
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (2): 229-236.DOI: 10.15541/jim20170312
• Orginal Article • Previous Articles Next Articles
LI Qiang1, SHI Wan-Yan1, ZHANG Chen2, JIANG Dan-Yu3
Received:2017-06-23
Revised:2017-11-24
Published:2018-02-26
Online:2018-01-26
CLC Number:
LI Qiang, SHI Wan-Yan, ZHANG Chen, JIANG Dan-Yu. SO2 Non-equilibrium Gas Sensor Based on Na3Zr2Si2PO12 Solid Electrolyte[J]. Journal of Inorganic Materials, 2018, 33(2): 229-236.
| 260℃ | 300℃ | 400℃ | 500℃ | 600℃ | |||||
|---|---|---|---|---|---|---|---|---|---|
| R | S | R | S | R | S | R | S | R | S |
| 0.99 | 160 | 0.99 | 59 | 0.99 | 37 | 0.99 | 106 | 0.99 | 101 |
Table 1 Linearity (R) and sensitivity (S) of the sensors based on Na2SO4-BaSO4 mixtures at different temperatures
| 260℃ | 300℃ | 400℃ | 500℃ | 600℃ | |||||
|---|---|---|---|---|---|---|---|---|---|
| R | S | R | S | R | S | R | S | R | S |
| 0.99 | 160 | 0.99 | 59 | 0.99 | 37 | 0.99 | 106 | 0.99 | 101 |
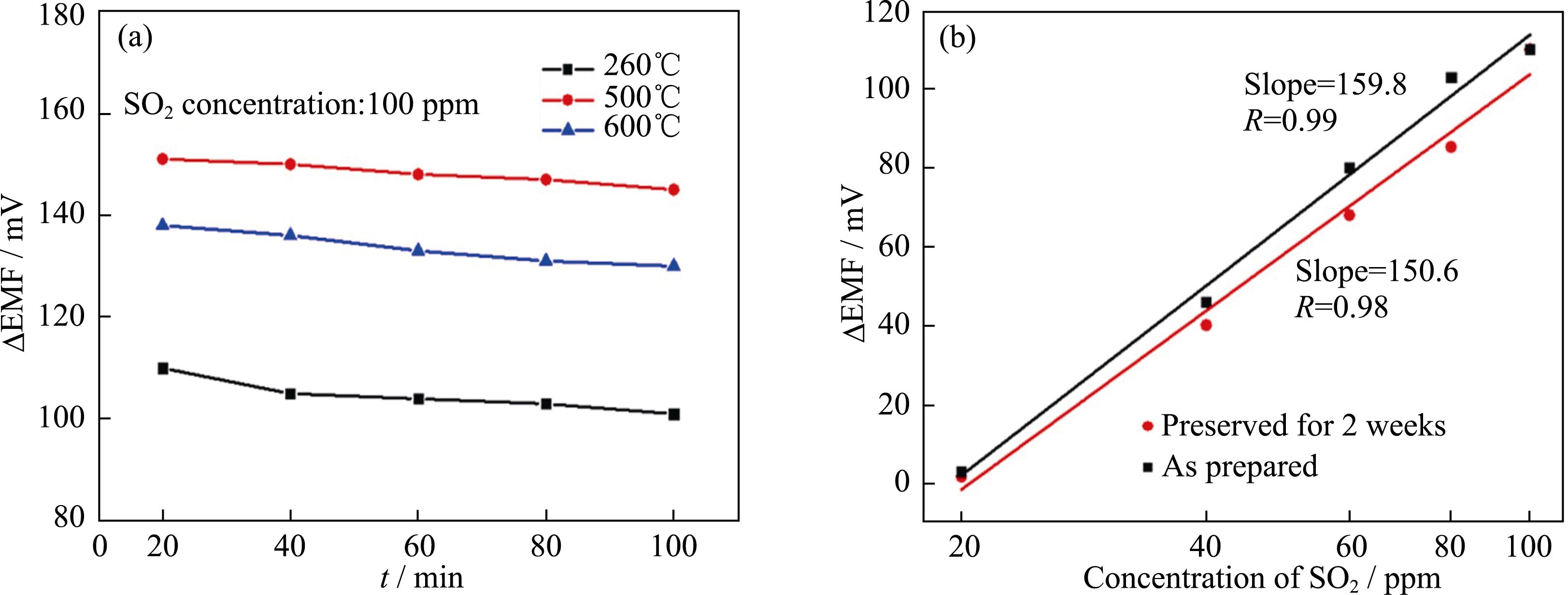
Fig. 4 Repeatability of the output electric potential for the sensor based on Na2SO4-BaSO4 mixtures (a) Different temperature; (b)Different preservation time(black-as prepared; red-preserved for two weeks)
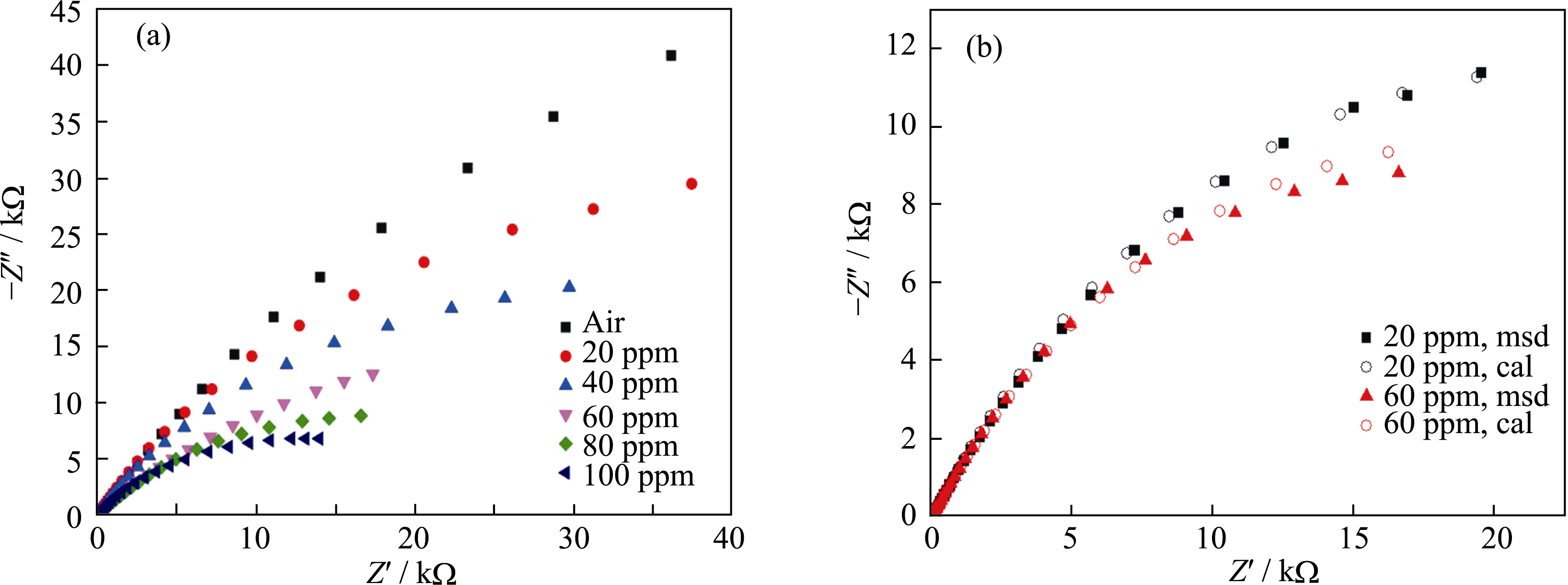
Fig. 6 (a) Impedance spectra and (b) equivalent circuit fitting curves of the sensor based on Na2SO4-BaSO4 mixtures under the atmophere with different concentrations of SO2
| SO2 | Rs/Ω | Rp/kΩ |
|---|---|---|
| 0 ppm | 43.02 | 248.90 |
| 20 ppm | 43.02 | 96.91 |
| 40 ppm | 44.12 | 67.15 |
| 60 ppm | 44.37 | 49.72 |
| 80 ppm | 44.05 | 37.81 |
| 100 ppm | 45.01 | 23.14 |
Table 2 Electrolyte resistance (Rs) and electrode resistance (Rp) of the sensor based on Na2SO4-BaSO4 mixtures at 260℃
| SO2 | Rs/Ω | Rp/kΩ |
|---|---|---|
| 0 ppm | 43.02 | 248.90 |
| 20 ppm | 43.02 | 96.91 |
| 40 ppm | 44.12 | 67.15 |
| 60 ppm | 44.37 | 49.72 |
| 80 ppm | 44.05 | 37.81 |
| 100 ppm | 45.01 | 23.14 |
| SO2 conc. | Rs/Ω | Rp/kΩ |
|---|---|---|
| 21% O2 | 77.02 | 172.9 |
| 20 ppm | 74.55 | 97.21 |
| 40 ppm | 73.85 | 66.76 |
| 60 ppm | 72.11 | 48.89 |
| 80 ppm | 70.06 | 34.75 |
| 100 ppm | 66.89 | 22.55 |
Table 3 Electrolyte resistance (Rs) and electrode resistance (Rp) of the sensor based on NaLa(SO4)2 at 260℃
| SO2 conc. | Rs/Ω | Rp/kΩ |
|---|---|---|
| 21% O2 | 77.02 | 172.9 |
| 20 ppm | 74.55 | 97.21 |
| 40 ppm | 73.85 | 66.76 |
| 60 ppm | 72.11 | 48.89 |
| 80 ppm | 70.06 | 34.75 |
| 100 ppm | 66.89 | 22.55 |
| [1] | BERGLEN T F, BERNTSEN T K, ISAKSEN I S A, et al. A global model of the coupled sulfur/oxidant chemistry in the troposphere: the sulfur cycle.Journal of Geophysical Research, 2004, 10(5): 1564-1567. |
| [2] | SCOTT BARRETT, KATHRYN GRADDY.Freedom, growth, and the environment.Environment and Development Economics, 2000, 5(4): 433-456. |
| [3] | LIU FANGMENG, WANG YINGLIN, BIN WANG, et al.Stabilized zirconia-based mixed potential type sensors utilizing MnNb2O6 sensing electrode for detection of low-concentration SO2. Sensors and Actuators B, 2017, 238: 1024-1031. |
| [4] | RITTE THOMAS, HAGEN GUNTER, KITA JAROSLAW, et al.Self-heated HTCC-based ceramic disc for mixed potential sensors and for direct conversion sensors for automotive catalysts.Sensors and Actuators B, 2017, 248: 793-802. |
| [5] | DAS S, RANA S, MURSALIN SK MD, et al.Sonochemically prepared nanosized BiFeO3 as novel SO2 sensor.Sensors and Actuators B, 2015, 218: 122-127. |
| [6] | CHOI SOON-DON, CHUNG WAN-YOUNG, LEE DUK- DONG. SO2 sensing characteristics of Nasicon electrolytes. Sensors and Actuators B, 1996, 35-36: 263-266. |
| [7] | SUGANUM SHIGEAKI, WATANABE MISA, KOBAYASHI TUYOSHI, et al.SO2 gas sensor utilizing stabilized zirconia and sulfate salts with a new working mechanism.Solid State Ionics, 1999, 126: 175-179. |
| [8] | SKEAFF J M, DUBREUIL A A.Electrochemical measurement of SO3-SO2 in process gas streams.Sensors and Actuators B, 1993, 10: 161-168. |
| [9] | WU JIAN, ZHANG CHENG, LI QIANG, et al.Application of TiO2 to amperometric NOx sensors based on NASICON.Solid State Ionics, 2016, 292: 32-37. |
| [10] | TAKEDA HIROTAKA, UEDA TARO, KAMADA KAI, et al.CO-sensing properties of a NASICON-based gas sensor attached with Pt mixed with Bi2O3 as a sensing electrode.Electrochimica Acta, 2015, 155: 8-15. |
| [11] | LORENC PIOTR, STRZELCZYK ANNA, CHACHULSKI BOGDAN, et al.Properties of Nasicon-based CO2 sensors with Bi8Nb2O17 reference electrode.Solid State Ionics, 2015, 271: 48-55. |
| [12] | HUANG LE-ZHI, WEN ZHAO-YIN, JIN JUN, et al.Preparation and characterization of PEO-LATP/LAGP ceramic composite electrolyte membrane for lithium batteries. Journal of Inorganic Materials, 2012, 27(3): 249-252. |
| [13] | ZHANG ZHI-ZHEN, SHI SI-QI, HU YONG-SHEN, et al.Sol-Gel synthesis and conductivity properties of sodium ion solid state electrolytes Na3Zr2Si2PO12.Journal of Inorganic Materials, 2013, 28(11): 1255-1260. |
| [14] | LIANG X, ZHONG T, QUAN B, et al.Solid-state potentiometric SO2 sensor combining NASICON with V2O5-doped TiO2 electrode.Sensors and Actuators B, 2008, 134: 25-30. |
| [15] | IZU N, HAGEN G, SCHÖNAUER D, et al. Planar potentiometric SO2 gas sensor for high temperatures using NASICON electrolyte combined with V2O5/WO3/TiO2 + Au or Pt electrode.Journal of the Ceramic Society of Japan, 2011, 119: 687-691. |
| [16] | CHIOU CHIOU-YEN, CHOU TSE-CHUAN.Amperometric SO2 gas sensors based on solid polymer electrolytes.Sensors and Actuators B, 2002, 87: 1-7. |
| [17] | MIN BONG-KI, CHOI SOON-DON.SO2-sensing characteristics of Nasicon sensors with Na2SO4-BaSO4 auxiliary electrolytes.Sensors and Actuators B, 2003, 93: 209-213. |
| [1] | WU Xing, LI Hai-Feng, ZHOU Jin-Ling, HUO Min-Feng, CHENG Cheng, SHEN Xu-Gen, YAN Chun-Jie. Fabrication and Properties of Highly Pure BiFeO3 Using A Method of Solid State Reaction-molten Salt Synthesis with Non-equilibrium Process [J]. Journal of Inorganic Materials, 2014, 29(11): 1151-1155. |
| [2] | HUANG Le-Zhi, WEN Zhao-Yin, JIN Jun, LIU Yu. Preparation and Characterization of PEO-LATP/LAGP Ceramic Composite Electrolyte Membrane for Lithium Batteries [J]. Journal of Inorganic Materials, 2012, 27(3): 249-252. |
| [3] | WANG Deng-Ke,HUANG Hao,YU Kuai,ZHANG Xue-Feng,DONG Xing-Long. Synthesis and Microwave Absorption of the Silica-coated Fe Nanocomposites [J]. Journal of Inorganic Materials, 2009, 24(2): 340-344. |
| [4] | ZHU Qi-Feng,QIU Fa-Bin,QUAN Yu-Jun1,WANG Yong-Wei,QUAN Bao-Fu,XU Bao-Kun. Preparation and Sintering Densification of Nanocrystalline NASICON Solid Material [J]. Journal of Inorganic Materials, 2004, 19(3): 510-516. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||