无机材料学报 ›› 2024, Vol. 39 ›› Issue (12): 1348-1356.DOI: 10.15541/jim20240172 CSTR: 32189.14.10.15541/jim20240172
所属专题: 【能源环境】氢能材料(202506)
肖文艳1,2( ), 付艳1,2, 杨书镔1,2, 朱洁1,2, 程照阳2, 温小煦1,2, 唐嘉繁1,2, 于亮1,2, 张骞2(
), 付艳1,2, 杨书镔1,2, 朱洁1,2, 程照阳2, 温小煦1,2, 唐嘉繁1,2, 于亮1,2, 张骞2( )
)
收稿日期:2024-04-08
修回日期:2024-07-25
出版日期:2024-09-02
网络出版日期:2024-09-02
通讯作者:
张 骞, 副教授. E-mail: zhangqian@swpu.edu.cn作者简介:肖文艳(1999-), 女, 硕士研究生. E-mail: xiao18780293769@163.com
基金资助:
XIAO Wenyan1,2( ), FU Yan1,2, YANG Shubin1,2, ZHU Jie1,2, CHENG Zhaoyang2, WEN Xiaoxu1,2, TANG Jiafan1,2, YU Liang1,2, ZHANG Qian2(
), FU Yan1,2, YANG Shubin1,2, ZHU Jie1,2, CHENG Zhaoyang2, WEN Xiaoxu1,2, TANG Jiafan1,2, YU Liang1,2, ZHANG Qian2( )
)
Received:2024-04-08
Revised:2024-07-25
Published:2024-09-02
Online:2024-09-02
Contact:
ZHANG Qian, associate professor. E-mail: zhangqian@swpu.edu.cnAbout author:Xiao Wenyan (1999-), female, Master candidate. E-mail: xiao18780293769@163.com
Supported by:摘要:
为了解决现有的能源危机, 实现持续的海水电解, 需要设计高效的电催化剂来应对阳极析氧反应缓慢与氯离子(Cl-)腐蚀的问题。本研究在泡沫镍(NF)骨架上采用一步式水热法制备了一种具有独特纳米结构的改性Ce-FeHPi/NF电极。实验结果表明, Ce掺杂调节了FeHPi/NF的表面形貌, 形成了非晶型纳米球, 这不仅使催化层生长为致密紧实的纳米结构, 而且大幅提高了电极的活性表面积, 从而明显提高了电催化活性。此外, 磷酸基可有效排斥电极表面的Cl-, 增强其耐腐蚀性, 使其在海水中长期稳定运行。10%Ce-FeHPi/NF电极在碱性模拟海水(1 mol·L-1 KOH + 0.5 mol·L-1 NaCl)电解液中, 仅需要较低的过电位(296 mV)即可达到100 mA·cm-2的电流密度。在1 mol·L-1 KOH + 1 mol·L-1 NaCl电解液中, 10%Ce-FeHPi/NF电极在恒电位1.774 V(vs. RHE)下实现了超过130 h的稳定运行。本研究所制备的改性纳米结构材料有效提高了电极的析氧活性, 为海水电解阳极催化材料的发展提供了一条新的途径。
中图分类号:
肖文艳, 付艳, 杨书镔, 朱洁, 程照阳, 温小煦, 唐嘉繁, 于亮, 张骞. 自支撑非晶Ce-FeHPi/NF电极的电解海水性能研究[J]. 无机材料学报, 2024, 39(12): 1348-1356.
XIAO Wenyan, FU Yan, YANG Shubin, ZHU Jie, CHENG Zhaoyang, WEN Xiaoxu, TANG Jiafan, YU Liang, ZHANG Qian. Seawater Electrolysis Performance of Self-supported Amorphous Ce-FeHPi/NF Electrode[J]. Journal of Inorganic Materials, 2024, 39(12): 1348-1356.
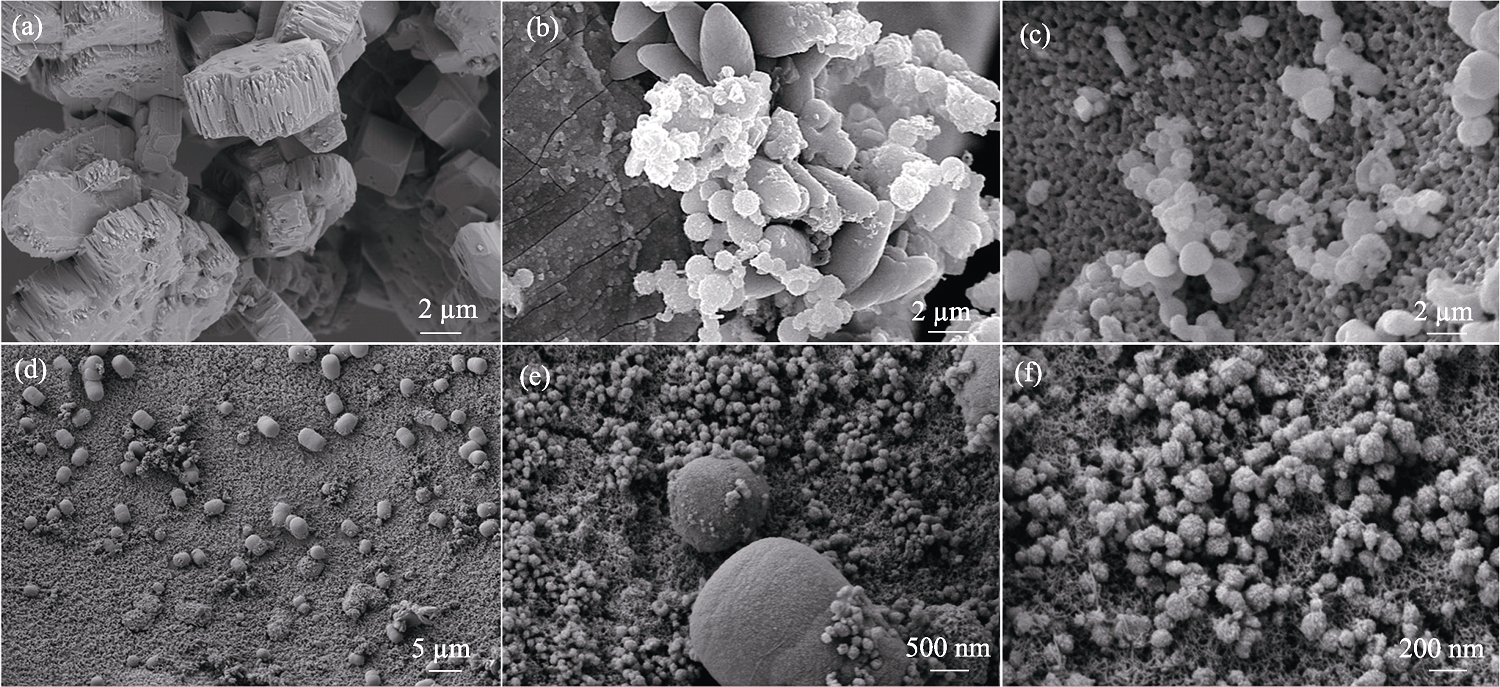
图2 (a)FeHPi/NF、(b)5%Ce-FeHPi/NF、(c)15%Ce-FeHPi/NF和(d~f)10%Ce-FeHPi/NF的SEM照片
Fig. 2 SEM images of (a) FeHPi/NF, (b) 5%Ce-FeHPi/NF, (c) 15%Ce-FeHPi/NF, and (d-f) 10%Ce-FeHPi/NF
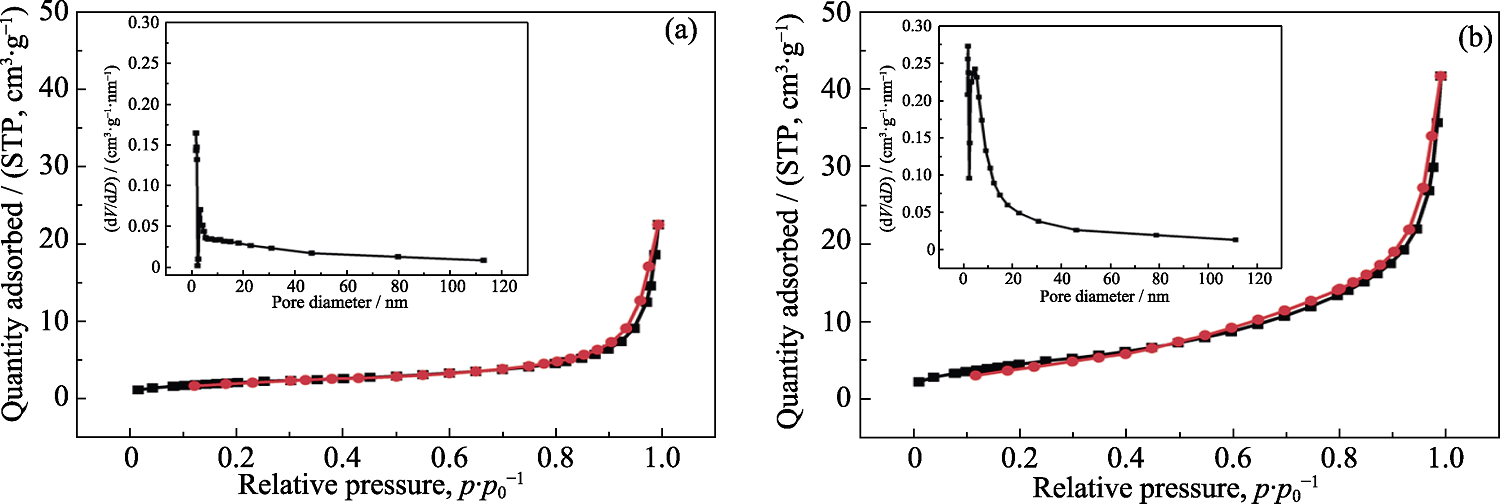
图3 (a)FeHPi/NF和(b)10%Ce-FeHPi/NF的氮气吸脱附曲线和孔径分布曲线
Fig. 3 Nitrogen adsorption/desorption curves and pore size distributions for (a) FeHPi/NF and (b) 10%Ce-FeHPi/NF
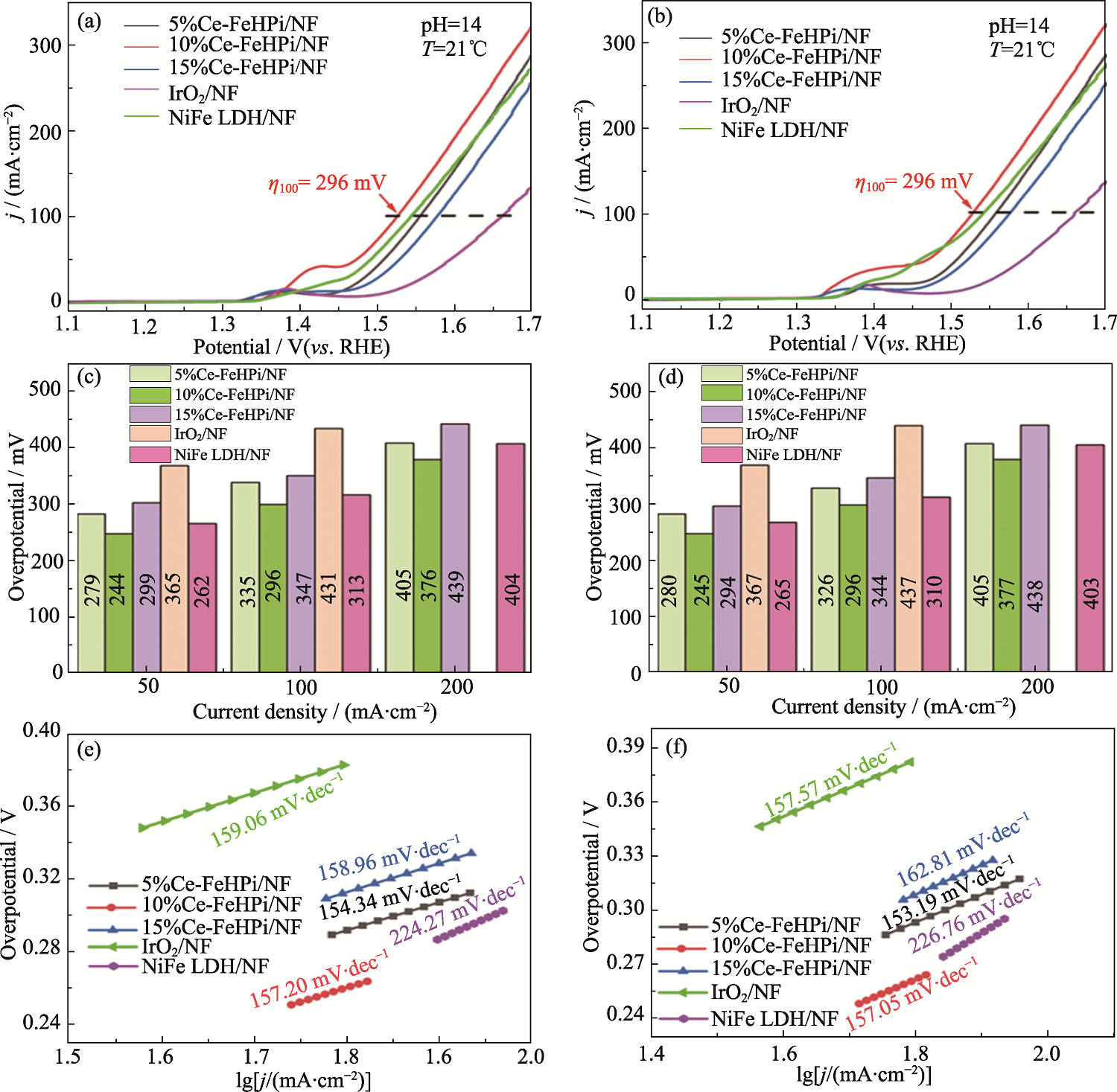
图7 Ce-FeHPi/NF在不同电解液中的(a, b)OER极化曲线、(c, d)过电位和(e, f)Tafel图谱
Fig. 7 (a, b) OER polarization curves, (c, d) overpotentials, and (e, f) Tafel plots of Ce-FeHPi/NF samples in different electrolytes (a, c, e) 1 mol·L-1 KOH; (b, d, f) 1 mol·L-1 KOH + 0.5 mol·L-1 NaCl; Colorful figures are available on website
| Element | Weight/% |
|---|---|
| Fe | 1.0341 |
| Ce | 0.2593 |
| P | 2.8334 |
表S1 ICP-OES分析10%Ce-FeHPi/NF中Fe、P和Ce的含量
Table S1 Amounts of Fe, P and Ce in 10%Ce-FeHPi/NF determined by ICP-OES
| Element | Weight/% |
|---|---|
| Fe | 1.0341 |
| Ce | 0.2593 |
| P | 2.8334 |

图S2 电极在不同电解液中的(a, b)OER极化曲线、(c, d)过电位和(e, f)Tafel图谱
Fig. S2 (a, b) OER polarization curves, (c, d) overpotentials, and (e, f) Tafel plots of electrodes in different electrolytes (a, c, e) 1 mol·L-1 KOH; (b, d, f) 1 mol·L-1 KOH + 0.5 mol·L-1 NaCl

图S3 在非法拉第反应区间不同扫描速率下的循环伏安曲线
Fig. S3 Cyclic voltammetric curves at different scanning rates in non-Faraday reaction intervals (a) FeHPi/NF; (b) 5%Ce-FeHPi/NF; (c) 10%Ce-FeHPi/NF; (d) 15%Ce-FeHPi/NF; 1 mol·L-1 KOH
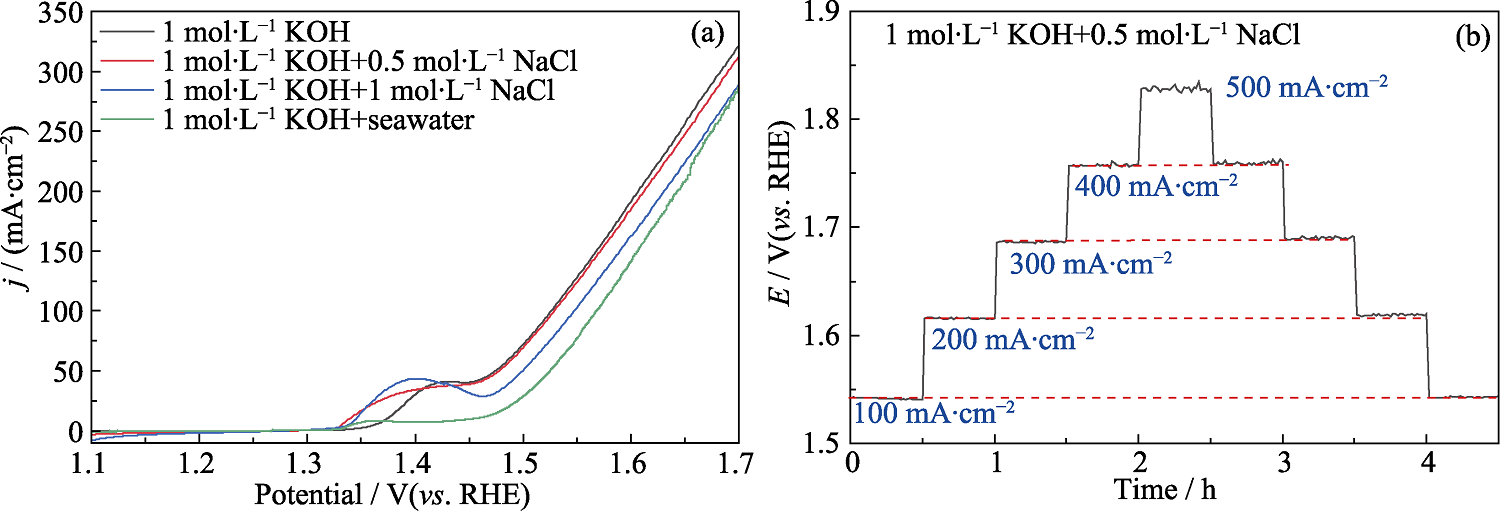
图S5 10%Ce-FeHPi/NF(a)在不同电解液中的OER极化曲线、(b)在1 mol·L-1 KOH + 0.5 mol·L-1 NaCl中的恒电流阶跃图
Fig. S5 (a) OER polarization curves in different electrolytes, and (b) constant current step plot of 10%Ce-FeHPi/NF in 1 mol·L-1 KOH + 0.5 mol·L-1 NaCl

图S6 长期稳定性测试后电解液的余氯检测结果(1 mol·L-1 KOH + 1 mol·L-1 NaCl, E=1.774 V(vs. RHE), 130 h)
Fig. S6 Residual chlorine testing for the electrolyte after the long-term stability test (1 mol·L-1 KOH + 1 mol·L-1 NaCl, E=1.774 V(vs. RHE), 130 h)
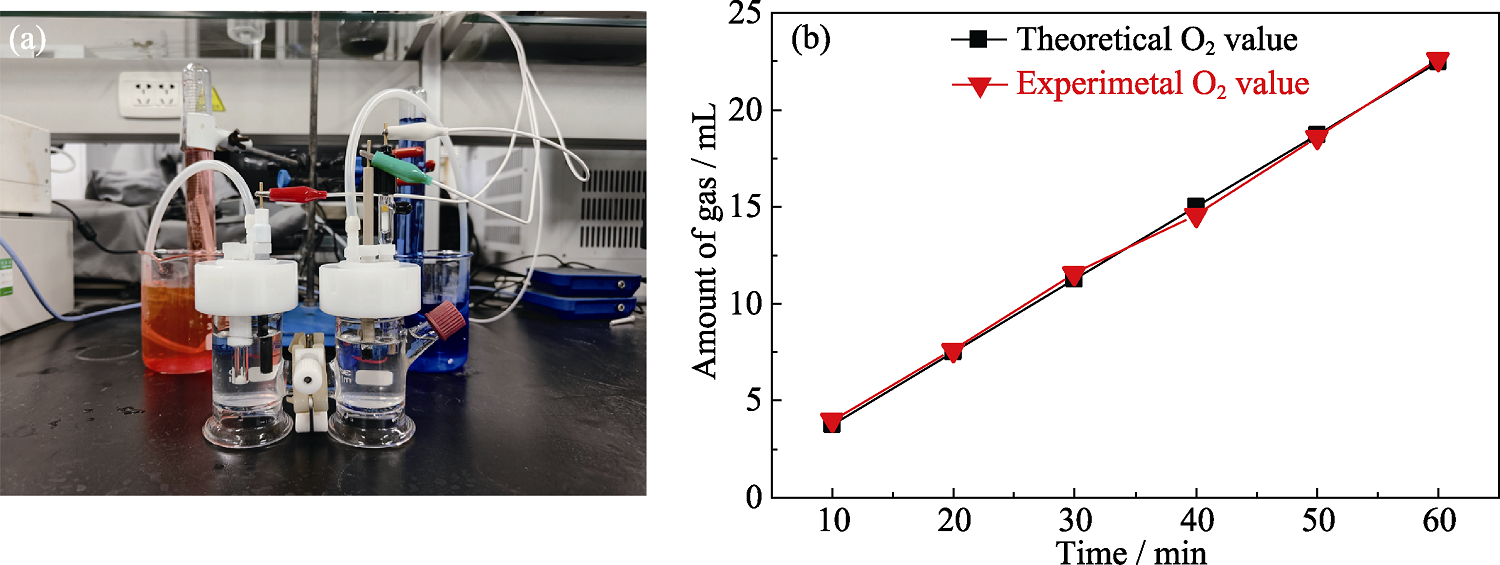
图S7 10%Ce-FeHPi/NF的法拉第效率测试
Fig. S7 Faraday efficiency test of 10%Ce-FeHPi/NF (a) Schematic diagram of testing equipment; (b) Faraday efficiency results
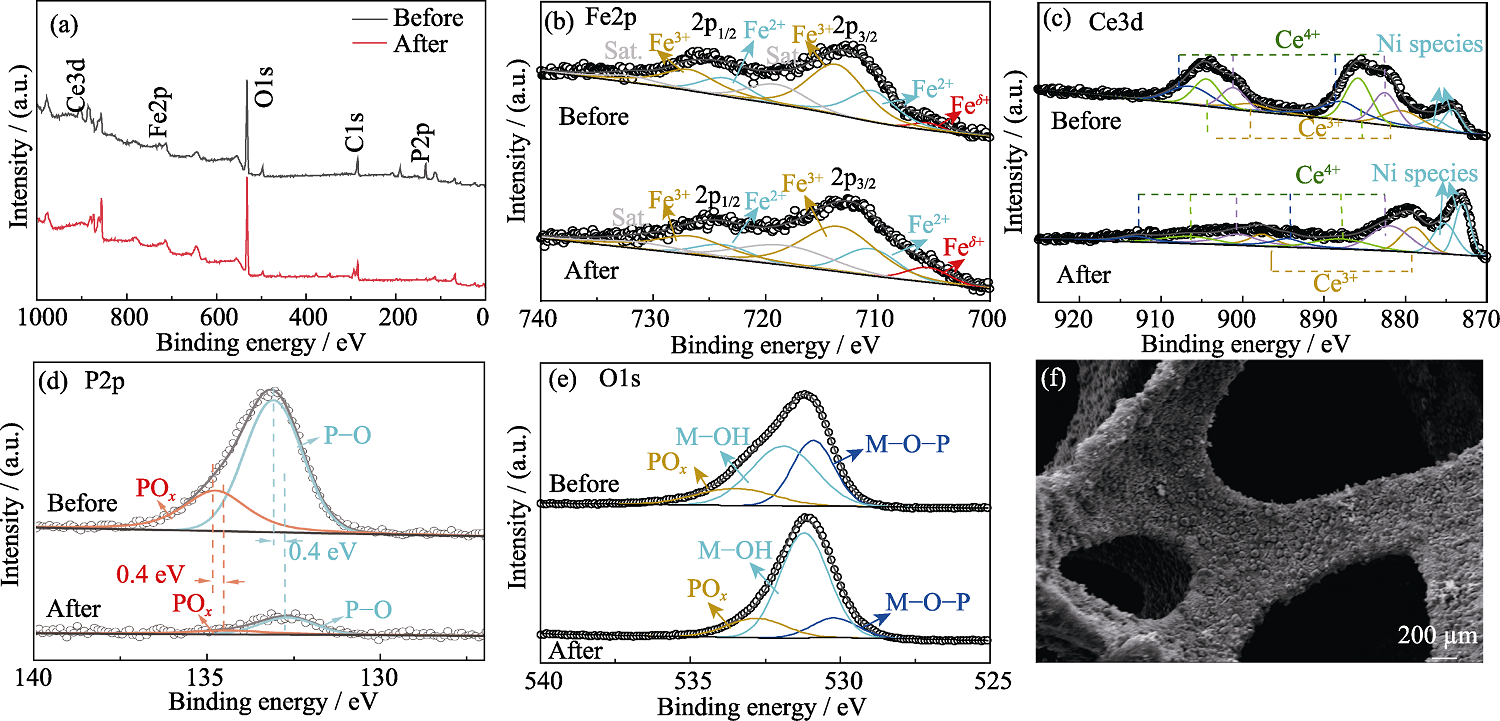
图S8 10%Ce-FeHPi/NF长时间反应后的(a~e)XPS谱图和(f)SEM照片
Fig. S8 (a-e) XPS spectra and (f) SEM image of 10%Ce-FeHPi/NF after long reaction (a) Full spectra; (b) Fe2p; (c) Ce3d; (d) P2p; (e) O1s
| [1] | YANG L, LIU Z, ZHU S, et al. Ni-based layered double hydroxide catalysts for oxygen evolution reaction. Materials Today Physics, 2021, 16: 100292. |
| [2] | ZHOU Q, LIAO L, BIAN Q, et al. Engineering in-plane nickel phosphide heterointerfaces with interfacial sp H-P hybridization for highly efficient and durable hydrogen evolution at 2 A·cm-2. Small, 2022, 18(4):2105642. |
| [3] |
MO Y, LIAO L, LI D, et al. Development prospects of metal-based two-dimensional nanomaterials in lithium-sulfur batteries. Chinese Chemical Letters, 2023, 34(1):107130.
DOI |
| [4] | AN P, YANG B, ZHANG N, et al. Hybrid TaON/LaTiO2N photoelectrode for water oxidation. Transportation Safety and Environment, 2019, 1(3):212. |
| [5] | WAN X, ZHAO Y, LI Z, et al. Emerging polymeric electrospun fibers: from structural diversity to application in flexible bioelectronics and tissue engineering. Exploration, 2022, 2(1):20210029. |
| [6] |
JIANG L L, XU S S, XIA B K, et al. Defect engineering of graphene hybrid catalysts for oxygen reduction reactions. Journal of Inorganic Materials, 2022, 37(2):215.
DOI |
| [7] |
ZHAO Z, CHENG G, ZHANG Y, et al. Metal-organic-framework based functional materials for uranium recovery: performance optimization and structure/functionality-activity relationships. ChemPlusChem, 2021, 86(8):1177.
DOI PMID |
| [8] |
TURNER J A. Sustainable hydrogen production. Science, 2004, 305(5686):972.
DOI PMID |
| [9] | RASHID M M, AL MESFER M K, NASEEM H, et al. Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high-temperature water electrolysis. International Journal of Engineering and Advanced Technology, 2015, 4(3):80. |
| [10] | STIBER S, SATA N, MORAWIETZ T, et al. A high-performance, durable and low-cost proton exchange membrane electrolyser with stainless steel components. Energy & Environmental Science, 2022, 15(1):109. |
| [11] | CARMO M, FRITZ D L, MERGEL J, et al. A comprehensive review on PEM water electrolysis. International Journal of Hydrogen Energy, 2013, 38(12):4901. |
| [12] | CHAE K J, CHOI M, AJAYI F F, et al. Mass transport through a proton exchange membrane (nafion) in microbial fuel cells. Energy & Fuels, 2008, 22(1):80. |
| [13] |
MAURITZ K A, MOORE R B. State of understanding of nafion. Chemical Reviews, 2004, 104(10):4535.
DOI PMID |
| [14] | LAGUNA-BERCERO M A. Recent advances in high-temperature electrolysis using solid oxide fuel cells: a review. Journal of Power Sources, 2012, 203: 4. |
| [15] | KUPKA J, BUDNIOK A. Electrolytic oxygen evolution on Ni- Co-P alloys. Journal of Applied Electrochemistry, 1990, 20(6):1015. |
| [16] | GUANG H L, ZHU S L, LIANG Y Q, et al. Highly efficient nanoporous CoBP electrocatalyst for hydrogen evolution reaction. Rare Metals, 2021, 40(5):1031. |
| [17] | WANG C, SHANG H, JIN L, et al. Advances in hydrogen production from electrocatalytic seawater splitting. Nanoscale, 2021, 13(17):7897. |
| [18] |
蒋博龙, 崔艳艳, 史顺杰, 等. 双金属氮化物NiMoN析氢催化剂制备及其电解海水析氢性能的研究. 化学学报, 2022, 80(10):1394.
DOI |
| [19] | CONG Y, CHEN X, MEI Y, et al. CeO2 decorated bimetallic phosphide nanowire arrays for enhanced oxygen evolution reaction electrocatalysis via interface engineering. Dalton Transactions, 2022, 51(7):2923. |
| [20] | YANG J, WANG Y, YANG J, et al. Quench-induced surface engineering boosts alkaline freshwater and seawater oxygen evolution reaction of porous NiCo2O4 nanowires. Small, 2022, 18(3):2106187. |
| [21] |
QIU Y, LI W, ZHAO W, et al. High-rate, ultralong cycle-life lithium/sulfur batteries enabled by nitrogen-doped graphene. Nano Letters, 2014, 14(8):4821.
DOI PMID |
| [22] | UL HAQ T, MANSOUR S, HAIK Y. Electronic and structural modification of Mn3O4 nanosheets for selective and sustained seawater oxidation. ACS Applied Materials & Interfaces, 2022, 14(18):20443. |
| [23] | ZHANG L, ZHANG R, GE R, et al. Facilitating active species generation by amorphous NiFe-Bi layer formation on NiFe-LDH nanoarray for efficient electrocatalytic oxygen evolution at alkaline pH. Chemistry — A European Journal, 2017, 23(48):11499. |
| [24] | LI P, ZHAO S, HUANG Y, et al. Corrosion resistant multilayered electrode comprising Ni3N nanoarray overcoated with NiFe-phytate complex for boosted oxygen evolution in seawater electrolysis. Advanced Energy Materials, 2024, 14(8):2303360. |
| [25] | SONG S, WANG Y, ZHOU S, et al. One-step synthesis of heterostructural MoS2-(FeNi)9S8 on Ni-Fe foam synergistically boosting for efficient fresh/seawater electrolysis. ACS Applied Energy Materials, 2022, 5(2): 1810. |
| [26] | SONG S, WANG Y, LIU X, et al. Synthesis of Mo-doped NiFe- phosphate hollow bird-nest architecture for efficient and stable seawater electrolysis. Applied Surface Science, 2022, 604: 154588. |
| [27] | SUN H, SUN J, SONG Y, et al. Nickel-cobalt hydrogen phosphate on nickel nitride supported on nickel foam for alkaline seawater electrolysis. ACS Applied Materials & Interfaces, 2022, 14(19):22061. |
| [28] | SONG S, ZANG J, ZHOU S, et al. Self-supported amorphous nickel-iron phosphorusoxides hollow spheres on Ni-Fe foam for highly efficient overall water splitting. Electrochimica Acta, 2021, 392: 138996. |
| [29] | WU L, YU L, ZHANG F, et al. Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater splitting. Advanced Functional Materials, 2021, 31(1):2006484. |
| [30] | LI M, PAN X, JIANG M, et al. Interface engineering of oxygen- vacancy-rich CoP/CeO2 heterostructure boosts oxygen evolution reaction. Chemical Engineering Journal, 2020, 395: 125160. |
| [31] | WANG B, XI P, SHAN C, et al. In situ growth of ceria on cerium- nitrogen-carbon as promoter for oxygen evolution reaction. Advanced Materials Interfaces, 2017, 4(13):1700272. |
| [32] | WANG Y, TAO S, LIN H, et al. NaBH4 induces a high ratio of Ni3+/Ni2+ boosting OER activity of the NiFe LDH electrocatalyst. RSC Advances, 2020, 10(55):33475. |
| [33] | MAHALA C, DEVI SHARMA M, BASU M. Fe-doped nickel hydroxide/nickel oxyhydroxide function as an efficient catalyst for the oxygen evolution reaction. ChemElectroChem, 2019, 6(13):3488. |
| [1] | 夏天, 孟燮, 骆婷, 占忠亮. 固体氧化物燃料电池LaxSr2-3x/2Fe1.5Ni0.1Mo0.4O6-δ阳极性能研究[J]. 无机材料学报, 2020, 35(5): 617-622. |
| [2] | 刘建哲, 郭鹏飞. VS2 纳米片: 一种十分有潜力的锂电池阳极材料[J]. 无机材料学报, 2015, 30(12): 1339-1344. |
| [3] | 常希望, 陈 宁, 王丽君, 卞刘振, 李福燊, 周国治. 固体氧化物燃料电池阳极材料成份的优化规律[J]. 无机材料学报, 2015, 30(10): 1043-1048. |
| [4] | 刘亚迪, 袁 春, 周玉存, 邹 杰, 辛显双, 王绍荣. Ni浸渍的LST-SSZ复合阳极及其直接甲烷固体氧化物燃料电池研究[J]. 无机材料学报, 2014, 29(11): 1121-1126. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||