
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (9): 963-968.DOI: 10.15541/jim20170554
• Orginal Article • Previous Articles Next Articles
FENG Shou-Ai1, ZHOU Jun1, YANG Xuan-Yu2, LIU Hong1, HUANG Jiang-Feng1, BAI Jia-Feng1, CHENG Xiao-Wei2, DENG Yong-Hui2
Received:2017-11-20
Revised:2018-02-24
Published:2018-09-20
Online:2018-08-14
About author:FENG Shou-Ai. E-mail: 897178472@qq.com
Supported by:CLC Number:
FENG Shou-Ai, ZHOU Jun, YANG Xuan-Yu, LIU Hong, HUANG Jiang-Feng, BAI Jia-Feng, CHENG Xiao-Wei, DENG Yong-Hui. Synthesis of Pure-silica BETA Zeolite by the Method of Seed-direct Steam-assisted Crystallization[J]. Journal of Inorganic Materials, 2018, 33(9): 963-968.
| Code | Sample | Molar composition | (Seed/SiO2)/wt% | Temp.(℃)/Time (h) | Product |
|---|---|---|---|---|---|
| 1 | BETA-0T10S150/48 | 1SiO2:0TEAOH : 0.4NaOH : 9.4H2O | 10 | 150/48 | Amorphous |
| 2 | BETA-0.1T10S140/48 | 1SiO2 : 0.1TEAOH : 0.3NaOH : 9.4H2O | 10 | 150/48 | Amorphous |
| 3 | BETA-0.1T2S150/48 | 1SiO2 : 0.1TEAOH : 0.3NaOH : 9.4H2O | 2 | 150/48 | Amorphous |
| 4 | BETA-0.1T5S150/48 | 1SiO2 : 0.1TEAOH : 0.3NaOH : 9.4H2O | 5 | 150/48 | BETA |
| 5 | BETA-0.1T8S150/48 | 1SiO2 : 0.1TEAOH : 0.3NaOH : 9.4H2O | 8 | 150/48 | BETA |
| 6 | BETA-0.1T10S150/48 | 1SiO2 : 0.1TEAOH : 0.3NaOH : 9.4H2O | 10 | 150/48 | BETA |
| 7 | BETA-0.2T2S150/48 | 1SiO2 : 0.2TEAOH : 0.2NaOH : 9.4H2O | 2 | 150/48 | Amorphous |
| 8 | BETA-0.2T5S150/48 | 1SiO2 : 0.2TEAOH : 0.2NaOH : 9.4H2O | 5 | 150/48 | BETA |
| 9 | BETA-0.2T8S150/48 | 1SiO2 : 0.2TEAOH : 0.2NaOH : 9.4H2O | 8 | 150/48 | BETA |
| 10 | BETA-0.2T10S150/48 | 1SiO2 : 0.2TEAOH : 0.2NaOH : 9.4H2O | 10 | 150/48 | BETA |
| 11 | BETA-0.3T2S150/48 | 1SiO2 : 0.3TEAOH : 0.1NaOH : 9.4H2O | 2 | 150/48 | Amorphous |
| 12 | BETA-0.3T5S150/48 | 1SiO2 : 0.3TEAOH : 0.1NaOH : 9.4H2O | 5 | 150/48 | BETA |
| 13 | BETA-0.3T8S150/48 | 1SiO2 : 0.3TEAOH : 0.1NaOH : 9.4H2O | 8 | 150/48 | BETA |
| 14 | BETA-0.3T10S150/48 | 1SiO2 : 0.3TEAOH : 0.1NaOH : 9.4H2O | 10 | 150/48 | BETA |
| 15 | BETA-0.3T10S150/48Ⅰ | 1SiO2 : 0.3TEAOH : 0NaOH : 9.4H2O | 10 | 150/48 | Amorphous |
| 16 | BETA-0.3T10S150/48Ⅱ | 1SiO2 : 0.3TEAOH : 0NaOH : 9.4H2O : 0.1NaCl | 10 | 150/48 | Amorphous |
| 17 | BETA-0.3T10S150/48Ⅲ | 1SiO2 : 0.3TEAOH : 0.2NaOH : 9.4H2O | 10 | 150/48 | BETA |
Table 1 Synthesis conditions and products of pure silica BETA zeolite
| Code | Sample | Molar composition | (Seed/SiO2)/wt% | Temp.(℃)/Time (h) | Product |
|---|---|---|---|---|---|
| 1 | BETA-0T10S150/48 | 1SiO2:0TEAOH : 0.4NaOH : 9.4H2O | 10 | 150/48 | Amorphous |
| 2 | BETA-0.1T10S140/48 | 1SiO2 : 0.1TEAOH : 0.3NaOH : 9.4H2O | 10 | 150/48 | Amorphous |
| 3 | BETA-0.1T2S150/48 | 1SiO2 : 0.1TEAOH : 0.3NaOH : 9.4H2O | 2 | 150/48 | Amorphous |
| 4 | BETA-0.1T5S150/48 | 1SiO2 : 0.1TEAOH : 0.3NaOH : 9.4H2O | 5 | 150/48 | BETA |
| 5 | BETA-0.1T8S150/48 | 1SiO2 : 0.1TEAOH : 0.3NaOH : 9.4H2O | 8 | 150/48 | BETA |
| 6 | BETA-0.1T10S150/48 | 1SiO2 : 0.1TEAOH : 0.3NaOH : 9.4H2O | 10 | 150/48 | BETA |
| 7 | BETA-0.2T2S150/48 | 1SiO2 : 0.2TEAOH : 0.2NaOH : 9.4H2O | 2 | 150/48 | Amorphous |
| 8 | BETA-0.2T5S150/48 | 1SiO2 : 0.2TEAOH : 0.2NaOH : 9.4H2O | 5 | 150/48 | BETA |
| 9 | BETA-0.2T8S150/48 | 1SiO2 : 0.2TEAOH : 0.2NaOH : 9.4H2O | 8 | 150/48 | BETA |
| 10 | BETA-0.2T10S150/48 | 1SiO2 : 0.2TEAOH : 0.2NaOH : 9.4H2O | 10 | 150/48 | BETA |
| 11 | BETA-0.3T2S150/48 | 1SiO2 : 0.3TEAOH : 0.1NaOH : 9.4H2O | 2 | 150/48 | Amorphous |
| 12 | BETA-0.3T5S150/48 | 1SiO2 : 0.3TEAOH : 0.1NaOH : 9.4H2O | 5 | 150/48 | BETA |
| 13 | BETA-0.3T8S150/48 | 1SiO2 : 0.3TEAOH : 0.1NaOH : 9.4H2O | 8 | 150/48 | BETA |
| 14 | BETA-0.3T10S150/48 | 1SiO2 : 0.3TEAOH : 0.1NaOH : 9.4H2O | 10 | 150/48 | BETA |
| 15 | BETA-0.3T10S150/48Ⅰ | 1SiO2 : 0.3TEAOH : 0NaOH : 9.4H2O | 10 | 150/48 | Amorphous |
| 16 | BETA-0.3T10S150/48Ⅱ | 1SiO2 : 0.3TEAOH : 0NaOH : 9.4H2O : 0.1NaCl | 10 | 150/48 | Amorphous |
| 17 | BETA-0.3T10S150/48Ⅲ | 1SiO2 : 0.3TEAOH : 0.2NaOH : 9.4H2O | 10 | 150/48 | BETA |
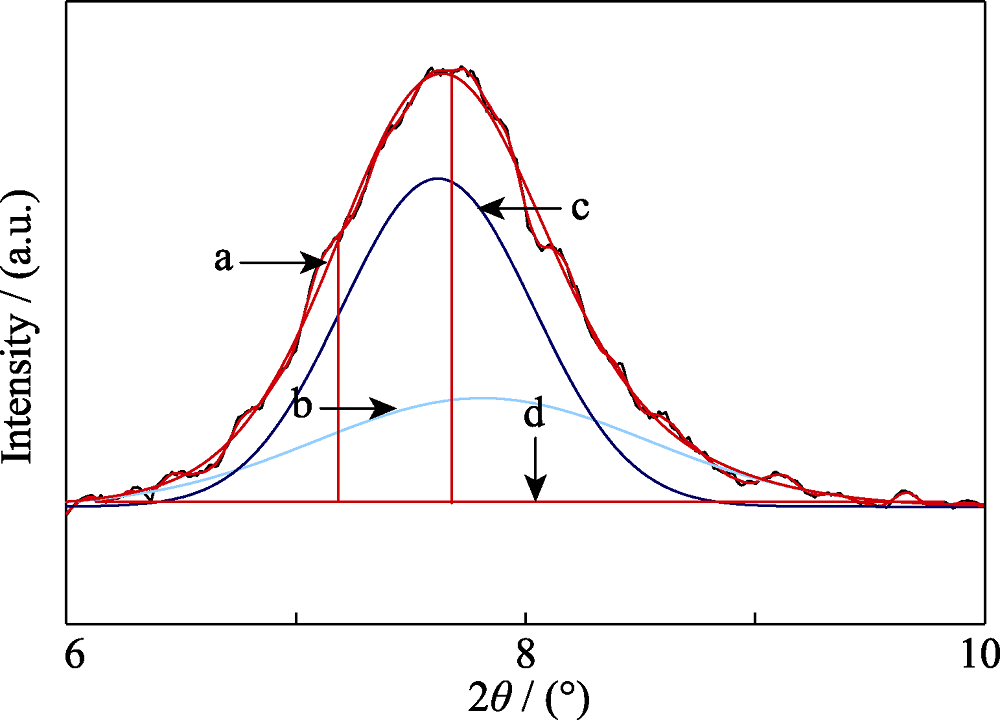
Fig. 3 Peak separation of the first low angle peak of pure silica zeolite beta(sample BETA-0.1T10S150/48)(a) Observed data; (b) Lower angle peak; (c) Higher angle peak; (d) Base line data
| Code | Sample | Height[cts] lower angle peak | Height[cts] higher angle peak | A/(A+B) peak ratio |
|---|---|---|---|---|
| 1 | BETA-0.3T 5S150/48 | 1000 | 1673 | 62.6% |
| 2 | BETA-0.3T 8S150/48 | 1102 | 1738 | 61.2% |
| 3 | BETA-0.3T 10S150/48 | 982 | 2073 | 67.9% |
| 4 | BETA-0.2T 5S150/48 | 1414 | 2235 | 61.2% |
| 5 | BETA-0.2T 8S150/48 | 945 | 2061 | 68.6% |
| 6 | BETA-0.2T 10S150/48 | 1139 | 2036 | 64.1% |
| 7 | BETA-0.1T 5S150/48 | 1358 | 1940 | 58.8% |
| 8 | BETA-0.1T 8S150/48 | 1211 | 2061 | 63.0% |
| 9 | BETA-0.1T 10S150/48 | 1252 | 2121 | 62.9% |
Table 2 Polymorphic enrichments in SAC samples
| Code | Sample | Height[cts] lower angle peak | Height[cts] higher angle peak | A/(A+B) peak ratio |
|---|---|---|---|---|
| 1 | BETA-0.3T 5S150/48 | 1000 | 1673 | 62.6% |
| 2 | BETA-0.3T 8S150/48 | 1102 | 1738 | 61.2% |
| 3 | BETA-0.3T 10S150/48 | 982 | 2073 | 67.9% |
| 4 | BETA-0.2T 5S150/48 | 1414 | 2235 | 61.2% |
| 5 | BETA-0.2T 8S150/48 | 945 | 2061 | 68.6% |
| 6 | BETA-0.2T 10S150/48 | 1139 | 2036 | 64.1% |
| 7 | BETA-0.1T 5S150/48 | 1358 | 1940 | 58.8% |
| 8 | BETA-0.1T 8S150/48 | 1211 | 2061 | 63.0% |
| 9 | BETA-0.1T 10S150/48 | 1252 | 2121 | 62.9% |
| Code | Sample | Average size/nm |
|---|---|---|
| 1 | BETA-0.3T5S150/48 | 310 |
| 2 | BETA-0.3T8S150/48 | 280 |
| 3 | BETA-0.3T10S150/48 | 200 |
| 4 | BETA-0.2T5S150/48 | 630 |
| 5 | BETA-0.2T8S150/48 | 420 |
| 6 | BETA-0.2T10S150/48 | 150 |
| 7 | BETA-0.1T5S150/48 | 300 |
| 8 | BETA-0.1T8S150/48 | 315 |
| 9 | BETA-0.1T10S150/48 | 190 |
Table 3 Crystal sizes of the pure BETA zeolite samples
| Code | Sample | Average size/nm |
|---|---|---|
| 1 | BETA-0.3T5S150/48 | 310 |
| 2 | BETA-0.3T8S150/48 | 280 |
| 3 | BETA-0.3T10S150/48 | 200 |
| 4 | BETA-0.2T5S150/48 | 630 |
| 5 | BETA-0.2T8S150/48 | 420 |
| 6 | BETA-0.2T10S150/48 | 150 |
| 7 | BETA-0.1T5S150/48 | 300 |
| 8 | BETA-0.1T8S150/48 | 315 |
| 9 | BETA-0.1T10S150/48 | 190 |
| Code | Sample | Si/Al | m1/g | m2/g | (m2-m1)/ m1 |
|---|---|---|---|---|---|
| 1 | BETA-0.3T10S150/48 | ∞ | 0.0698 | 0.0715 | 2.4% |
| 2 | BETA seeds | 27 | 0.0417 | 0.0558 | 33.8% |
Table 4 Water absorption amount of selected pure silica BETA zeolite sample and BETA seeds
| Code | Sample | Si/Al | m1/g | m2/g | (m2-m1)/ m1 |
|---|---|---|---|---|---|
| 1 | BETA-0.3T10S150/48 | ∞ | 0.0698 | 0.0715 | 2.4% |
| 2 | BETA seeds | 27 | 0.0417 | 0.0558 | 33.8% |
| [1] | WADLINGER R L, KERR G T, ROSINSKI E J,et al. Catalytic composition of a crystalline zeolite. U. S. A., C1, US 3308069. 1967. 03.07. |
| [2] | BENSLAMA R, FRAISSARD J, ABIZANE A,et al. An example of the technique of studying adsorbed xenon by 129Xe N.M.R.: aproximate determination of the internal void space of zeolite beta. Zeolites, 1988, 8(3): 196-198. |
| [3] | MARCILLA A, GÓMEZ-SIURANA A, BERENGUER D, et al. Reduction of tobacco smoke components yield in commercial cigarette brands by addition of HUSY, NaY and Al-MCM-41 to the cigarette rod. Toxico. Rep., 2015, 2(28): 152-164. |
| [4] | MARCILLA A, BELTRÁN M I, GÓMEZ-SIURANA A, et al. Effect of the concentration of siliceous materials added to tobacco cigarettes on the composition of the smoke generated during smoking. Ind. Eng. Chem. Res., 2015, 54(6): 1916-1929. |
| [5] | LI Y Y, CAO Y, YUE M B,et al. Hierarchical composites to reduce N-nitrosamines in cigarette smoke. Materials, 2015, 8(3): 1325-1340. |
| [6] | KUBIČKA D, KIKHTYANIN O. Opportunities for zeolites in biomass upgrading—lessons from the refining and petrochemical industry.Catal. Today, 2015, 243(243): 10-22. |
| [7] | KHALIL U, MURAZA O, KONDOH H,et al. Robust surface- modified beta zeolite for selective production of lighter fuels by steam-assisted catalytic cracking from heavy oil. Fuel, 2016, 168(1): 61-67. |
| [8] | VASILIADOU E S, GOULD, N S, LOBO R F.Zeolite-catalyzed formaldehyde-propylene prins condensation.ChemCatChem, 2017, 9(23): 4417-4425. |
| [9] | NEWSAM J M, TREACY M M J, KOETSIER W T, et al. Structural characterization of zeolite beta. Proc. R. Soc. Lond. A, 1988, 420(1859): 375-405. |
| [10] | HIGGINS J B, LAPIERRE R B, SCHLENKER J L,et al. The framework topology of zeolite beta. Zeolites, 1988, 8(6): 446-452. |
| [11] | YOSHIHIRO K, SHINYA T, KEIJI I,et al. Crystallization behavior of zeolite beta in OSDA-free, seed-assisted synthesis. J. Phys. Chem. C, 2011, 115(3): 744-750. |
| [12] | XU R R.Zeolite and Porous Materials, Beijing: Science Press, 2015: 171-175. |
| [13] | YOU H S, JIN H, MO Y,et al. CO2 adsorption behavior of microwave synthesized zeolite beta. Mater. Lett., 2013, 108(5): 106-109. |
| [14] | HONDA K, YASHIKI A, ITAKURA M,et al. Influence of seeding on FAU-*BEA interzeolite conversions. Micropor. Mesopor. Mater., 2011, 142(1): 161-167. |
| [15] | YANG H, YANG P, LIU X,et al. Space-confined synthesis of zeolite beta microspheres via steam-assisted crystallization. Chem. Eng. J., 2016, 299(10): 112-119. |
| [16] | VAN DER WAAL J C, GIGUTTO M S, VAN BEKKUM H. Synthesis of all-silica zeolite beta.J. Chem. Soc. Chem. Commun., 1994, 10(10): 1241-1242. |
| [17] | MÖLLER K, YILMAZ B, JACUBINAS R M,et al. One-step synthesis of hierarchical zeolite beta via network formation of uniform nanocrystals. J. Am. Chem. Soc., 2011, 133(14): 5284-5295. |
| [18] | WU Q, LIU X, ZHU L,et al. Solvent-free synthesis of zeolites from anhydrous starting raw solids. J. Am. Chem. Soc., 2015, 137(3): 1052-1055. |
| [19] | ZHU Z, XU H, JIANG J,et al. Structural reconstruction: a milestone in the hydrothermal synthesis of highly active Sn-beta zeolites. Chem. Commun., 2017, 53(93): 12516-12519. |
| [20] | TAKAGI Y, KOMATSU T, KITABANA Y.Crystallization of zeolite beta in the presence of chiral amine or rhodium complex.Micropor. Mesopor. Mater., 2008, 109(1/2/3): 567-576. |
| [21] | PETUSHKOV A, MERILIS G, LARSEN S C.From nanoparticles to hierarchical structures: controlling the morphology of zeolite beta.Micropor. Mesopor. Mater., 2011, 143(1): 97-103. |
| [22] | GONZÁLES-RIVERA JOSÉ, GALINDO-ESQUIVEL IGNACIO R, ONOR MASSIMO,et al. Heterogeneous catalytic reaction of microcrystalline cellulose in hydrothermal microwave-assisted decomposition: effect of modified zeolite beta. Green Chem., 2014, 16(3): 1417-1425. |
| [1] | CHANG Li-Na,QU Shu-Xin, LIN Sun-Zhong, DUAN Ke, WENGJie. Preparation and Biological Properties of HA Containing Astragalus Polysaccharides [J]. Journal of Inorganic Materials, 2011, 26(1): 22-28. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||