无机材料学报 ›› 2020, Vol. 35 ›› Issue (12): 1349-1356.DOI: 10.15541/jim20200023 CSTR: 32189.14.10.15541/jim20200023
所属专题: 能源材料论文精选(四):光催化与电催化(2020)
林海1( ),苏玮韬1,朱玉1,彭湃1,冯苗1,2(
),苏玮韬1,朱玉1,彭湃1,冯苗1,2( ),于岩1,2(
),于岩1,2( )
)
收稿日期:2020-01-13
修回日期:2020-03-18
出版日期:2020-12-20
网络出版日期:2020-03-20
作者简介:林 海(1994–), 男, 硕士研究生. E-mail: linhaifj@outlook.com
基金资助:
LIN hai1( ),SU Weitao1,ZHU Yu1,PENG Pai1,FENG Miao1,2(
),SU Weitao1,ZHU Yu1,PENG Pai1,FENG Miao1,2( ),YU Yan1,2(
),YU Yan1,2( )
)
Received:2020-01-13
Revised:2020-03-18
Published:2020-12-20
Online:2020-03-20
About author:LIN Hai(1994–), male, Master candidate. E-mail: linhaifj@outlook.com
Supported by:摘要:
为研究热处理过程与异质结构筑对WO3的光电化学效应的影响机制, 采用低温溶剂热法制备纳米花状WO3, 通过热处理精确调控WO3纳米花的活性晶面、晶粒尺寸及结晶度。进一步借助循环化学浴法, 构筑WO3/CdS/α-S异质结, 并研究其光电化学性能与浓度效应。结果表明, (200)晶面是WO3纳米花的主要暴露晶面, 且比例随热处理温度升高而增大。350 ℃热处理的WO3纳米花表现出最高的光响应电流。通过构筑WO3/CdS/α-S梯形异质结, 增强材料在可见光区的吸收, 以牺牲少部分载流子的方式提高整体光生载流子的分离效率, 促进WO3的宏观光电化学效应的提升。
中图分类号:
林海, 苏玮韬, 朱玉, 彭湃, 冯苗, 于岩. WO3纳米花的热处理晶格调控及WO3/CdS/α-S异质结的构筑[J]. 无机材料学报, 2020, 35(12): 1349-1356.
LIN hai, SU Weitao, ZHU Yu, PENG Pai, FENG Miao, YU Yan. Lattice Control of WO3 Nanoflowers by Heat Treatment and Construction of WO3/CdS/α-S Heterojuntion[J]. Journal of Inorganic Materials, 2020, 35(12): 1349-1356.
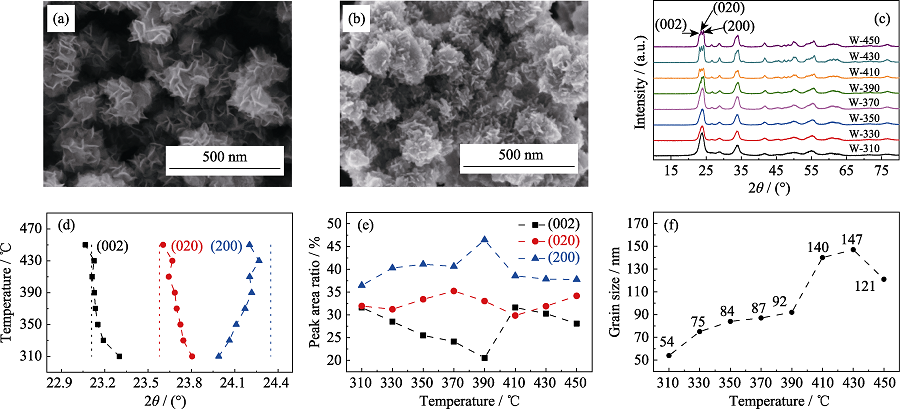
图1 WO3纳米花前驱体(a)和W-350(b)的SEM照片; 不同温度热处理样品的XRD图谱(c); Rietveld精修的(002)、(020)、(200)晶面衍射峰位(d)、衍射峰积分面积占比(e)和晶粒尺寸(f)与热处理温度的关系曲线
Fig. 1 SEM images of WO3 nanoflower precursor (a) and W-350 (b); XRD patterns of the samples heat-treated at different temperatures (c); (002), (020), and (200) crystal plane diffraction peak positions (d), diffraction peak integrated area ratios (e) and grain sizes (f) obtained by Rietveld refinement varied as functions of heat treatment temperature
图S1 前驱体(a)、W-310(b)、W-330(c)、W-370(d)、W-390(e)、W-410(f)、W-430(g)、W-450(h)的SEM照片
Fig. S1 SEM images of precursor (a), W-310 (b), W-330 (c), W-370 (d), W-390 (e), W-410 (f), W-430 (g), and W-450 (h).
图S2 W-310 (a)、W-330 (b)、W-350 (c)、W-370 (d)、W-390 (e)、W-410 (f)、W-430 (g)、W-450 (h)的XRD数据Rietveld精修结果
Fig. S2 Rietveld refinement results of XRD data from W-310 (a), W-330 (b), W-350 (c), W-370 (d), W-390 (e), W-410 (f), W-430 (g), W-450 (h)
| Sample | (002) 2θ/(°) | (020) 2θ/(°) | (200) 2θ/(°) | (002) Peak area ratio/% | (020) Peak area ratio/% | (200) Peak area ratio/% | R/%a | E/%b |
|---|---|---|---|---|---|---|---|---|
| W-310 | 23.302 | 23.805 | 23.989 | 31.60736 | 31.93592 | 36.45672 | 7.96 | 7.22 |
| W-330 | 23.191 | 23.744 | 24.063 | 28.51362 | 31.21225 | 40.27413 | 8.45 | 9.08 |
| W-350 | 23.152 | 23.724 | 24.110 | 25.48277 | 33.41551 | 41.10171 | 8.71 | 8.87 |
| W-370 | 23.136 | 23.698 | 24.171 | 24.13370 | 35.22923 | 40.63707 | 7.77 | 8.89 |
| W-390 | 23.128 | 23.685 | 24.217 | 20.52815 | 33.02325 | 46.4486 | 8.74 | 8.72 |
| W-410 | 23.113 | 23.643 | 24.203 | 31.60641 | 29.83773 | 38.55586 | 9.62 | 11.08 |
| W-430 | 23.126 | 23.668 | 24.268 | 30.26410 | 31.89507 | 37.84083 | 8.92 | 8.88 |
| W-450 | 23.066 | 23.604 | 24.202 | 28.08145 | 34.16319 | 37.75536 | 8.84 | 9.0 |
表1 不同温度热处理样品的XRD数据Rietveld精修结果
Table 1 Rietveld refinement results of XRD data of the samples heat-treated at different temperatures
| Sample | (002) 2θ/(°) | (020) 2θ/(°) | (200) 2θ/(°) | (002) Peak area ratio/% | (020) Peak area ratio/% | (200) Peak area ratio/% | R/%a | E/%b |
|---|---|---|---|---|---|---|---|---|
| W-310 | 23.302 | 23.805 | 23.989 | 31.60736 | 31.93592 | 36.45672 | 7.96 | 7.22 |
| W-330 | 23.191 | 23.744 | 24.063 | 28.51362 | 31.21225 | 40.27413 | 8.45 | 9.08 |
| W-350 | 23.152 | 23.724 | 24.110 | 25.48277 | 33.41551 | 41.10171 | 8.71 | 8.87 |
| W-370 | 23.136 | 23.698 | 24.171 | 24.13370 | 35.22923 | 40.63707 | 7.77 | 8.89 |
| W-390 | 23.128 | 23.685 | 24.217 | 20.52815 | 33.02325 | 46.4486 | 8.74 | 8.72 |
| W-410 | 23.113 | 23.643 | 24.203 | 31.60641 | 29.83773 | 38.55586 | 9.62 | 11.08 |
| W-430 | 23.126 | 23.668 | 24.268 | 30.26410 | 31.89507 | 37.84083 | 8.92 | 8.88 |
| W-450 | 23.066 | 23.604 | 24.202 | 28.08145 | 34.16319 | 37.75536 | 8.84 | 9.0 |
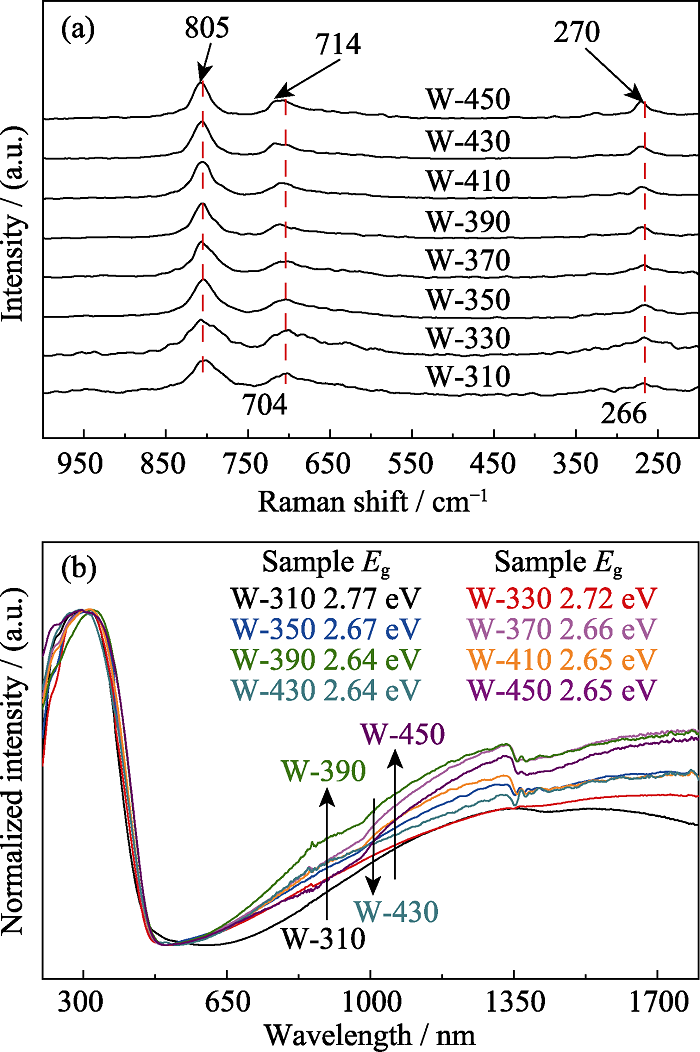
图2 不同温度热处理样品的Raman谱图(a)和UV-Vis-IR吸收光谱图(b)
Fig. 2 Raman spectra (a) and UV-Vis-IR absorption spectra (b) of the samples heat-treated at different temperatures
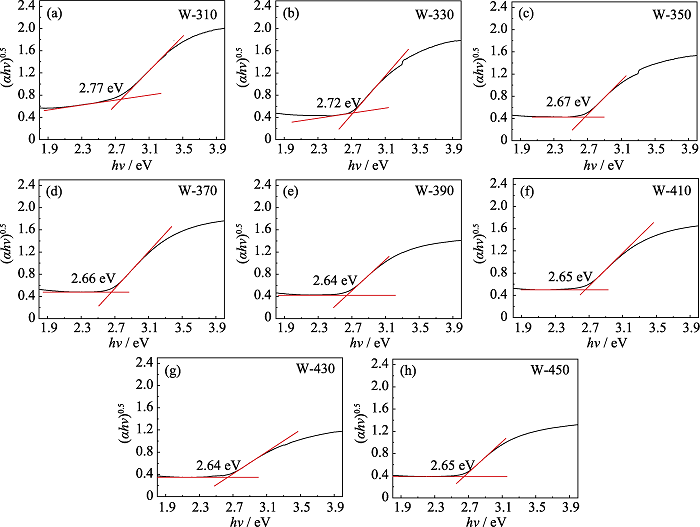
图S3 W-310 (a)、W-330 (b)、W-350 (c)、W-370 (d)、W-390 (e)、W-410 (f)、W-430 (g)、W-450 (h)的Tauc曲线及根据吸收边截线交点得出的样品带隙Eg
Fig. S3 Tauc plots of W-310 (a), W-330 (b), W-350 (c), W-370 (d), W-390 (e), W-410 (f), W-430 (g), W-450 (h) and the band gaps Eg obtained from the intersection of the absorption edge intercept line
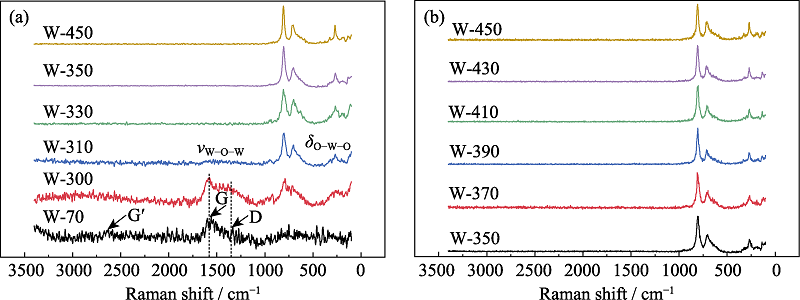
图S4 WO3前驱体与代表性的热处理样品(a), 350 ℃以上热处理样品(b)的Raman谱图
Fig. S4 Raman spectra of WO3 precursor and representative heat-treated samples (a) and the WO3 sample heat-treated above 350 ℃ (b)
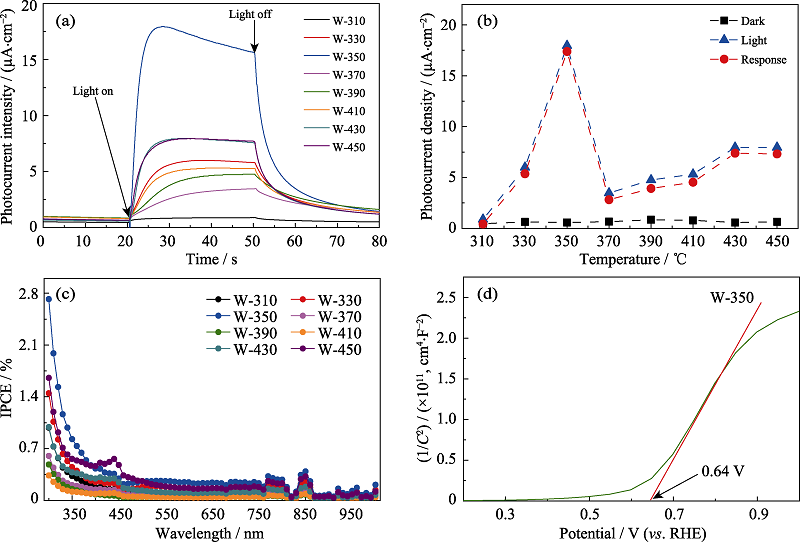
图3 不同温度热处理样品的光电流响应曲线(a)、光电流峰值(b)和IPCE谱图(c); W-350的Mott-Schottky曲线(d)
Fig. 3 Photocurrent response curves (a), photocurrent response peak values (b) and IPCE plots (c) of the samples heat-treated at different temperatures; Mott-Schottky curve of W-350 (d) Colourful version is available on offical website
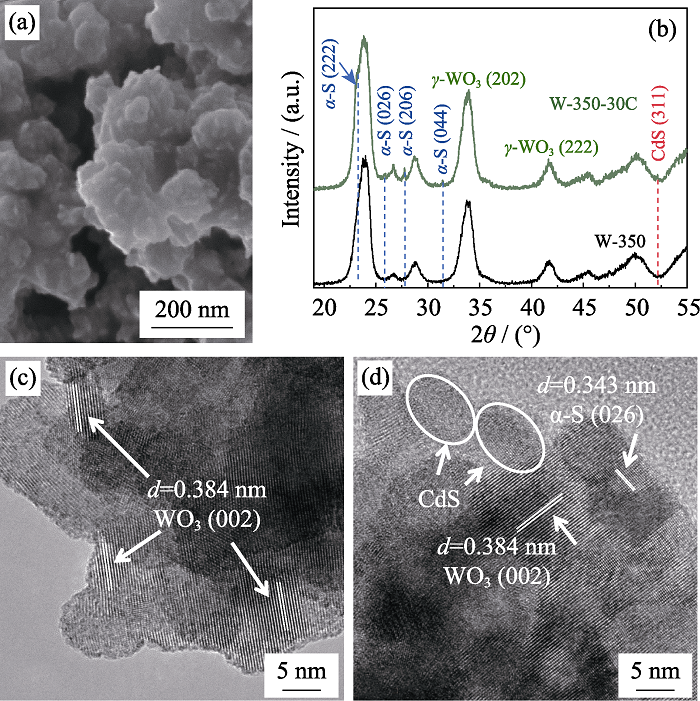
图4 W-350-30C的SEM照片(a); W-350和W-350-30C的XRD图谱(b); W-350(c)和W-350-30C(d)的TEM照片
Fig. 4 SEM image of W-350-30C (a); XRD patterns of W-350 and W-350-30C(b); TEM images of W-350-30C (c) and W-350-30C (d)
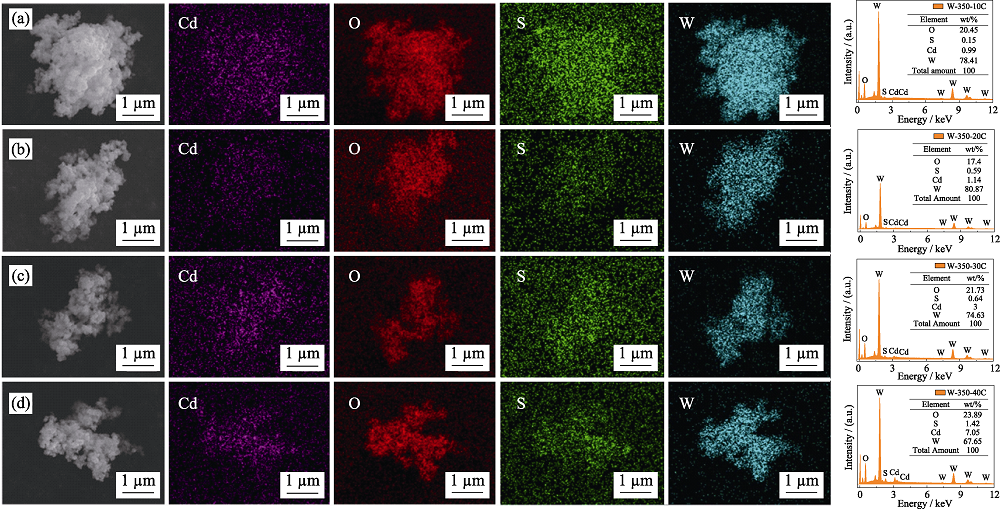
图S6 W-350-10C (a)、W-350-20C (b)、W-350-30C (c)、W-350-40C (d)的EDX图谱
Fig. S6 EDX results of W-350-10C (a), W-350-20C (b), W-350-30C (c), and W-350-40C (d)
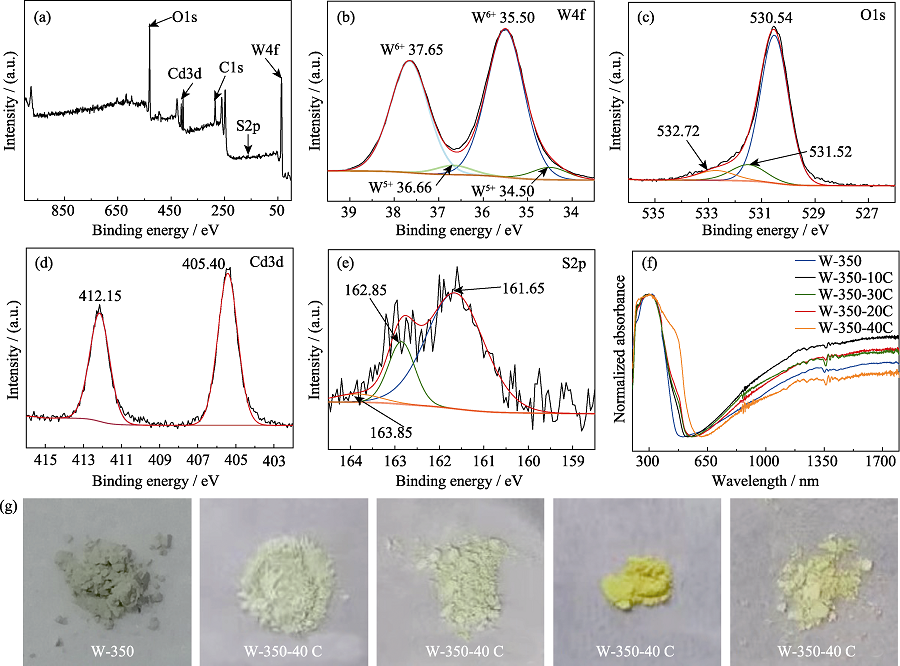
图5 W-350-30C的XPS全谱(a)、W4f (b)、O1s (c)、Cd3d(d)、S2p(e)的XPS高分辨谱; 不同CdS/α-S表面修饰浓度的γ-WO3纳米花的UV-Vis-IR吸收光谱图(f)和样品照片(g)
Fig. 5 XPS spectrum of sample W-350-30C (a); XPS high-resolution spectra of W4f (b), O1s (c), Cd3d (d) and S2p (e) for sample W- 350-30C; UV-Vis-IR absorption spectra (f) and photos (g) of γ-WO3 nanoflowers with different amounts of CdS/α-S modified on the surface Colourful version is available on offical website
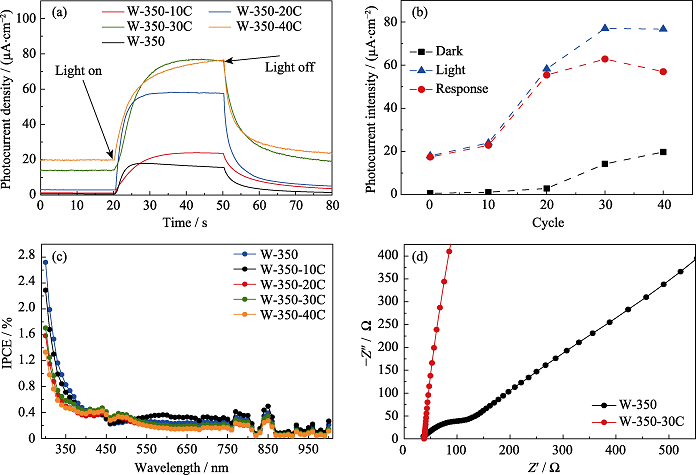
图6 不同CdS/α-S异质结浓度的γ-WO3纳米花的光电流响应曲线(a)、光电流峰值(b)和IPCE测试结果(c); W-350和W-350-30C的EIS曲线(d)
Fig. 6 Photocurrent response curves(a), photocurrent response peak values(b), and IPCE plots(c) of γ-WO3 nanoflowers modified with different amounts of CdS/α-S on the surface; EIS plots of W-350 and W-350-30C(d) Colourful version is available on offical website
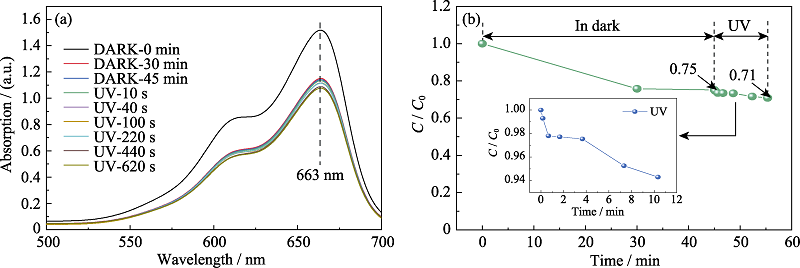
图S7 亚甲基蓝溶液经W-350避光吸附与不同时间紫外光照射后的UV-Vis光谱图(a); 亚甲基蓝降解率随时间的变化曲线(b)
Fig. S7 UV-Vis spectra of methylene blue solution after absorbed by W-350 in the dark and UV irradiation for different time (a); Variation of methylene blue degradation rate with different time (b)
| [1] | YE S, DING C M, LIU M Y, et al. Water oxidation catalysts for artificial photosynthesis. Advanced Materials, 2019,31(50):1902069. |
| [2] |
LUO Z B, WANG T, GONG J L. Single-crystal silicon-based electrodes for unbiased solar water splitting: current status and prospects. Chemical Society Reviews, 2019,48:2158-2181.
DOI URL PMID |
| [3] | WU Y S, LIU X J, HAN D D, et al. Electron density modulation of NiCo2S4 nanowires by nitrogen incorporation for highly efficient hydrogen evolution catalysis. Nature Communications, 2018,9(1425):1-9. |
| [4] | WEI J M, LÜ Q, WANG B C, et al. Synthesis of cubic-relievo Ag3PO4 with high visible-light photocatalytic activity. Journal of Inorganic Materials, 2019,34(7):786-790. |
| [5] | WANG Y D, TIAN W, CHEN C, et al. Tungsten trioxide nanostructures for photoelectrochemical water splitting: material engineering and charge carrier dynamic manipulation. Advanced Functional Materials, 2019,29(23):1809036. |
| [6] | ZHANG J J, CHANG X X, LI C C, et al. WO3 photoanodes with controllable bulk and surface oxygen vacancies for photoelectrochemical water oxidation. Journal of Materials Chemistry A, 2018,6:3350-3354. |
| [7] | FU J W, XU Q L, LOW J X, et al. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Applied Catalysis B: Environmental, 2019,243:556-565. |
| [8] |
LI H J, GAO Y Y, ZHOU Y, et al. Construction and nanoscale detection of interfacial charge transfer of elegant Z-scheme WO3/Au/ In2S3 nanowire arrays. Nano Letters, 2016,16(9):5547-5552.
DOI URL PMID |
| [9] | CAO F R, MENG L X, WANG M, et al. Gradient energy band driven high-performance self-powered perovskite/CdS photodetector. Advanced Materials, 2019,30(12):1806725-1-7. |
| [10] |
LIU G, NIU P, YIN L C, et al. α-Sulfur crystals as a visible-light- active photocatalyst. Journal of the American Chemical Society, 2012,134(22):9070-9073.
DOI URL PMID |
| [11] |
HU W, LIN L, ZHANG R Q, et al. Highly efficient photocatalytic water splitting over edge-modified phosphorene nanoribbons. Journal of the American Chemical Society, 2017,139(43):15429-15436.
DOI URL PMID |
| [12] |
ZHANG M Y, LIN H L, CAO J, et al. Construction of novel S/CdS type II heterojunction for photocatalytic H2 production under visible light: the intrinsic positive role of elementary α-S. Chemical Engineering Journal, 2017,321:484-494.
DOI URL |
| [13] |
SUN W T, YU Y, PAN H Y, et al. CdS quantum dots sensitized TiO2 nanotube-array photoelectrodes. Journal of the American Chemical Society, 2008,130(4):1124-1125.
DOI URL PMID |
| [14] |
CHANDRASEKARAN S, ZHANG P X, PENG F, et al. Tailoring the geometric and electronic structure of tungsten oxide with manganese or vanadium doping toward highly efficient electrochemical and photoelectrochemical water splitting. Journal of Materials Chemistry A, 2019,7:6161-6172.
DOI URL |
| [15] |
ZHANG H F, ZHOU W W, YANG Y P, et al. 3D WO3/BiVO4/cobalt phosphate composites inverse opal photoanode for efficient photoelectrochemical water splitting. Small, 2017,13(16):1603840-1-7.
DOI URL |
| [16] |
LI W J, DA P M, ZHANG Y Y, et al. WO3 nanoflakes for enhanced photoelectrochemical conversion. ACS Nano, 2014,8(11):11770-11777.
DOI URL PMID |
| [17] |
ZHENG T T, SANG W, HE Z H, et al. Conductive tungsten oxide nanosheets for highly efficient hydrogen evolution. Nano Letters, 2017,17(12):7968-7973.
DOI URL PMID |
| [18] |
CONG S, GENG F X, ZHAO Z G. Tungsten oxide materials for optoelectronic applications. Advanced Materials, 2016,28(47):10518-10528.
DOI URL PMID |
| [19] | CHEN S, ZENG L, TIAN H, et al. Enhanced lattice oxygen reactivity over Ni-modified WO3-based redox catalysts for chemical looping partial oxidation of methane. ACS Catalysis, 2017,7(5):3548-3559. |
| [20] | WANG F G, VALENTIN C D, PACCHIONI G. Semiconductor-to- metal transition in WO3-x: nature of the oxygen vacancy. Physical Review B, 2011,84(7):073103-1-5. |
| [21] | MA Y L, FENG B, LANG J Y, et al. Synthesis of semi-metallic tungsten trioxide for infrared light photoelectrocatalytic water splitting. The Journal of Physical Chemistry C, 2019,123(42):25833-25843. |
| [22] |
YAN J Q, WANG T, WU G J, et al. Tungsten oxide single crystal nanosheets for enhanced multichannel solar light harvesting. Advanced Materials, 2019,27(9):1580-1586.
DOI URL PMID |
| [23] | BAI S, ZHANG N, GAO C, et al. Defect engineering in photocatalytic materials. Nano Energy, 2018,53:296-336. |
| [24] |
HUANG Z F, SONG J J, PAN L, et al. Tungsten oxides for photocatalysis, electrochemistry, and phototherapy. Advanced Materials, 2015,27(36):5309-5327.
DOI URL PMID |
| [25] |
FORMAL F L, PENDLEBURY S R, CORNUZ M, et al. Back electron-hole recombination in hematite photoanodes for water splitting. Journal of the American Chemical Society, 2014,136(6):2564-2574.
DOI URL PMID |
| [26] | FORMAL F L, SIVULA K, GRATZEL M. The transient photocurrent and photovoltage behavior of a hematite photoanode under working conditions and the influence of surface treatments. The Journal of Physical Chemistry C, 2012,116:26707-26720. |
| [27] |
ZHANG N, CHEN C, MEI Z W, et al. Monoclinic tungsten oxide with {100} facet orientation and tuned electronic band structure for enhanced photocatalytic oxidations. ACS Applied Materials & Interfaces, 2016,8(16):10367-10374.
DOI URL PMID |
| [28] | 张青莲, 姚凤仪, 郭德威, 桂明德. 无机化学丛书. 第五卷, 氧、硫、硒分族. 北京: 科学出版社, 1990: 173-237. |
| [29] | WANG G M, LING Y C, WANG H Y, et al. Hydrogen-treated WO3 nanoflakes show enhanced photostability. Energy & Environmental Science, 2012,5:6180-6187. |
| [30] | ZHANG J J, ZHANG P, WANG T, et al. Monoclinic WO3 nanomultilayers with preferentially exposed (002) facets for photoelectrochemical water splitting. Nano Energy, 2015,11:189-195. |
| [31] |
MENG J, LIN Q Y, CHEN T, et al. Oxygen vacancy regulation on tungsten oxides with specific exposed facets for enhanced visible- light-driven photocatalytic oxidation. Nanoscale, 2018,10:2908-2915.
URL PMID |
| [32] | LÜ Y, ZHU Y, ZHU Y. Enhanced photocatalytic performance for the BiPO4-x nanorod induced by surface oxygen vacancy. The Journal of Physical Chemistry C, 2013,117(36):18520-18528. |
| [33] | WANG J, JIANG W, LIU D et al. Photocatalytic performance enhanced via surface bismuth vacancy of Bi6S2O15 core/shell nanowires. Applied Catalysis B: Environmental, 2015,176-177:306-314. |
| [34] | LI Y S, TANG Z L, ZHANG J Y et al. Defect engineering of air- treated WO3 and its enhanced visible light-driven photocatalytic and electrochemical performance. The Journal of Physical Chemistry C, 2016,120:9750-9763. |
| [35] | ZHONG Y Y, ZHAO G, MA F K, et al. Utilizing photocorrosion- recrystallization to prepare a highly stable and efficient CdS/WS2 nanocomposite photocatalyst for hydrogen evolution. Applied Catalysis B: Environmental, 2016,199:466-472. |
| [36] | JIN J, YU J G, GUO D P, et al. A hierarchical Z-scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity. Small, 2015,11(39):5262-5271. |
| [37] |
WANG M Y, CAI L J, WANG Y, et al. Graphene-draped semiconductors for enhanced photocorrosion resistance and photocatalytic properties. Journal of the American Chemical Society, 2017,139(11):4144-4151.
DOI URL PMID |
| [38] | ZONG X, HAN J F, MA G J, et al. Photocatalytic H2 evolution on CdS loaded with WS2 as cocatalyst under visible light irradiation. The Journal of Physical Chemistry C, 2011,115(24):12202-12208. |
| [39] |
LI J J, WANG Y A, GUO W Z, et al. Large-scale synthesis of nearly monodisperse CdSe/CdS core/shell nanocrystals using air-stable reagents via successive ion layer adsorption and reaction. Journal of the American Chemical Society, 2003,125(41):12567-12575.
DOI URL PMID |
| [40] | MENG S G, CAO Z S, FU X L, et al. Fabrication of hydrophilic S/In2O3 core-shell nanocomposite for enhancement of photocatalytic performance under visible light irradiation. Applied Surface Science, 2015,324:188-197. |
| [41] |
LIN R, WAN J W, XIONG Y, et al. Quantitative study of charge carrier dynamics in well-defined WO3 nanowires and nanosheets: insight into the crystal facet effect in photocatalysis. Journal of the American Chemical Society, 2018,140(29):9078-9082.
DOI URL |
| [1] | 吴秋琴, 姚奋发, 金传洪, 郑遗凡. 碳纳米管内填充生长超细一维亚化学计量比氧化钨纳米线[J]. 无机材料学报, 2022, 37(4): 413-419. |
| [2] | 李文博, 钱荣, 卓尚军, 江鸿, 盛成, 朱月琴. 不同形貌MoS2的制备及对NH3气敏性能的研究[J]. 无机材料学报, 2022, 37(10): 1135-1140. |
| [3] | 武琦, 丛杉, 赵志刚. 多彩氧化钨薄膜的红外电致变色性能研究[J]. 无机材料学报, 2021, 36(5): 485-491. |
| [4] | 钟晓岚, 刘雪晴, 刁训刚. 基于氧化钨和氧化镍的电致变色器件研究进展[J]. 无机材料学报, 2021, 36(2): 128-139. |
| [5] | 赵林艳, 刘阳思, 席晓丽, 马立文, 聂祚仁. 基于第一性原理计算的纳米氧化钨研究进展[J]. 无机材料学报, 2021, 36(11): 1125-1136. |
| [6] | 王美涵, 温佳星, 陈昀, 雷浩. 掠射角溅射沉积纳米结构氧化钨薄膜[J]. 无机材料学报, 2018, 33(12): 1303-1308. |
| [7] | 喻 洋, 佟明兴, 何玉兰, 陈 辉, 高 静, 李国华. 介孔TiO2/WO3空心球的制备及其可见光光催化性能[J]. 无机材料学报, 2017, 32(4): 365-371. |
| [8] | 彭明栋, 章俞之, 宋力昕, 尹小富, 王盼盼, 吴岭南, 胡行方. 钛掺杂三氧化钨薄膜结构与电致变色性能研究[J]. 无机材料学报, 2017, 32(3): 287-292. |
| [9] | 金 冲, 张卫国, 姚素薇, 王宏智. 热处理工艺对TiO2纳米管阵列结构及其光电性能的影响[J]. 无机材料学报, 2012, 27(1): 54-58. |
| [10] | 钱柏太,沈自求. 控制表面氧化法制备超疏水 CuO纳米花膜[J]. 无机材料学报, 2006, 21(3): 747-752. |
| [11] | 边继明,李效民,赵俊亮,于伟东. PLD法生长高质量 ZnO薄膜及其光电导特性研究[J]. 无机材料学报, 2006, 21(3): 701-706. |
| [12] | 曹广胜,俞庆森,董喜贵,宋旭春,谭非. 过渡元素钼掺杂氧化钨纳米棒的合成[J]. 无机材料学报, 2005, 20(4): 815-820. |
| [13] | 牛新书,魏少红,许亚杰,蒋凯. SiO2-WO3纳米粉体的合成及其气敏特性[J]. 无机材料学报, 2003, 18(4): 782-786. |
| [14] | 黄银松,章俞之,宋力昕,胡行方. 多晶氧化钨薄膜的制备及其红外反射调制性能研究[J]. 无机材料学报, 2002, 17(6): 1263-1268. |
| [15] | 方国家,刘祖黎,姚凯伦. WO3/Si纳米晶薄膜的脉冲准分子激光沉积及结构分析[J]. 无机材料学报, 2002, 17(1): 139-144. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||