
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (3): 277-283.DOI: 10.15541/jim20190377
Special Issue: 2020年环境材料论文精选(三)有机小分子去除; 【虚拟专辑】污染物吸附水处理(2020~2021)
Previous Articles Next Articles
ZHAO Chaofeng1,JIN Jiaren1,HUO Yingzhong1,SUN Lu2,AI Yuejie1( )
)
Received:2019-07-23
Revised:2019-09-23
Published:2020-03-20
Online:2019-12-04
About author:ZHAO Chaofeng (1995-), male, Master candidate. E-mail:cfzhao@ncepu.edu.cn
Supported by:CLC Number:
ZHAO Chaofeng, JIN Jiaren, HUO Yingzhong, SUN Lu, AI Yuejie. Adsorption of Phenolic Organic Pollutants on Graphene Oxide: Molecular Dynamics Study[J]. Journal of Inorganic Materials, 2020, 35(3): 277-283.
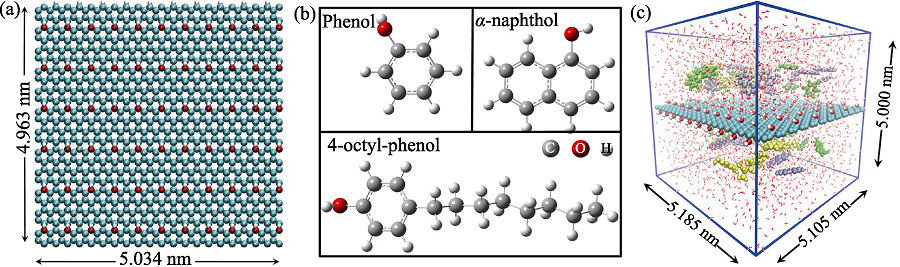
Fig. 1 (a) GO model, (b) structures of phenol, α-naphthol and 4-octyl-phenol molecules in MD simulations, and (c) initial configuration of phenol (green), α-naphthol (purple) and 4-octyl-phenol (yellow) molecules in the competitive system
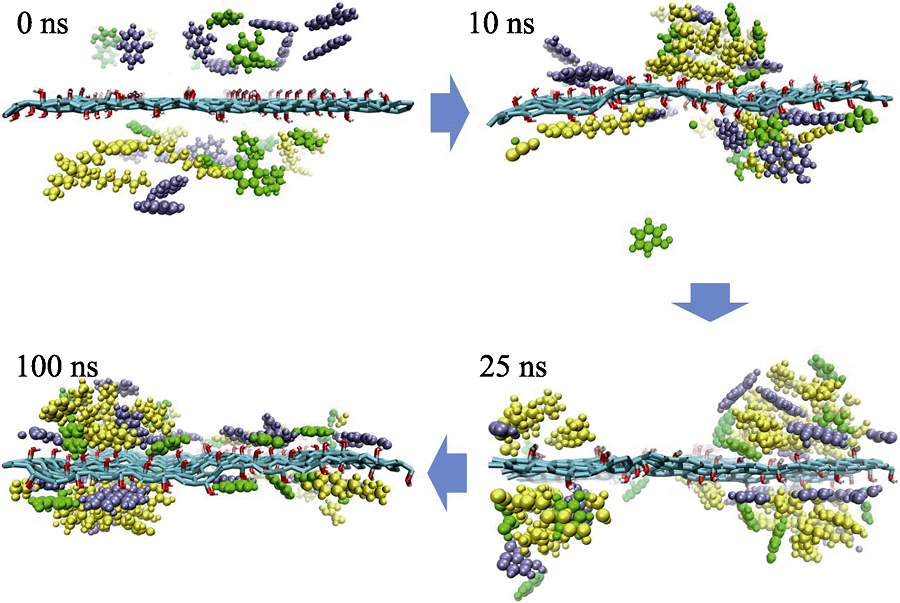
Fig. 3 Snapshots of competitive system from simulation process at different time Phenol, α-naphthol and 4-octyl-phenol molecules are shown as green, purple and yellow molecules, respectively. Water molecules are not shown to highlight the configuration
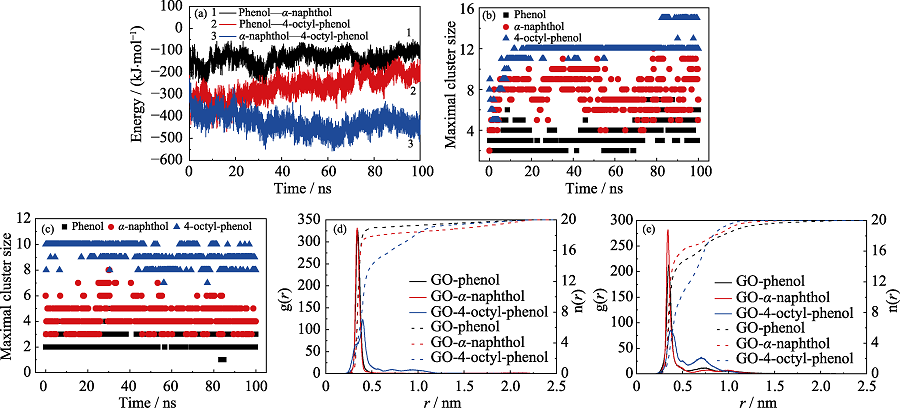
Fig. 5 (a) Interaction energies between different POPs molecules in competitive system; The maximal cluster size of POPs in (b) independent and (c) competitive systems, respectively; The radial distribution functions (g(r)) and coordination numbers (n(r)) of POPs in (d) independent and (e) competitive systems, respectively
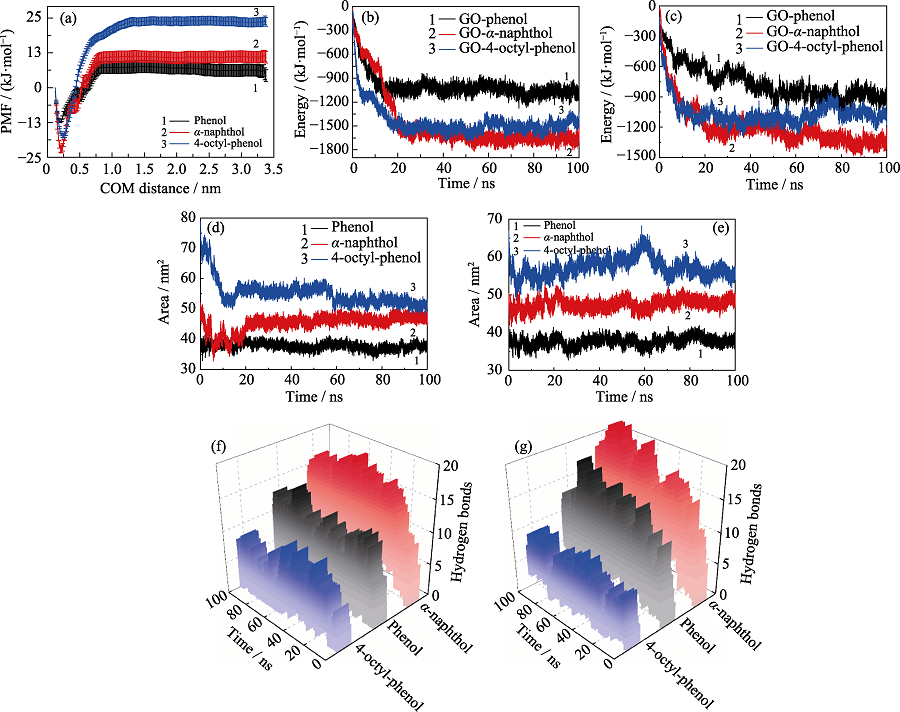
Fig. 6 (a) Potential of mean force of POPs molecules; The interaction energies between GO and POPs molecules in (b) independent and (c) competitive systems, respectively; The hydrophobic areas of POPs molecules in (d) independent and (e) competitive systems, respectively; The hydrogen bonds between GO and POPs molecules in (f) independent and (g) competitive systems, respectively
| Time/ns | Phenol/(kJ•mol-1) | α-naphthol/(kJ•mol-1) | 4-octyl-phenol/(kJ•mol-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coulomb interaction | L-J Potential | Total | Coulomb interaction | L-J Potential | Total | Coulomb interaction | L-J Potential | Total | |
| 20 | -44.10 | -866.02 | -910.12 | -317.12 | -1172.76 | -1489.87 | -195.98 | -1311.17 | -1507.15 |
| 40 | -174.71 | -877.44 | -1052.16 | -388.46 | -1276.89 | -1665.35 | -162.60 | -1359.01 | -1521.60 |
| 60 | -124.19 | -897.25 | -1021.45 | -405.37 | -1243.64 | -1649.01 | -96.07 | -1340.12 | -1436.19 |
| 80 | -167.61 | -864.44 | -1032.05 | -358.80 | -1313.62 | -1672.42 | -125.83 | -1454.64 | -1580.47 |
| 100 | -237.23 | -880.63 | -1117.87 | -251.36 | -1338.97 | -1590.33 | -62.78 | -1344.93 | -1407.71 |
Table S1 Interaction energies between GO and POPs molecules in independent system at different periods
| Time/ns | Phenol/(kJ•mol-1) | α-naphthol/(kJ•mol-1) | 4-octyl-phenol/(kJ•mol-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coulomb interaction | L-J Potential | Total | Coulomb interaction | L-J Potential | Total | Coulomb interaction | L-J Potential | Total | |
| 20 | -44.10 | -866.02 | -910.12 | -317.12 | -1172.76 | -1489.87 | -195.98 | -1311.17 | -1507.15 |
| 40 | -174.71 | -877.44 | -1052.16 | -388.46 | -1276.89 | -1665.35 | -162.60 | -1359.01 | -1521.60 |
| 60 | -124.19 | -897.25 | -1021.45 | -405.37 | -1243.64 | -1649.01 | -96.07 | -1340.12 | -1436.19 |
| 80 | -167.61 | -864.44 | -1032.05 | -358.80 | -1313.62 | -1672.42 | -125.83 | -1454.64 | -1580.47 |
| 100 | -237.23 | -880.63 | -1117.87 | -251.36 | -1338.97 | -1590.33 | -62.78 | -1344.93 | -1407.71 |
| Time/ns | Phenol/(kJ•mol-1) | α-naphthol/(kJ•mol-1) | 4-octyl-phenol/(kJ•mol-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coulomb interaction | L-J Potential | Total | Coulomb interaction | L-J Potential | Total | Coulomb interaction | L-J Potential | Total | |
| 20 | -160.11 | -448.40 | -608.51 | -235.85 | -914.54 | -1150.38 | -71.34 | -980.56 | -1051.90 |
| 40 | -240.86 | -533.62 | -774.48 | -288.30 | -960.43 | -1248.73 | -182.74 | -928.42 | -1111.16 |
| 60 | -334.12 | -573.27 | -907.39 | -354.11 | -993.34 | -1347.46 | -152.72 | -1013.83 | -1166.55 |
| 80 | -215.40 | -672.38 | -887.78 | -363.20 | -959.82 | -1323.02 | -201.10 | -912.11 | -1113.20 |
| 100 | -214.74 | -674.05 | -888.79 | -305.51 | -1069.33 | -1374.84 | -179.30 | -918.63 | -1097.92 |
Table S2 Interaction energies between GO and POPs molecules in competitive system at different periods
| Time/ns | Phenol/(kJ•mol-1) | α-naphthol/(kJ•mol-1) | 4-octyl-phenol/(kJ•mol-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coulomb interaction | L-J Potential | Total | Coulomb interaction | L-J Potential | Total | Coulomb interaction | L-J Potential | Total | |
| 20 | -160.11 | -448.40 | -608.51 | -235.85 | -914.54 | -1150.38 | -71.34 | -980.56 | -1051.90 |
| 40 | -240.86 | -533.62 | -774.48 | -288.30 | -960.43 | -1248.73 | -182.74 | -928.42 | -1111.16 |
| 60 | -334.12 | -573.27 | -907.39 | -354.11 | -993.34 | -1347.46 | -152.72 | -1013.83 | -1166.55 |
| 80 | -215.40 | -672.38 | -887.78 | -363.20 | -959.82 | -1323.02 | -201.10 | -912.11 | -1113.20 |
| 100 | -214.74 | -674.05 | -888.79 | -305.51 | -1069.33 | -1374.84 | -179.30 | -918.63 | -1097.92 |
| [1] | AHMARUZZAMAN M . Adsorption of phenolic compounds on low-cost adsorbents: a review. Advances in Colloid and Interface Science, 2008,143(1/2):48-67. |
| [2] | BABICH H, DAVIS D L . Phenol: a review of environmental and health risks. Regulatory Toxicology and Pharmacology, 1981,1(1):90-109. |
| [3] | KARTHIKEYAN K G, CHOROVER JON, BORTIATYNSKI JACKIE M , et al. Interaction of 1-naphthol and its oxidation products with aluminum hydroxide. Environmental Science & Technology, 1999,33(22):4009-4015. |
| [4] | PONZO OSVALDO J, SILVIA CARBONE . Evidence of reproductive disruption associated with neuroendocrine changes induced by UV-B filters, phthalates and nonylphenol during sexual maturation in rats of both gender. Toxicology, 2013,311(1/2):41-51. |
| [5] | YU SHU-JUN, WANG XIANG-XUE, YAO WEN , et al. Macroscopic, spectroscopic, and theoretical investigation for the interaction of phenol and naphthol on reduced graphene oxide. Environmental Science & Technology, 2017,51(6):3278-3286. |
| [6] | YU SHU-JUN, WANG XIANG-XUE, AI YUE-JIE , et al. Experimental and theoretical studies on competitive adsorption of aromatic compounds on reduced graphene oxides. Journal of Materials Chemistry A, 2016,4(15):5654-5662. |
| [7] | PAN BO, LIN DAO-HUI, MASHAYEKHI HAMID , et al. Adsorption and hysteresis of bisphenol a and 17α-ethinyl estradiol on carbon nanomaterials. Environmental Science & Technology, 2008,42(15):5480-5485. |
| [8] | RUESGAS-RAMON MARIANA, FIGUEROA-ESPINOZA MARIA CRUZ, DURAND ERWANN , Application of deep eutectic solvents (des) for phenolic compounds extraction: overview, challenges, and opportunities. Journal of Agricultural and Food Chemistry, 2017,65(18):3591-3601. |
| [9] | CIULU MARCO, CADIZ-GURREA MARIA DE LA LUZ, SEGURA-CARRETERO ANTONIO . Extraction and analysis of phenolic compounds in rice: a review. Molecules, 2018, 23(11):2890-1-20. |
| [10] | CASTRO-MUNOZ ROBERTO, YANEZ-FERNANDEZ JORGE, FILA VLASTIMIL . Phenolic compounds recovered from agro-food by-products using membrane technologies: an overview. Food Chemistry, 2016,213:753-762. |
| [11] | RAZA WASEEM, LEE JECHAN, RAZA NADEEM , et al. Removal of phenolic compounds from industrial waste water based on membrane-based technologies. Journal of Industrial and Engineering Chemistry, 2019,71:1-18. |
| [12] | ZYSZKA-HABERECHT BEATA, NIEMCZYK EMILIA, LIPOK JACEK . Metabolic relation of cyanobacteria to aromatic compounds. Applied Microbiology and Biotechnology, 2019,103(3):1167-1178. |
| [13] | SINGH PRIYARAGINI, KUMAR RAKESH . Critical review of microbial degradation of aromatic compounds and exploring potential aspects of furfuryl alcohol degradation. Journal of Polymers and the Environment, 2019,27(5):901-916. |
| [14] | LUO XU-BIAO, DENG FANG, MIN LU-JUAN , et al. Facile one-step synthesis of inorganic-framework molecularly imprinted TiO2/WO3 nanocomposite and its molecular recognitive photocatalytic degradation of target contaminant. Environmental Science & Technology, 2013,47(13):7404-7412. |
| [15] | SHAO PENG-HUI, TIAN JIA-YU, YANG FENG , et al. Identification and regulation of active sites on nanodiamonds: establishing a highly efficient catalytic system for oxidation of organic contaminants. Advanced Functional Materials, 2018,28(13):1705295. |
| [16] | BORTHAKUR PRIYAKSHREE, BORUAH PURNA K, DAS MANASH R , et al. Adsorption of 17α-ethynyl estradiol and β-estradiol on graphene oxide surface: an experimental and computational study. Journal of Molecular Liquids, 2018,269:160-168. |
| [17] | MOLLA ANIRUDDHA, LI YUAN-YUAN, MANDAL BIKASH , et al. Selective adsorption of organic dyes on graphene oxide: theoretical and experimental analysis. Applied Surface Science, 2019,464:170-177. |
| [18] | THAKUR KIRTI, KANDASUBRAMANIAN BALASUBRAMANIAN . Graphene and graphene oxide-based composites for removal of organic pollutants: a review. Journal of Chemical & Engineering Data, 2019,64(3):833-867. |
| [19] | MUKHERJEE MALOSHREE, GOSWAMI SUDIPTA, BANERJEE PRIYA , et al. Ultrasonic assisted graphene oxide nanosheet for the removal of phenol containing solution. Environmental Technology & Innovation, 2019,13:398-407. |
| [20] | ZHOU QING-XIANG, WANG YU-QIN, XIAO JUN-PING , et al. Fabrication and characterisation of magnetic graphene oxide incorporated Fe3O4@polyaniline for the removal of bisphenol A, t-octyl-phenol, and alpha-naphthol from water. Scientific Reports, 2017,7(1):11316. |
| [21] | TANG HUAN, ZHAO YING, SHAN SU-JIE , et al. Theoretical insight into the adsorption of aromatic compounds on graphene oxide. Environmental Science: Nano, 2018,5(10):2357-2367. |
| [22] | ZHENG HUI-LING, GAO YANG, ZHU KAI-RUO , et al. Investigation of the adsorption mechanisms of Pb(II) and 1-naphthol by beta-cyclodextrin modified graphene oxide nanosheets from aqueous solution. Journal of Colloid and Interface Science, 2018,530:154-162. |
| [23] | AMINPOUR MARAL, MONTEMAGNO CARLO, TUSZYNSKI JACK A . An overview of molecular modeling for drug discovery with specific illustrative examples of applications. Molecules, DOI: 10.3390/molecules24091693 |
| [24] | FENG DAI-LI, FENG YAN-HUI, QIU LIN , et al. Review on nanoporous composite phase change materials: fabrication, characterization, enhancement and molecular simulation. Renewable & Sustainable Energy Reviews, 2019,109:578-605. |
| [25] | SPONER JIRI, BUSSI GIOVANNI, KREPL MIROSLAV , et al. RNA structural dynamics as captured by molecular simulations: a comprehensive overview. Chemical Reviews, 2018,118(8):4177-4338. |
| [26] | MA ZHAO-YANG, PATHEGAMA GAMAGE RANJITH, RATHNAWEERA THARAKA , et al. Review of application of molecular dynamic simulations in geological high-level radioactive waste disposal. Applied Clay Science, 2019,168:436-449. |
| [27] | LIU LU-MENG, LIU JUN-JIE, PEI JING-JING . Towards a better understanding of adsorption of indoor air pollutants in porous media— from mechanistic model to molecular simulation. Building Simulation, 2018,11(5):997-1010. |
| [28] | TANG HUAN, ZHAO YING, YANG XIAO-NAN , et al. Understanding the pH-dependent adsorption of ionizable compounds on graphene oxide using molecular dynamics simulations. Environmental Science: Nano, 2017,4(10):1935-1943. |
| [29] | TANG HUAN, ZHAO YING, SHAN SU-JIE , et al. Wrinkle- and edge-adsorption of aromatic compounds on graphene oxide as revealed by atomic force microscopy, molecular dynamics simulation, and density functional theory. Environmental Science & Technology, 2018,52(14):7689-7697. |
| [30] | CHEN XIAO-XIAO, CHEN BAO-LIANG . Macroscopic and spectroscopic investigations of the adsorption of nitroaromatic compounds on graphene oxide, reduced graphene oxide, and graphene nanosheets. Environmental Science & Technology, 2015,49(10):6181-6189. |
| [31] | MART NEZ JOS MARIO, MART NEZ LEANDRO . Packing optimization for automated generation of complex system's initial configurations for molecular dynamics and docking. Journal of Computational Chemistry, 2003,24(7):819-825. |
| [32] | MART NEZ LEANDRO, ANDRADE RICARDO, BIRGIN ERNESTO G , et al. PACKMOL: a package for building initial configurations for molecular dynamics simulations. Journal of Computational Chemistry, 2009,30(13):2157-2164. |
| [33] | BERENDSEN H J C, GRIGERA J R, STRAATSMA T P . The missing term in effective pair potentials. Journal of Physical Chemistry, 1987,91(24):6269-6271. |
| [34] | JORGENSEN WILLIAM L, MAXWELL DAVID S, TIRADO- RIVES JULIAN . Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. Journal of the American Chemical Society, 1996,118(45):11225-11236. |
| [35] | WANG JUN-MEI, HOU TING-JUN . Application of molecular dynamics simulations in molecular property prediction I: density and heat of vaporization. Journal of Chemical Theory and Computation, 2011,7(7):2151-2165. |
| [36] | SUBASINGHEGE DON VISAL, DAVID ROLF, DU PU , et al. Interfacial water at graphene oxide surface: ordered or disordered? The Journal of Physical Chemistry B, 2019,123(7):1636-1649. |
| [37] | PRICE DANIEL J, BROOKS CHARLES L . Detailed considerations for a balanced and broadly applicable force field: a study of substituted benzenes modeled with OPLS-AA. Journal of Computational Chemistry, 2005,26(14):1529-1541. |
| [38] | KAMINSKI GEORGE A . Accurate prediction of absolute acidity constants in water with a polarizable force field: substituted phenols, methanol, and imidazole. The Journal of Physical Chemistry B, 2005,109(12):5884-5890. |
| [39] | BERENDSEN HERMAN J C, VAN DER SPOEL DAVID, VAN DRUNEN RUDI . GROMACS: a message-passing parallel molecular dynamics implementation. Computer Physics Communications, 1995,91(1/2/3):43-56. |
| [40] | VAN DER SPOEL DAVID, LINDAHL ERIK, HESS BERK , et al. GROMACS: fast, flexible, and free. Journal of Computational Chemistry, 2005,26(16):1701-1718. |
| [41] | WANG JUN, CHEN BAO-LIANG . Adsorption and coadsorption of organic pollutants and a heavy metal by graphene oxide and reduced graphene materials. Chemical Engineering Journal, 2015,281:379-388. |
| [1] | GAO Chenguang, SUN Xiaoliang, CHEN Jun, LI Daxin, CHEN Qingqing, JIA Dechang, ZHOU Yu. SiBCN-rGO Ceramic Fibers Based on Wet Spinning Technology: Microstructure, Mechanical and Microwave-absorbing Properties [J]. Journal of Inorganic Materials, 2025, 40(3): 290-296. |
| [2] | WANG Yue, WANG Xin, YU Xianli. Room-temperature Ferromagnetic All-carbon Films Based on Reduced Graphene Oxide [J]. Journal of Inorganic Materials, 2025, 40(3): 305-313. |
| [3] | DONG Yiman, TAN Zhan’ao. Research Progress of Recombination Layers in Two-terminal Tandem Solar Cells Based on Wide Bandgap Perovskite [J]. Journal of Inorganic Materials, 2023, 38(9): 1031-1043. |
| [4] | SUN Ming, SHAO Puzhen, SUN Kai, HUANG Jianhua, ZHANG Qiang, XIU Ziyang, XIAO Haiying, WU Gaohui. First-principles Study on Interface of Reduced Graphene Oxide Reinforced Aluminum Matrix Composites [J]. Journal of Inorganic Materials, 2022, 37(6): 651-659. |
| [5] | DONG Shurui, ZHAO Di, ZHAO Jing, JIN Wanqin. Effect of Ionized Amino Acid on the Water-selective Permeation through Graphene Oxide Membrane in Pervaporation Process [J]. Journal of Inorganic Materials, 2022, 37(4): 387-394. |
| [6] | LI Hao, TANG Zhihong, ZHUO Shangjun, QIAN Rong. High Performance of Room-temperature NO2 Gas Sensor Based on ZIF8/rGO [J]. Journal of Inorganic Materials, 2021, 36(12): 1277-1282. |
| [7] | ZHANG Sai, ZOU Yingtong, CHEN Zhongshen, LI Bingfeng, GU Pengcheng, WEN Tao. Visible-light-driven Activation of Persulfate by RGO/g-C3N4 Composites for Degradation of BPA in Wastewater [J]. Journal of Inorganic Materials, 2020, 35(3): 329-336. |
| [8] | LIN Qimin, CUI Jiangong, YAN Xin, YUAN Xueguang, CHEN Xiaoyu, LU Qichao, LUO Yanbin, HUANG Xue, ZHANG Xia, REN Xiaomin. First-principles Study on Electronic Structure and Optical Properties of Single Point Defect Graphene Oxide [J]. Journal of Inorganic Materials, 2020, 35(10): 1117-1122. |
| [9] | Feng ZHANG, Kai-Li ZHANG, Ming-Ming ZHOU, Chao CHEN, Zhi-Wei CAI, Guo-Hui WEI, Xing-Mao JIANG, Cheng ZHANG, RUHLMANN Laurent, Yao-Kang LÜ. A New Polyethylene Composite Material Based on Nano Silver Particels Loaded Graphene Oxide [J]. Journal of Inorganic Materials, 2019, 34(6): 633-640. |
| [10] | Zhi-Jun MA, Chang-Ye MANG, Hai-Tao ZHAO, Zhi-Hao GUAN, Liang CHENG. Comparison of Electromagnetism Behavior of Different Content Cobalt-zinc Ferrite Loaded with Graphene [J]. Journal of Inorganic Materials, 2019, 34(4): 407-416. |
| [11] | WANG Gui-Xin, PEI Zhi-Bin, YE Chang-Hui. Inkjet-printing and Performance Investigation of Self-powered Flexible Graphene Oxide Humidity Sensors [J]. Journal of Inorganic Materials, 2019, 34(1): 114-120. |
| [12] | QIN Shi-Lin, LI Ji-Cheng, LI Zhao-Hui, HU Zhong-Liang, DING Yan-Huai, LEI Gang-Tie, XIAO Qi-Zhen. Ferric Oxide-reduced Graphene Oxide Composite Material: Synthesis Based on Covalent Binding and Its Lithium-storage Property [J]. Journal of Inorganic Materials, 2018, 33(7): 741-748. |
| [13] | YAN Xin, LU Jin-Hua, HUI Xiao-Yan, YAN Cong-Xiang, GAO Qiang, SUN Guo-Dong. Preparation and Visible Light Photocatalytic Property of g-C3N4/MoS2 Nanosheets/GO Ternary Composite Photocatalyst [J]. Journal of Inorganic Materials, 2018, 33(5): 515-520. |
| [14] | WANG Yong, YU Yun, FENG Ai-Hu, JIANG Feng, HU Xue-Bing, SONG Li-Xin. Nafion Modified Graphene Aerogel with Hierarchical Porous Structures [J]. Journal of Inorganic Materials, 2018, 33(4): 469-474. |
| [15] | HUANG Yi-Hua, JIANG Dong-Liang, CHEN Zhong-Ming, LIU Xue-Jian, ZHANG Xian-Feng, LIAO Zhen-Kui, HUANG Zheng-Ren. Fabrication and Property of rGO/SiC Composite [J]. Journal of Inorganic Materials, 2018, 33(11): 1147-1153. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||