无机材料学报 ›› 2023, Vol. 38 ›› Issue (10): 1216-1222.DOI: 10.15541/jim20230161 CSTR: 32189.14.10.15541/jim20230161
所属专题: 【能源环境】污染物催化去除(202506)
张瑞阳1,2( ), 王壹1,2, 欧博文2, 周莹1,2(
), 王壹1,2, 欧博文2, 周莹1,2( )
)
收稿日期:2023-04-03
修回日期:2023-05-11
出版日期:2023-10-20
网络出版日期:2023-05-14
通讯作者:
周 莹, 教授. E-mail: yzhou@swpu.edu.cn作者简介:张瑞阳(1990-), 男, 博士. E-mail: ryzhang@swpu.edu.cn
基金资助:
ZHANG Ruiyang1,2( ), WANG Yi1,2, OU Bowen2, ZHOU Ying1,2(
), WANG Yi1,2, OU Bowen2, ZHOU Ying1,2( )
)
Received:2023-04-03
Revised:2023-05-11
Published:2023-10-20
Online:2023-05-14
Contact:
ZHOU Ying, professor. E-mail: yzhou@swpu.edu.cnAbout author:ZHANG Ruiyang (1990-), male, PhD. E-mail: ryzhang@swpu.edu.cn
Supported by:摘要:
室内甲醛污染已成为影响人类生命健康的重要问题之一。以氧气为氧化剂的催化氧化甲醛技术以其条件温和、无毒副产物等优势而受到广泛关注, 但是开发经济高效的催化材料仍然面临巨大的挑战。本工作通过一步水热法制备了α-Ni(OH)2, 并研究了其催化氧化甲醛机理。测试结果表明, 以水为溶剂、硝酸镍为镍源制备的α-Ni(OH)2在室温下催化氧化甲醛效率最高, 达到71.2%。原位红外和理论计算分析发现, 由于α-Ni(OH)2表面丰富的羟基官能团, 吸附的甲醛与α-Ni(OH)2表面羟基之间存在强烈的相互作用, 增强了对甲醛的活化, 在无氧气条件下实现了甲醛氧化。另一方面, 不同条件处理的α-Ni(OH)2的XPS分析证实了催化氧化甲醛的活性位点为Ni3+, 且氧气可加速Ni3+活性位点的回复。α-Ni(OH)2表面羟基协同活性位点Ni3+促进了甲醛的催化氧化, 这与传统氧气解离为速控步的甲醛氧化反应路径明显不同。本研究提出了表面羟基协同活性位点促进甲醛氧化的反应机理, 为催化氧化甲醛技术的实际应用提供了理论基础。
中图分类号:
张瑞阳, 王壹, 欧博文, 周莹. α-Ni(OH)2表面羟基协同Ni3+位点催化氧化甲醛机理研究[J]. 无机材料学报, 2023, 38(10): 1216-1222.
ZHANG Ruiyang, WANG Yi, OU Bowen, ZHOU Ying. α-Ni(OH)2 Surface Hydroxyls Synergize Ni3+ Sites for Catalytic Formaldehyde Oxidation[J]. Journal of Inorganic Materials, 2023, 38(10): 1216-1222.
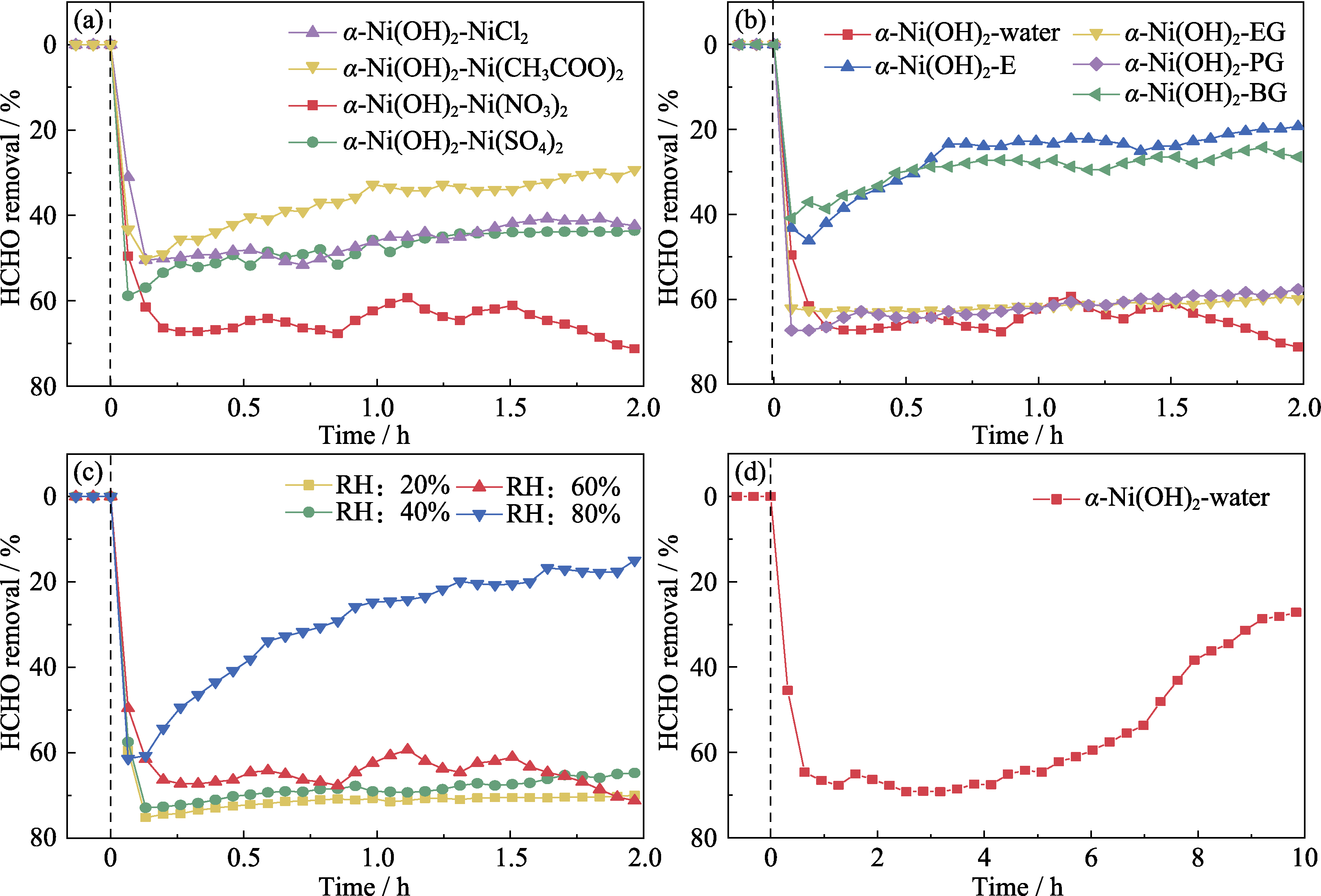
图1 α-Ni(OH)2催化氧化甲醛活性测试
Fig. 1 Catalytic HCHO oxidation over α-Ni(OH)2 (a) Different nickel sources; (b) Different solvents; (c) Different relative humidity; (d) Long time test Mass: 0.1 g; Temperature: 25 ℃; GHSV: 900 h-1; Relative humidity: 60%; Initial concentration of formaldehyde: 2 μL/L
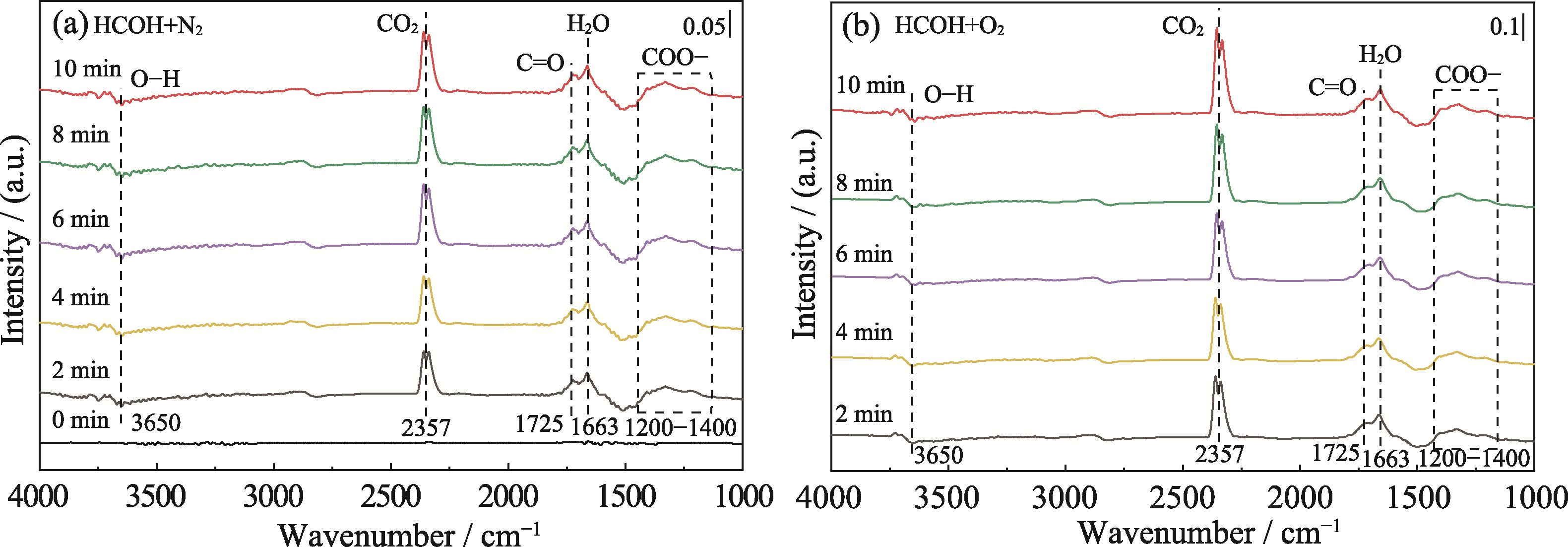
图5 α-Ni(OH)2-water催化氧化甲醛的原位红外光谱图
Fig. 5 In situ DRIFTS of catalytic HCHO oxidation over α-Ni(OH)2-water under different conditions (a) HCHO and N2; (b) HCHO and O2
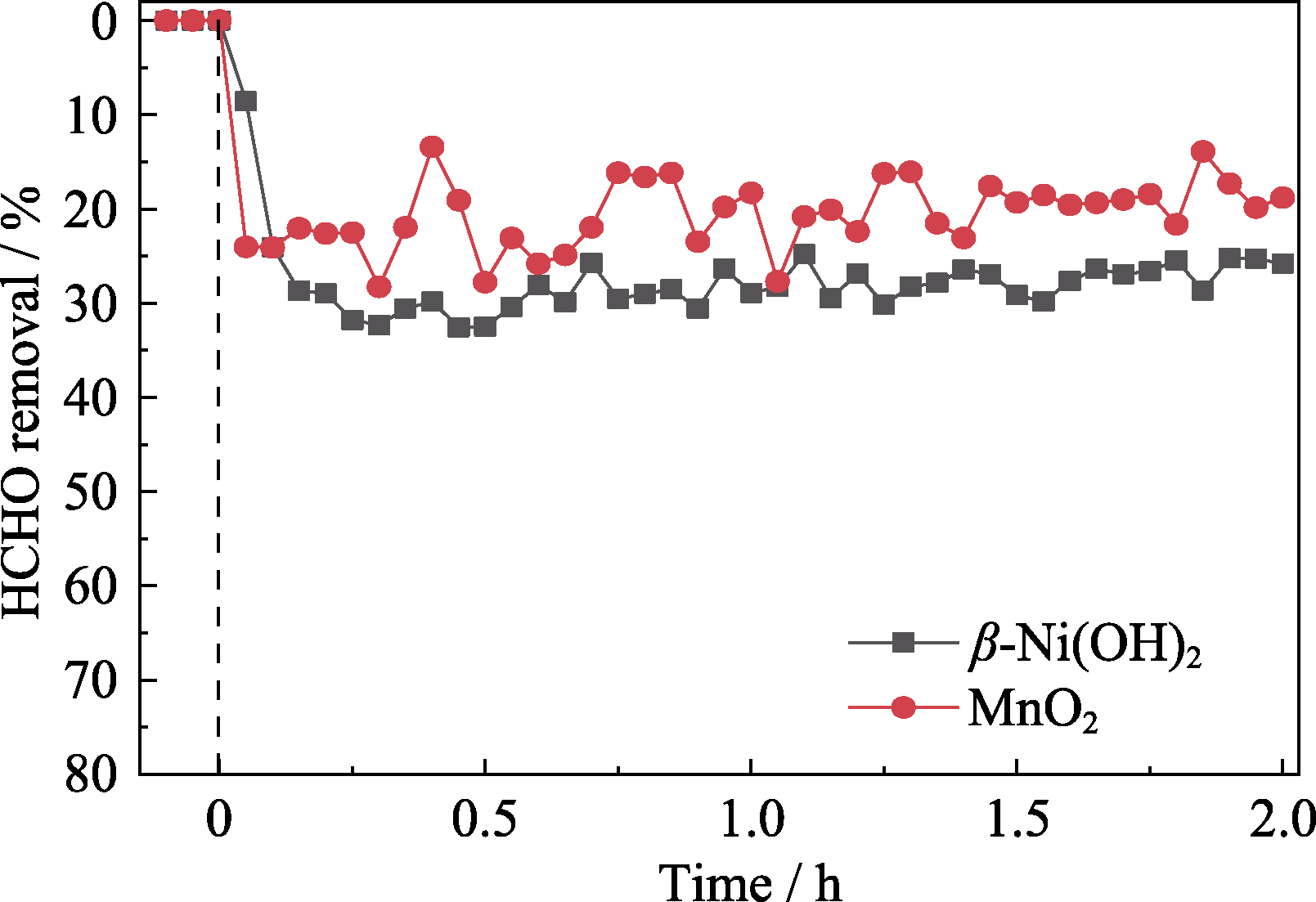
图S2 甲醛净化性能对比图
Fig. S2 Comparison of HCHO removal ratio Temperature: 25 ℃; GHSV: 900 h-1; Relative humidity: 60%; Initial concentration of formaldehyde: 2×10-6
| [1] | BOURDIN D, MOCHO P, DESAUZIERS V, et al. Formaldehyde emission behavior of building materials: on-site measurements and modeling approach to predict indoor air pollution. Journal of Hazardous Materials, 2014, 280: 164. |
| [2] |
QIN D, GUO B, ZHOU J, et al. Indoor air formaldehyde (HCHO) pollution of urban coach cabins. Scientific Reports, 2020, 10(1): 332.
DOI PMID |
| [3] | 刘洪霞, 吕功煊. 甲醛催化制氢的研究进展. 分子催化, 2020, 34(3): 210. |
| [4] |
ZHANG Y, YANG Y, HE X, et al. The cellular function and molecular mechanism of formaldehyde in cardiovascular disease and heart development. Journal of Cellular and Molecular Medicine, 2021, 25(12): 5358.
DOI PMID |
| [5] | KIM W, YOUNIS S A, KIM K. The control on adsorption kinetics and selectivity of formaldehyde in relation to different surface- modification approaches for microporous carbon bed systems. Separation and Purification Technology, 2022, 283: 120178. |
| [6] |
LI R, HUANG Y, ZHU D, et al. Improved oxygen activation over a carbon/Co3O4 nanocomposite for efficient catalytic oxidation of formaldehyde at room temperature. Environmental Science & Technology, 2021, 55(6): 4054.
DOI URL |
| [7] | VIKRANT K, KIM K, DONG F, et al. Deep oxidation of gaseous formaldehyde at room-temperature by a durable catalyst formed through the controlled addition of potassium to platinum supported on waste eggshell. Chemical Engineering Journal, 2022, 428: 131177. |
| [8] |
WU F, ZHAO Z, LI B, et al. Interfacial oxygen vacancy of Bi2O2CO3/PPy and its visible-light photocatalytic NO oxidation mechanism. Journal of Inorganic Materials, 2020, 35(5): 541.
DOI URL |
| [9] |
SUN P, YU H, LIU T, et al. Efficiently photothermal conversion in a MnOx-based monolithic photothermocatalyst for gaseous formaldehyde elimination. Chinese Chemical Letters, 2022, 33(5): 2564.
DOI URL |
| [10] | 张珍珍, 李鑫恒. 基于催化氧化技术去除甲醛的研究进展. 分子催化, 2019, 33(4): 382. |
| [11] |
YE J, ZHU B, CHENG B, et al. Synergy between platinum and gold nanoparticles in oxygen activation for enhanced room- temperature formaldehyde oxidation. Advanced Functional Materials, 2022, 32(15): 2110423.
DOI URL |
| [12] | JI J, LU X, CHEN C, et al. Potassium-modulated δ-MnO2 as robust catalysts for formaldehyde oxidation at room temperature. Applied Catalysis B: Environmental, 2020, 260: 118210. |
| [13] |
ZENG X, SHAN C, SUN M, et al. Graphene enhanced α-MnO2 for photothermal catalytic decomposition of carcinogen formaldehyde. Chinese Chemical Letters, 2022, 33(11): 4771.
DOI URL |
| [14] |
ZHA K, SUN W, HUANG Z, et al. Insights into high-performance monolith catalysts of Co3O4 nanowires grown on nickel foam with abundant oxygen vacancies for formaldehyde oxidation. ACS Catalysis, 2020, 10(20): 12127.
DOI URL |
| [15] | ZHU S, ZHENG J, XIN S, et al. Preparation of flexible Pt/TiO2/ γ-Al2O3 nanofiber paper for room-temperature HCHO oxidation and particulate filtration. Chemical Engineering Journal, 2022, 427: 130951. |
| [16] |
ZHANG Z, HE G, LI Y, et al. Effect of hydroxyl groups on metal anchoring and formaldehyde oxidation performance of Pt/Al2O3. Environmental Science & Technology, 2022, 56(15): 10916.
DOI URL |
| [17] |
ZHANG L, BAO Q, ZHANG B, et al. Distinct role of surface hydroxyls in single-atom Pt1/CeO2 catalyst for room-temperature formaldehyde oxidation: acid-base versus redox. JACS Au, 2022, 2(7): 1651.
DOI URL |
| [18] | YANG M, ZHANG J, ZHANG W, et al. Pt nanoparticles/Fe-doped α-Ni(OH)2 nanosheets array with low Pt loading as a high- performance electrocatalyst for alkaline hydrogen evolution reaction. Journal of Alloys and Compounds, 2020, 823: 153790. |
| [19] |
WU D, SHEN X, LIU X, et al. Insight into Fe activating one-dimensional α-Ni(OH)2 nanobelts for efficient oxygen evolution reaction. The Journal of Physical Chemistry C, 2021, 125(37): 20301.
DOI URL |
| [20] |
ZHANG R, RAN T, CAO Y, et al. Surface hydrogen atoms promote oxygen activation for solar light-driven NO oxidization over monolithic α-Ni(OH)2/Ni foam. Environmental Science & Technology, 2020, 54(24): 16221.
DOI URL |
| [21] | ZHANG A, ZHANG R, FEI L, et al. Tunable microstructure of α-Ni(OH)2 for highly-efficient surface adsorbates activation to promote catalytic NO oxidation. Chemical Engineering Journal, 2021, 425: 130663. |
| [22] | ZHANG R, RAN T, CAO Y, et al. Oxygen activation of noble- metal-free g-C3N4/α-Ni(OH)2 to control the toxic byproduct of photocatalytic nitric oxide removal. Chemical Engineering Journal, 2020, 382: 123029. |
| [23] | JIA D, GAO H, DONG W, et al. Hierarchical α-Ni(OH)2 composed of ultrathin nanosheets with controlled interlayer distances and their enhanced catalytic performance. ACS Applied Materials & Interfaces, 2017, 9(24): 20476. |
| [24] | LI H, RAMESHAN C, BUKHTIYAROV A V, et al. CO2 activation on ultrathin ZrO2 film by H2O co-adsorption: in situ NAP-XPS and IRAS studies. Surface Science, 2019, 679: 139. |
| [25] | ZHU J, YANG J, ZHOU J, et al. A stable organic-inorganic hybrid layer protected lithium metal anode for long-cycle lithium-oxygen batteries. Journal of Power Sources, 2017, 366: 265. |
| [26] |
LEE S, KIM S, LEE W J, et al. Boosting activity and durability of an electrodeposited Ni(OH)2 catalyst using carbon nanotube- grafted substrates for the alkaline oxygen evolution reaction. ACS Applied Nano Materials, 2021, 4(10): 10267.
DOI URL |
| [27] |
HOU J, YANG Y, ZHOU J, et al. Flexible CdS and PbS nanoparticles sensitized TiO2 nanotube arrays lead to significantly enhanced photocatalytic performance. Ceramics International, 2020, 46(18): 28785.
DOI URL |
| [28] |
CAI J, ZHANG D, DING W, et al. Promising rice-husk-derived carbon/Ni(OH)2 composite materials as a high-performing supercapacitor electrode. ACS Omega, 2020, 5(46): 29896.
DOI URL |
| [29] |
HUANG J, SUN Y, DU X, et al. Cytomembrane-structure-inspired active Ni-N-O interface for enhanced oxygen evolution reaction. Advanced Materials, 2018, 30(39): 1803367.
DOI URL |
| [30] |
TILL N A, TIAN L, DONG Z, et al. Mechanistic analysis of metallaphotoredox C-N coupling: photocatalysis initiates and perpetuates Ni(I)/Ni(III) coupling activity. Journal of the American Chemical Society, 2020, 142(37): 15830.
DOI PMID |
| [31] | YANG X, ZHANG H, XU W, et al. A doping element improving the properties of catalysis: in situ Raman spectroscopy insights into Mn-doped NiMn layered double hydroxide for the urea oxidation reaction. Catalysis Science & Technology, 2022, 12(14): 4471. |
| [32] | 韩高伟, 徐飞燕, 程蓓, 等. 反蛋白石结构ZnO@PDA用于增强光催化产H2O2性能. 物理化学学报, 2022, 38(7): 2112037. |
| [33] | LAN G, LI J, ZHANG G, et al. Thermal decomposition mechanism study of 3-nitro-1, 2, 4-triazol-5-one (NTO): combined TG-FTIR- MS techniques and ReaxFF reactive molecular dynamics simulations. Fuel, 2021, 295: 120655. |
| [34] | XUE H, WANG C, MAHMOOD A, et al. Two-dimensional g-C3N4 compositing with Ag-TiO2 as deactivation resistant photocatalyst for degradation of gaseous acetaldehyde. Journal of Inorganic Materials, 2022, 37(8): 865. |
| [35] |
CHEN X, CHEN Y, YUAN X. Decomposition of cyclohexyl hydroperoxide catalyzed by core-shell material Co3O4@SiO2. Journal of Inorganic Materials, 2022, 37(1): 65.
DOI URL |
| [36] | 雷卓楠, 马心怡, 胡晓云, 等. Ni2P-NiS双助剂促进g-C3N4光催化产氢动力学. 物理化学学报, 2022, 38(7): 2110049. |
| [37] |
ZHANG C, WANG Y, SONG W, et al. Synthesis of MnO2 modified porous carbon spheres by preoxidation-assisted impregnation for catalytic oxidation of indoor formaldehyde. Journal of Porous Materials, 2020, 27(3): 801.
DOI |
| [38] | WANG H, GUO W, JIANG Z, et al. New insight into the enhanced activity of ordered mesoporous nickel oxide in formaldehyde catalytic oxidation reactions. Journal of Catalysis, 2018, 361: 370. |
| [39] | WANG C, ZOU X, LIU H, et al. A highly efficient catalyst of palygorskite-supported manganese oxide for formaldehyde oxidation at ambient and low temperature: performance, mechanism and reaction kinetics. Applied Surface Science, 2019, 486: 420. |
| [40] | WANG C, LI Y, ZHANG C, et al. A simple strategy to improve Pd dispersion and enhance Pd/TiO2 catalytic activity for formaldehyde oxidation: the roles of surface defects. Applied Catalysis B: Environmental, 2021, 282: 119540. |
| [41] | SONG I, LEE H, JEON S W, et al. Understanding the dynamic behavior of acid sites on TiO2-supported vanadia catalysts via operando DRIFTS under SCR-relevant conditions. Journal of Catalysis, 2020, 382: 269. |
| [42] | BU Y, CHEN Y, JIANG G, et al. Understanding of Au-CeO2 interface and its role in catalytic oxidation of formaldehyde. Applied Catalysis B: Environmental, 2020, 260: 118138. |
| [43] | CHEN J, TANG H, HUANG M, et al. Surface lattice oxygen activation by nitrogen-doped manganese dioxide as an effective and longevous catalyst for indoor HCHO decomposition. ACS Applied Materials & Interfaces, 2021, 13(23): 26960. |
| [1] | 高娃, 熊宇杰, 吴聪萍, 周勇, 邹志刚. 基于超薄纳米结构的光催化二氧化碳选择性转化[J]. 无机材料学报, 2022, 37(1): 3-14. |
| [2] | 郑燕宁, 季军荣, 梁雪玲, 赖正杰, 陈启帆, 廖丹葵. 氮掺杂中空碳球氧化物模拟酶性能研究[J]. 无机材料学报, 2021, 36(5): 527-534. |
| [3] | 许云青,王海增. EDTA辅助水热法制备不同形貌的氟化镁钠[J]. 无机材料学报, 2019, 34(9): 933-937. |
| [4] | 鱼银虎, 汪涛, 廖秋平, 缪润杰, 潘剑锋, 张度宝. 低温固相反应合成纳米级TiB2-TiC复合粉体[J]. 无机材料学报, 2016, 31(3): 324-328. |
| [5] | 鱼银虎, 汪 涛, 张洪敏, 张度宝, 潘剑锋. PTFE促发TiC陶瓷粉体低温固相合成研究[J]. 无机材料学报, 2015, 30(3): 272-276. |
| [6] | 豆志河, 张廷安, 文 明, 史冠勇, 赫冀成. 燃烧合成法制备NdB6超细粉体及反应机理[J]. 无机材料学报, 2014, 29(7): 711-716. |
| [7] | 史晓睿, 王 群, 吕羚源, 李 洋, 俞 潇, 陈 刚. 碲化铜纳米材料的液相可控合成及其电导率[J]. 无机材料学报, 2012, 27(4): 433-438. |
| [8] | 穆云超, 梁宝岩, 郭基凤. 金刚石表面形成Ti3SiC2的反应机理[J]. 无机材料学报, 2012, 27(10): 1099-1104. |
| [9] | 徐秀华, 肖汉宁, 郭文明, 高朋召, 彭苏华. 常压固相反应合成 LaB6 粉末及其反应机理[J]. 无机材料学报, 2011, 26(4): 417-421. |
| [10] | 吴 皓1,2, 陈 诚1, 蒋丹宇2, 李 强1. 纳米铌镁酸铅机械化学法低温快速合成[J]. 无机材料学报, 2010, 25(5): 541-545. |
| [11] | 刘学建,李会利,黄政仁,王士维,江东亮. 高温固相反应工艺制备AlON粉体[J]. 无机材料学报, 2009, 24(6): 1159-1162. |
| [12] | 徐顺建,乔冠军,王红洁,李涤尘,卢天健. 微孔碳陶瓷化反应机理的研究[J]. 无机材料学报, 2009, 24(2): 291-296. |
| [13] | 李江鸿,张红波,熊翔,肖鹏,赵磊,黄伯. 含钽树脂先驱体转变生成TaC的过程研究[J]. 无机材料学报, 2007, 22(5): 973-978. |
| [14] | 符史流,尹涛,柴飞. Ca2SnO4:Eu3+的固相反应形成机理及发光性质研究[J]. 无机材料学报, 2007, 22(4): 647-651. |
| [15] | 刘金华,王大志. 乌贼骨及其水热改性制备羟基磷灰石的研究[J]. 无机材料学报, 2006, 21(2): 433-440. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||