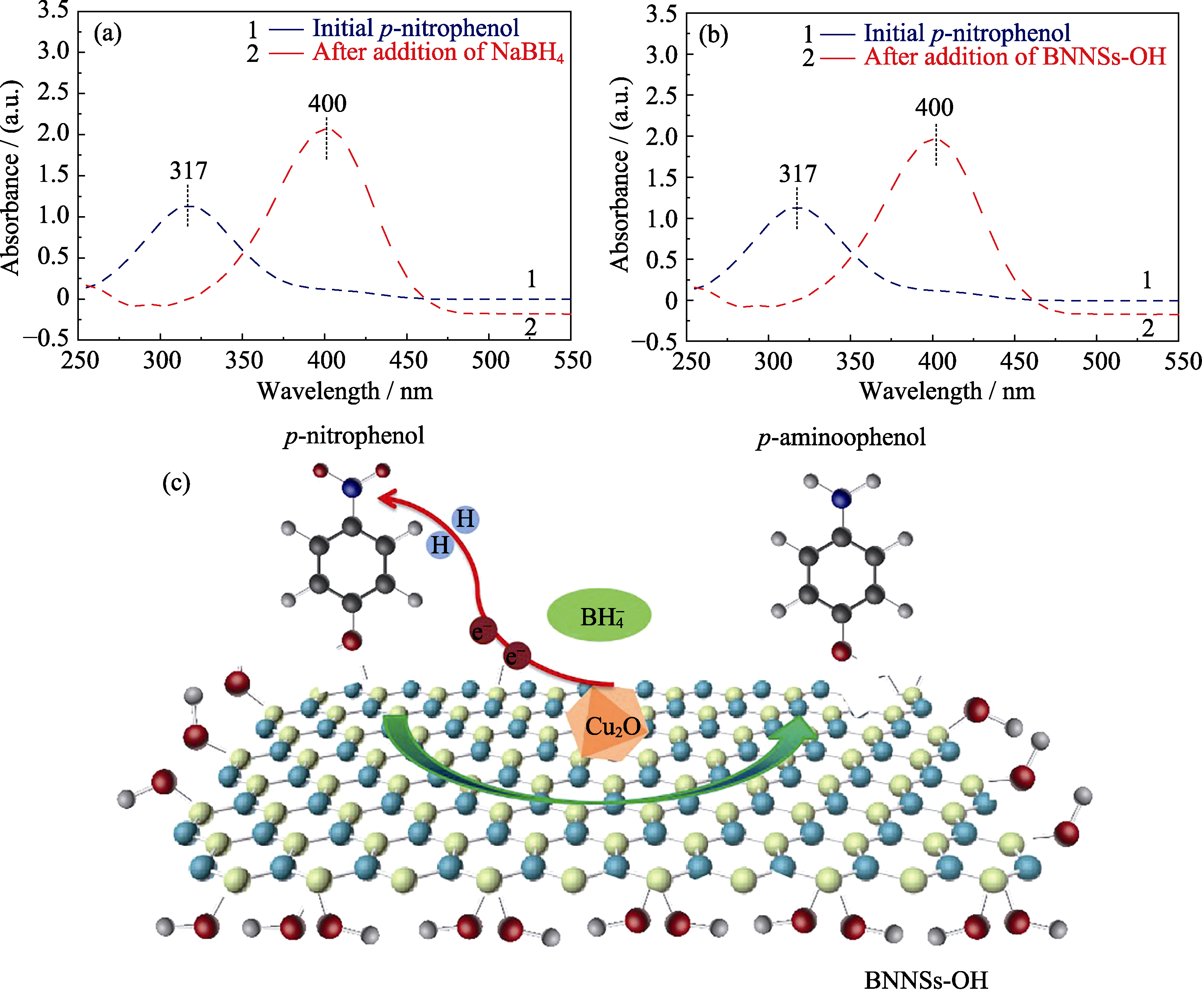
Fig. 10 UV-Vis absorption spectra of 4-NP solution before and after NaBH4 (a) and BNNSs-OH (b) additions, and schematic of the reduction of 4-NP to 4-AP over the Cu2O/ BNNSs-OH (c)
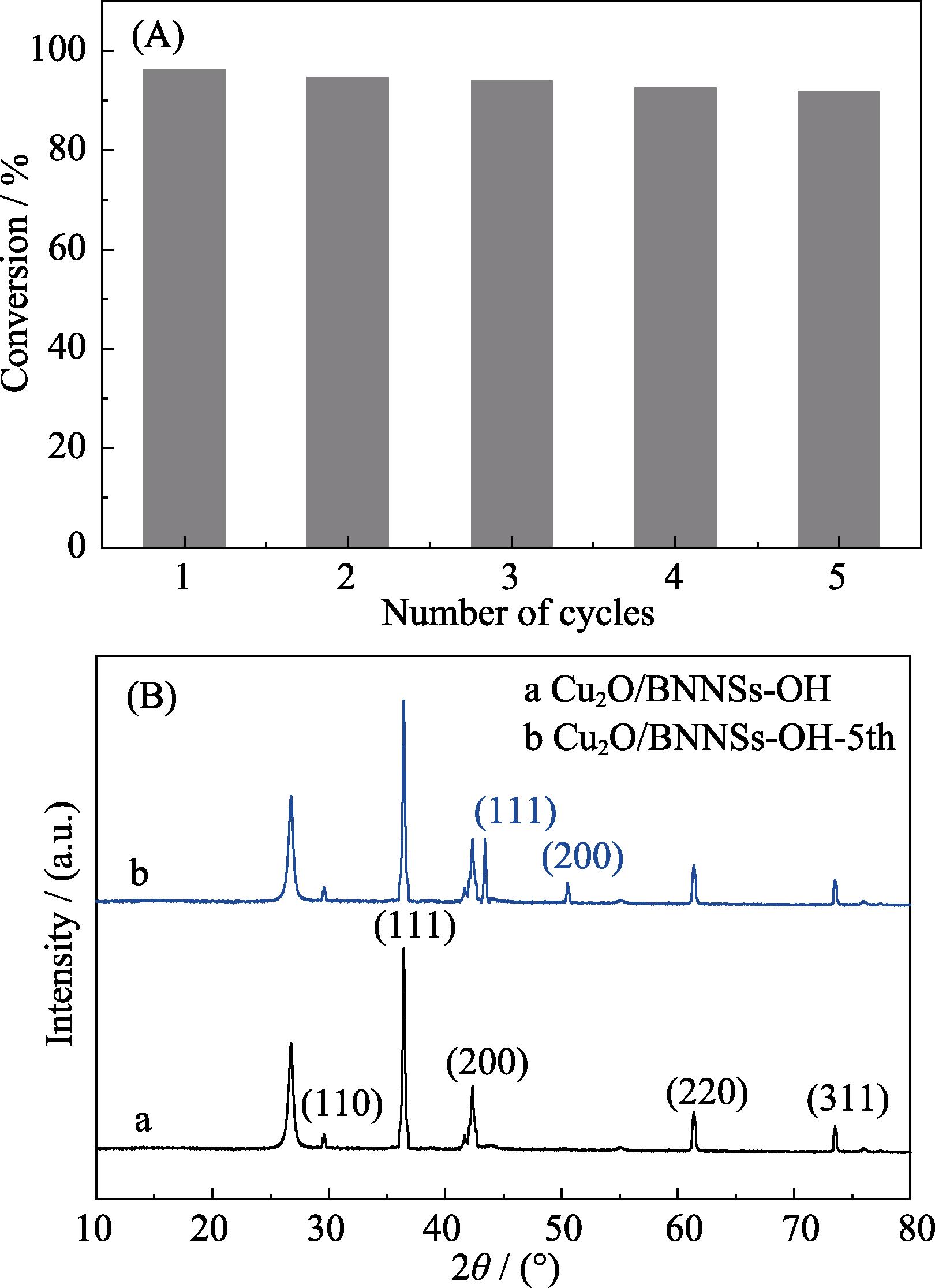
Fig. 11 Reusability of Cu2O/BNNSs-OH catalyst for the reduction of 4-NP with NaBH4 (A), XRD patterns of Cu2O/BNNSs- OH catalyst before and after five usages (B)
| [1] | RATH P C, SAIKIA D, MISHRA M , et al. Exceptional catalytic performance of ultrafine Cu2O nanoparticles confined in cubic mesoporous carbon for 4-nitrophenol reduction. Applied Surface Science, 2018,427:1217-1226. |
| [2] | NIU H, LIU S, CAI Y , et al. MOF derived porous carbon supported Cu/Cu2O composite as high performance non-noble catalyst. Microporous and Mesoporous Materials, 2016,219:48-53. |
| [3] | ROCHA M, COSTA P, PEREIRA C , et al. L-serine-functionalized montmorillonite decorated with Au nanoparticles: a new highly efficient catalyst for the reduction of 4-nitrophenol. Journal of Catalysis, 2018,361:143-155. |
| [4] | MAHAM M, NASROLLAHZADEH M, SAJADI S M , et al. Biosynthesis of Ag/reduced graphene oxide/Fe3O4 using Lotus garcinii leaf extract and its application as a recyclable nanocatalyst for the reduction of 4-nitrophenol and organic dyes. Journal of Colloid & Interface Science, 2017,497:33-42. |
| [5] | ZOU P P, WANG M S, ZHAO L , et al. One-step synthesis of Pt@three-dimensional graphene composite hydrogel: an efficient recyclable catalyst for reduction of 4-nitrophenol. Applied Organometallic Chemistry, 2016,30(8):722-725. |
| [6] | COCCIA F, TNUCCI L, BOSCO D , et al. One-pot synthesis of lignin-stabilised platinum and palladium nanoparticles and their catalytic behaviour in oxidation and reduction reaction. Green Chemistry, 2012,14(4):1073-1078. |
| [7] |
GACEM N, DIAO P . Effect of solvent polarity on the assembly behavior of PVP coated rhodium nanoparticles. Colloids and Surfaces A, 2013,417:32-38.
DOI URL |
| [8] | YANG X F, WANG A, QIAO B , et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Accounts of Chemical Research, 2013,46(8):1740-1748. |
| [9] | OH S D, KIM M R, CHOI S H , et al. Radiolytic synthesis of Pd-M (M=Ag, Au, Cu, Ni and Pt) alloy nanoparticles and their use in reduction of 4-nitrophenol. Journal of Industrial and Engineering Chemistry, 2008,14(5):687-692. |
| [10] | MUNNIK P, DE JONGH P E, DE JONG K P . Recent developments in the synthesis of supported catalysts. Chemical Reviews, 2015,46(38):6687-6714. |
| [11] | YAN X, WANG X, TANG Y , et al. Unusual loading-dependent sintering-resistant properties of gold nanoparticles supported within extra-large mesopores. Chemistry of Materials, 2013,25(9):1556-1563. |
| [12] | NAG A, RAIDOGIA K, HEMBRAM K P , et al. Graphene analogues of BN: novel synthesis and properties. ACS Nano, 2010,4(3):1539-1544. |
| [13] | LASKOWSKI R, BLAHA P, SCHWARZ K . Bonding of hexagonal BN to transition metal surfaces: an ab initio density-functional theory study. Physical Review B Condensed Matter, 2008,78(78):1436-1446. |
| [14] | HUANG C, CHEN C, YE X , et al. Stable colloidal boron nitride nanosheet dispersion and its potential application in catalysis. Journal of Materials Chemistry A, 2013,1(39):12192-12197. |
| [15] | ZHENG M, LIU Y, GU Y , et al. Synthesis and characterization of boron nitride sponges as a novel support for metal nanoparticles. Science in China Series B: Chemistry, 2008,51(3):205-210. |
| [16] | LIANG H L . Research on the physical properties of fully hydrogenated boron nitride films. Jinan: Shandong University. 2012:1-24. |
| [17] | LI C, WANG T L, WU Y Z , et al. Fabrication of two-dimensional nanosheets via water freezing expansion exfoliation. Nanotechnology, 2014,25(49):1-6. |
| [18] | GUARDIA L, PAREDES J I, ROZADA R , et al. Production of aqueous dispersions of inorganic graphene analogues by exfoliation and stabilization with non-ionic surfactants. RSC Advances, 2014,4(27):14115-14127. |
| [19] | LIU Q, KAZUAKI N, KENSUKE K , et al. Effects of reaction parameters on the preparation of submicron Cu particles by liquid phase reduction method and the study of reaction mechanism. Powder Technology, 2013,241(3):98-104. |
| [20] | SONG H, LI T, ZHANG J , et al. Highly anisotropic Sb2Se3 nanosheets: gentle exfoliation from the bulk precursors possessing 1D crystal structurep. Advanced Materials, 2017,29(29):1-7. |
| [21] | SMITH R J, KIBG P J, LOTYA M , et al. Large-scale exfoliation of inorganic layered compounds in aqueous surfactant solutions. Advanced Materials, 2011,23(34):3944-3948. |
| [22] |
MA P, SPENCER J T . Non-covalent stabilization and functionalization of boron nitride nanosheets (BNNSs) by organic polymers: formation of complex BNNSs-containing structures. Journal of Materials Science, 2015,50(1):313-323.
DOI URL |
| [23] |
GAO W, ZHAO Y, YIN H . Lateral size selection of liquid exfoliated hexagonal boron nitride nanosheets. RSC Advances, 2018,8:5976-5983.
DOI URL |
| [24] | HUMINIC G, HUMINIC A . Application of nanofluids in heat exchangers: a review. Renewable & Sustainable Energy Reviews, 2012,16(8):5625-5638. |
| [25] | PARK K S, LEE D Y, KIM K J , et al. Observation of a hexagonal BN surface layer on the cubic BN film grown by dual ion beam sputter deposition. Applied Physics Letters, 1997,70(3):315-317. |
| [26] | WAGNER C D, RIGGS W M, Davis L E , et al. Muilenber, handbook of X-ray Photoelectron Spectroscopy. erkin Elmer Corporation Physical Electronics Division, USA, 1979: 1-190. |
| [27] | ESPINOS J P, MORALES J, BARRANCO A , et al. Interface effects for Cu, CuO, and Cu2O deposited on SiO2 and ZrO2. XPS determination of the valence state of copper in Cu/SiO2 and Cu/ZrO2 Catalysts. Journal of Physical Chemistry B, 2002,106(27):6921-6929. |
| [28] | SUN Q, LI Y, SUN X , et al. Improved photoelectrical performance of single-crystal TiO2 nanorod arrays by surface sensitization with copper quantum dots. ACS Sustainable Chemistry & Engineering, 2013,1(7):798-804. |
| [29] | YAN X Y, TONG X L, ZHANG Y F , et al. Cuprous oxide nanoparticles dispersed on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. Chemical Communications, 2012,48(13):1892-1894. |
| [30] | MICHIKAZU H, TAKESHI K, MUTSUKO K , et al. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chemical Communication, 1998,3:357-358. |
| [31] | HUANG J, VOGEHR S, TANG S , et al. Highly catalytic Pd-Ag bimetallic dendrites. Journal of Physical Chemistry C, 2010,114(35):15005-15010. |
| [1] | Ren-Jie GENG, Song-Feng E, Chao-Wei LI, Tao-Tao LI, Jun WU, Ya-Gang YAO. High Crystallinity Boron Nitride Nanosheets: Preparation and the Property of BNNSs/Polyvinyl Alcohol Composite Film [J]. Journal of Inorganic Materials, 2019, 34(4): 401-406. |
| [2] | WU Shuang, LIU Bo, QIU Zhi-Che, CHEN Shi-Wei, ZHANG Juan-Nan, LIU Xiao-Lin, GU Mu, HUANG Shi-Ming, NI Chen. Improved Preparation Method and Luminescence Properties of LuTaO4:Ln3+(Ln=Eu,Tb) Thin Film [J]. Journal of Inorganic Materials, 2016, 31(4): 372-376. |
| [3] | ZHANG Hao, ZHANG Gao-Xiao, WU Xiang-Wei, WEN Zhao-Yin. Synthesis of Na-β-Al2O3 Nanopowders by PVP Sol-Gel Process [J]. Journal of Inorganic Materials, 2013, 28(9): 916-920. |
| [4] | ZHU Ding,LIU Heng,YAO Ya-Dong,LI Da-Cheng. Fabrication and Characterization of Nanostructural Vanadium Pentoxide Hollow Microspheres [J]. Journal of Inorganic Materials, 2008, 23(1): 43-48. |
| [5] | YAO Jian-Xi,ZHAO Gao-Ling,HAN Gao-Rong. Synthesis and Characterization of CdS Nanoparticles Modified with Organic [J]. Journal of Inorganic Materials, 2003, 18(2): 445-450. |
| [6] | MA Jing-Tao,XIE Zhi-Peng,MIAO He-Zhuo,HUANG Yong,CHENG Yi-Bing. Inhibitive Role and Mechanism of Water-Soluble Polymer PVP on the Surface-Exfoliation Problem of Ceramic Green Bodies Prepared byGelcasting [J]. Journal of Inorganic Materials, 2002, 17(3): 480-488. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||