无机材料学报 ›› 2020, Vol. 35 ›› Issue (12): 1340-1348.DOI: 10.15541/jim20200133 CSTR: 32189.14.10.15541/jim20200133
所属专题: 环境材料论文精选(2020); 【虚拟专辑】放射性污染物去除(2020~2021)
王旭聪1,2,3( ),邓浩3,姜忠义1,2(
),邓浩3,姜忠义1,2( ),袁立永3(
),袁立永3( )
)
收稿日期:2020-03-16
修回日期:2020-04-20
出版日期:2020-12-20
网络出版日期:2020-06-09
作者简介:王旭聪(1994–), 男, 硕士研究生. E-mail: wangxc@ihep.ac.cn
基金资助:
WANG Xucong1,2,3( ),DENG Hao3,JIANG Zhongyi1,2(
),DENG Hao3,JIANG Zhongyi1,2( ),YUAN Liyong3(
),YUAN Liyong3( )
)
Received:2020-03-16
Revised:2020-04-20
Published:2020-12-20
Online:2020-06-09
About author:WANG Xucong(1994–), male, Master candidate. E-mail: wangxc@ihep.ac.cn
Supported by:摘要:
为探究不同N源对无定形TiO2/g-C3N4(TCN)复合材料光催化还原Re(VII)的影响, 通过热分解不同前驱体(尿素Urea、硫脲Thiourea和三聚氰胺Melamine)制备g-C3N4, 再分别与无定形TiO2复合, 制备了三种TCN复合光催化剂。通过不同分析手段对材料进行表征, 并比较了不同TCN复合材料光催化还原去除Re(VII)的活性差异。结果表明, U-TCN(尿素为N源)具有更均匀的表观形貌, 最大的比表面积(474 m2/g), 最优异的光吸收性能, 对Re(VII)的光催化还原效率(90%)明显高于T-TCN(20%)和M-TCN(15%)。通过复合材料的瞬态光电流和电化学阻抗(EIS)分析光催化机理, 证明U-TCN光生电子空穴分离效率最高; 电子顺磁共振波谱(EPR)分析表明U-TCN产生的羟基自由基(?OH)更多, 因此与甲酸反应产生的强还原性?CO2-自由基更多, 从而更有利于Re(VII)的还原; 利用同步辐射X射线吸收光谱分析Ti元素价态及配位环境, 表明U-TCN还具有优异的光化学稳定性。本研究揭示了不同N源对所制备TCN复合材料光催化性能的影响, 并发现了一种可用于实际废水中光催化还原去除Tc(VII)的优选材料。
中图分类号:
王旭聪, 邓浩, 姜忠义, 袁立永. 不同N源无定形TiO2/g-C3N4光催化还原Re(VII)性能[J]. 无机材料学报, 2020, 35(12): 1340-1348.
WANG Xucong, DENG Hao, JIANG Zhongyi, YUAN Liyong. Photocatalytic Reduction of Re (VII) on Amorphous TiO2/g-C3N4 Derived from Different N Sources[J]. Journal of Inorganic Materials, 2020, 35(12): 1340-1348.
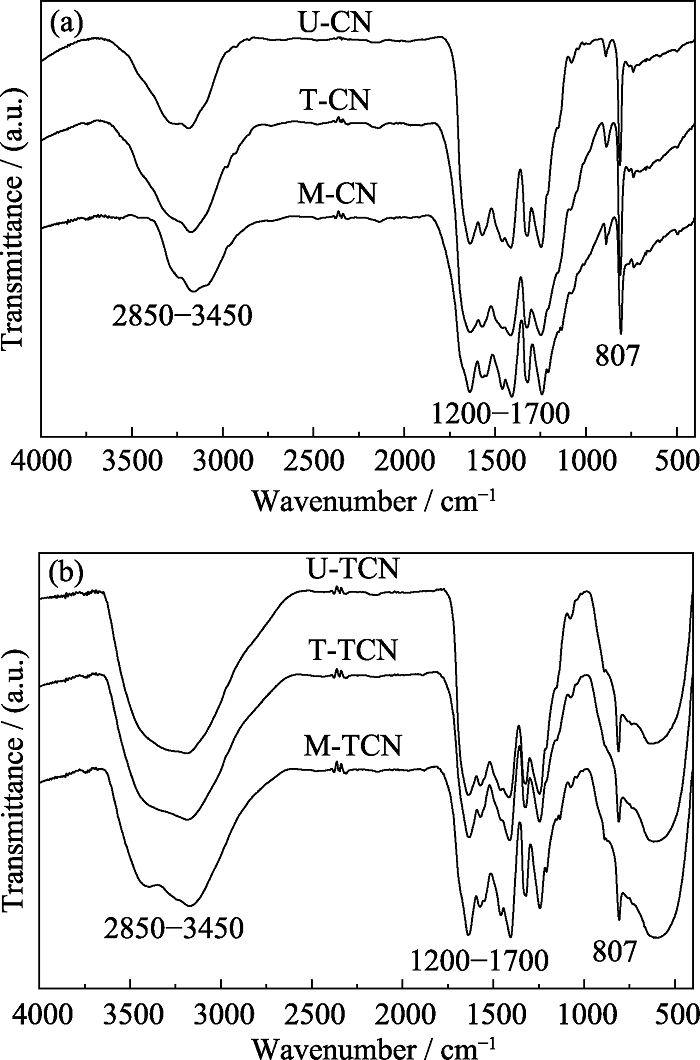
图2 (a) M-CN、T-CN、U-CN样品和(b)M-TCN、T-TCN和U-TCN样品的FT-IR图谱
Fig. 2 FT-IR spectra of (a) M-CN, T-CN and U-CN samples, and (b) M-TCN, T-TCN and U-TCN samples
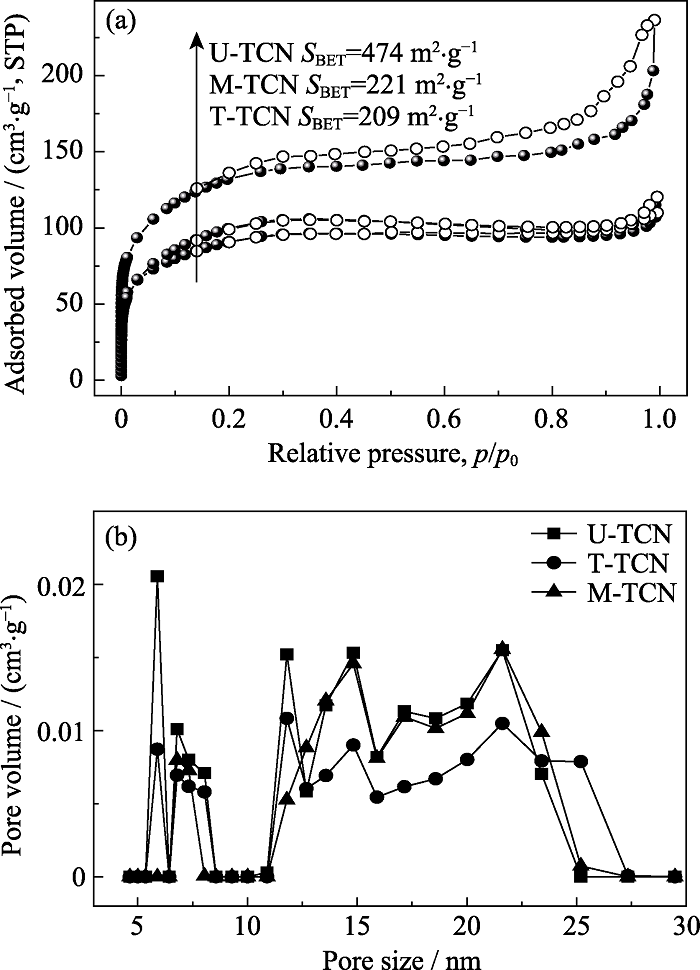
图4 M-TCN、T-TCN和U-TCN样品的(a)N2吸附-脱附等温线和(b)孔径分布
Fig. 4 (a) N2 adsorption-desorption isotherms and (b) corresponding pore size distribution curves of M-TCN, T-TCN and U-TCN samples
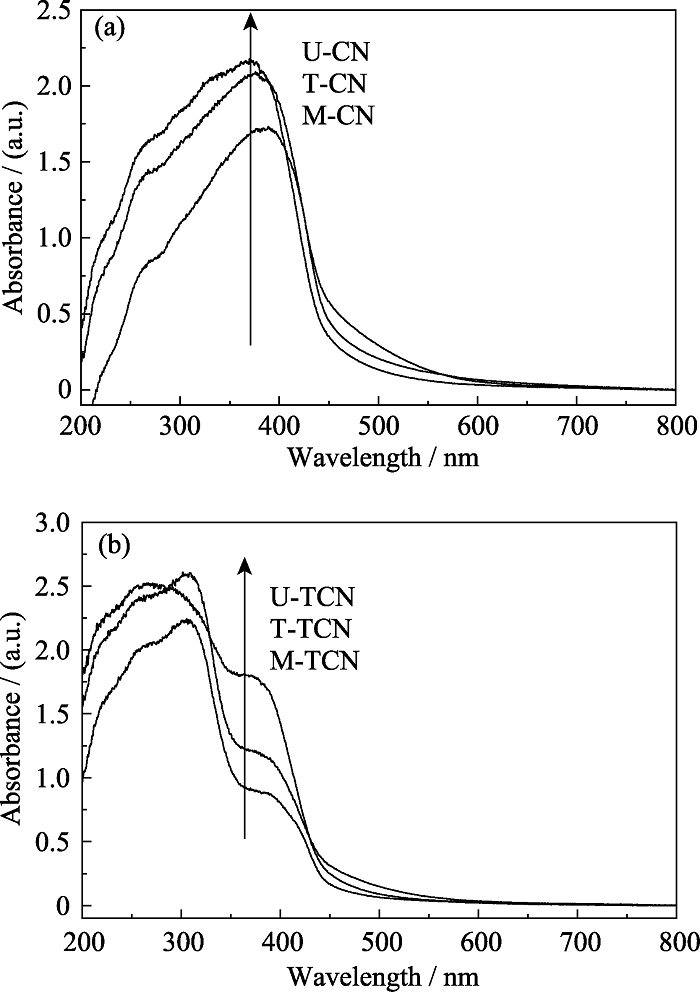
图5 (a) M-CN、T-CN和U-CN样品和(b) M-TCN、T-TCN和U-TCN样品的UV-Vis光谱
Fig. 5 UV-Vis spectra of (a) M-CN, T-CN and U-CN samples and (b) M-TCN, T-TCN and U-TCN samples
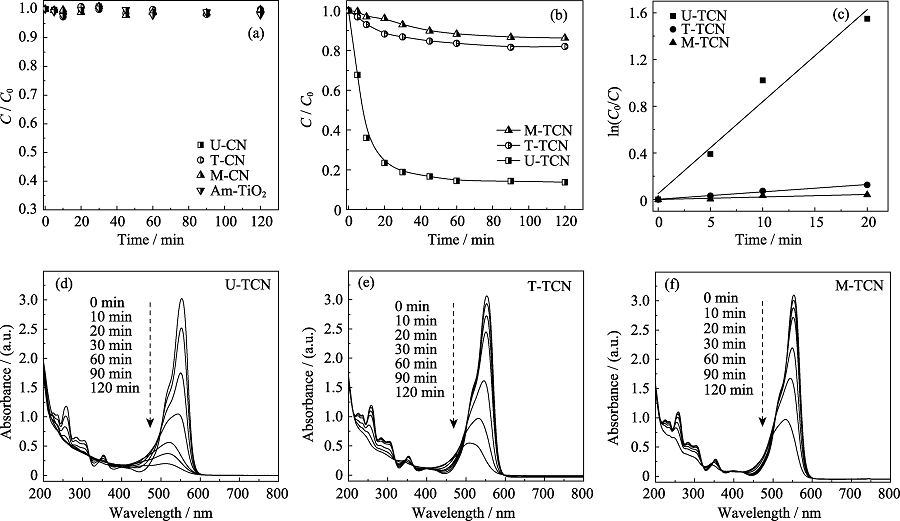
图7 (a)U-CN、T-CN、M-CN、Am-TiO2和(b)U-TCN、T-TCN、M-TCN在UV-Vis下还原和去除Re(VII); (c)Langmuir- Hinshelwood动力学方程的线性拟合; (d)U-TCN、(e)T-TCN和(f)M-TCN在UV-Vis光照下降解RhB
Fig. 7 Photocatalytic reduction and removal of Re(VII) using (a) U-CN, T-CN, M-CN, Am-TiO2 and (b) U-TCN, T-TCN and M-TCN composites under UV-Vis irradiation; (c) Linear fitting of Langmuir-Hinshelwood equation; The photodegradation of RhB using 0.4 g?L-1 (d) U-TCN, (e) T-TCN and (f) M-TCN under UV-Vis irradiation
| Sample | Re(VII) removed | k/min-1 | R2 |
|---|---|---|---|
| U-TCN | 90% | 0.0788 | 0.9524 |
| T-TCN | 20% | 0.0063 | 0.9861 |
| M-TCN | 15% | 0.0021 | 0.7954 |
表1 TCN材料光催化还原和去除Re(VII)的动力学参数
Table 1 Kinetic parameters of photocatalytic reduction and removal of Re(VII) using TCN composites
| Sample | Re(VII) removed | k/min-1 | R2 |
|---|---|---|---|
| U-TCN | 90% | 0.0788 | 0.9524 |
| T-TCN | 20% | 0.0063 | 0.9861 |
| M-TCN | 15% | 0.0021 | 0.7954 |
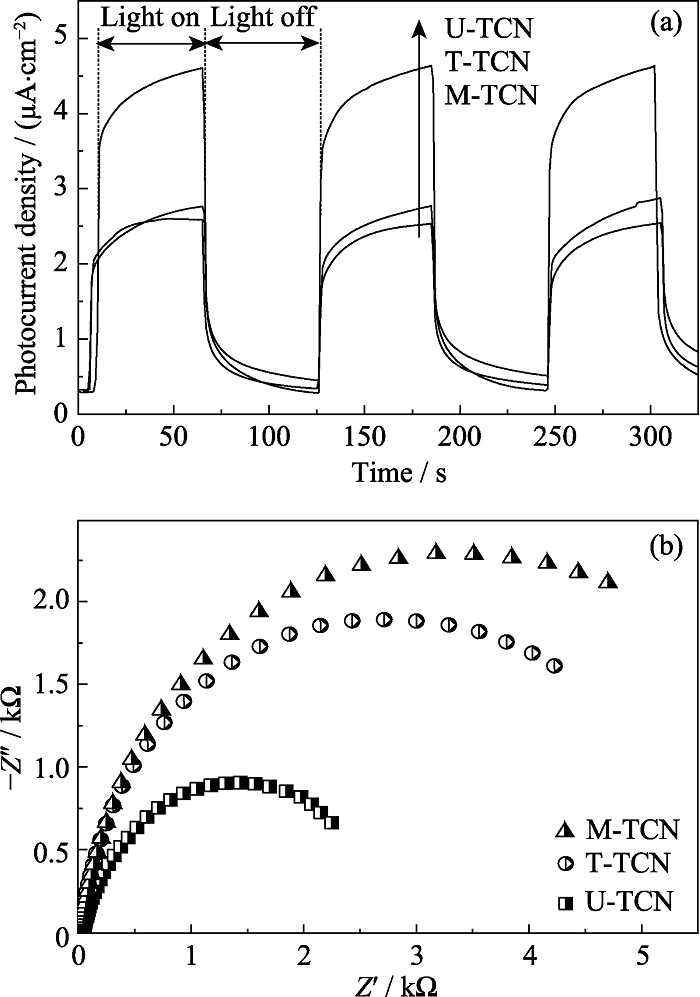
图8 U-TCN、T-TCN和M-TCN的(a)瞬态光电流响应和(b)在UV-Vis光照下的EIS曲线
Fig. 8 (a) Transient photocurrent responses and (b) EIS curves of U-TCN, T-TCN and M-TCN composites under UV-Vis irradiation
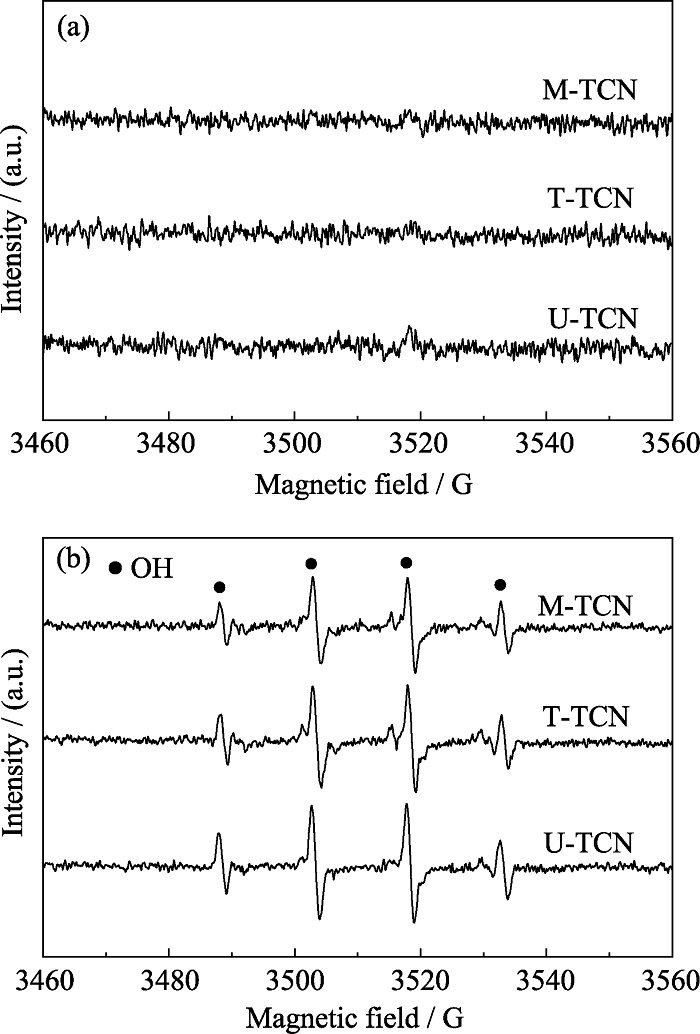
图9 U-TCN、T-TCN和M-TCN在(a)黑暗条件和(b)紫外可见光照射下EPR谱图
Fig. 9 EPR spectra of U-TCN, T-TCN and M-TCN composites (a) in the dark and (b) under UV-visible light irradiation
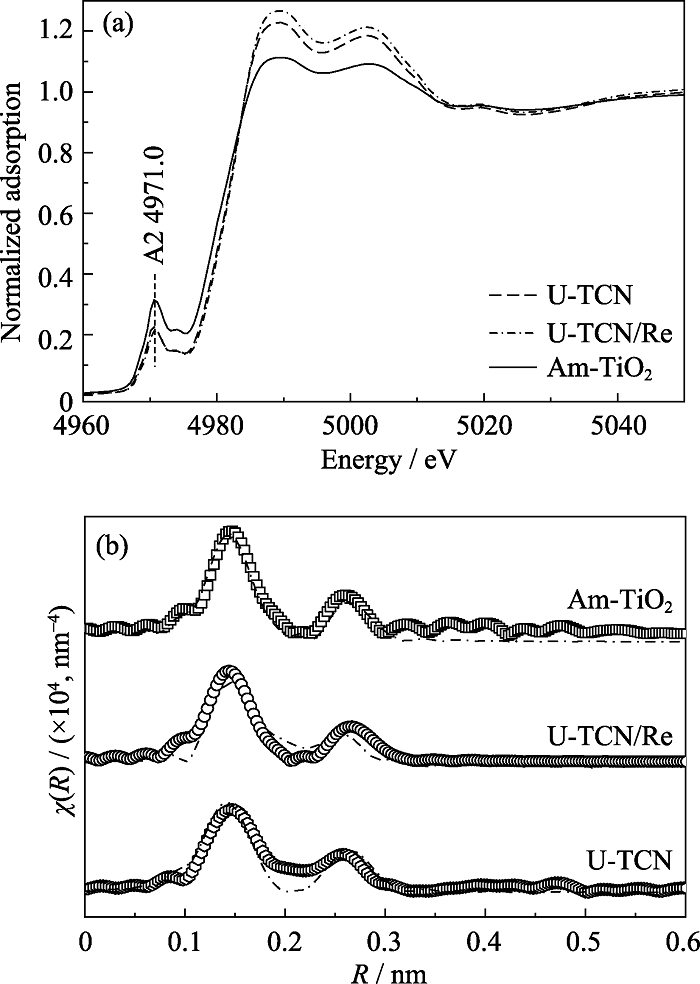
图10 Am-TiO2、U-TCN和U-TCN/Re的Ti K-edge XANES分析(a)和EXAFS分析(b)
Fig. 10 (a) Ti K-edge XANES analysis and (b) EXAFS analysis for the Am-TiO2, U-TCN and U-TCN/Re
| Sample | Bond | Na | R/nmb | σ2/nm2 c | R-factord |
|---|---|---|---|---|---|
| Am-TiO2 | Ti-O | 1.2 | 0.198(3) | 3×10-6 | |
| Ti-O | 1.2 | 0.183(2) | 0 | 0.001 | |
| Ti-Ti | 1.0 | 0.306(2) | 6.5×10-5 | ||
| U-TCN | Ti-O | 1.5 | 0.192(2) | 2×10-5 | |
| Ti-O | 1.0 | 0.184(3) | 0 | 0.017 | |
| Ti-Ti | 1.7 | 0.312(2) | 8.9×10-5 | ||
| U-TCN/Re | Ti-O | 1.2 | 0.203(3) | 1.0×10-5 | |
| Ti-O | 1.2 | 0.188(2) | 3×10-6 | 0.017 | |
| Ti-Ti | 1.6 | 0.314(2) | 9.8×10-5 |
表2 EXAFS数据分析的拟合参数
Table 2 Fitting parameters from the analysis of EXAFS data
| Sample | Bond | Na | R/nmb | σ2/nm2 c | R-factord |
|---|---|---|---|---|---|
| Am-TiO2 | Ti-O | 1.2 | 0.198(3) | 3×10-6 | |
| Ti-O | 1.2 | 0.183(2) | 0 | 0.001 | |
| Ti-Ti | 1.0 | 0.306(2) | 6.5×10-5 | ||
| U-TCN | Ti-O | 1.5 | 0.192(2) | 2×10-5 | |
| Ti-O | 1.0 | 0.184(3) | 0 | 0.017 | |
| Ti-Ti | 1.7 | 0.312(2) | 8.9×10-5 | ||
| U-TCN/Re | Ti-O | 1.2 | 0.203(3) | 1.0×10-5 | |
| Ti-O | 1.2 | 0.188(2) | 3×10-6 | 0.017 | |
| Ti-Ti | 1.6 | 0.314(2) | 9.8×10-5 |
| [1] | SHEN D, FAN X, SU X, et al. Study of sorption of technetium on pyrrhotine. Journal of Nuclear and Radiochemistry, 2001,23(2):72-78. |
| [2] |
MEI L, LI F Z, LAN J H, et al. Anion-adaptive crystalline cationic material for 99TcO4- trapping. Nature Communications, 2019,10(1):1532.
DOI URL PMID |
| [3] |
MEENA A H, ARAI Y. Environmental geochemistry of technetium. Environmental Chemistry Letters, 2017,15(2):241-263.
DOI URL |
| [4] |
ZACHARA J M, HEALD S M, JEON B H, et al. Reduction of pertechnetate [Tc(VII)] by aqueous Fe(II) and the nature of solid phase redox products. Geochimica et Cosmochimica Acta, 2007,71(9):2137-2157.
DOI URL |
| [5] |
PEARCE C I, ICENHOWER J P, ASMUSSEN R M, et al. Technetium stabilization in low-solubility sulfide phases: a review. ACS Earth and Space Chemistry, 2018,2(6):532-547.
DOI URL |
| [6] |
SHANG Y, XIAO J, WENG H, et al. Efficient separation of Re(VII) by radiation-induced reduction from aqueous solution. Chemical Engineering Journal, 2018,341:317-326.
DOI URL |
| [7] |
WANG L, SONG H, YUAN L, et al. Effective removal of anionic Re(VII) by surface-modified Ti2CTx MXene nanocomposites: implications for Tc(VII) sequestration. Environmental Science & Technology, 2019,53(7):3739-3747.
DOI URL PMID |
| [8] |
DENG H, LI Z, WANG X, et al. Efficient photocatalytic reduction of aqueous perrhenate and pertechnetate. Environmental Science & Technology, 2019,53(18):10917-10925.
DOI URL PMID |
| [9] |
BURTON-PYE B P, RADIVOJEVIC I, MCGREGOR D, et al. Photoreduction of 99Tc pertechnetate by nanometer-sized metal oxides: new strategies for formation and sequestration of low-valent technetium . Journal of the American Chemical Society, 2011,133(46):18802-18815.
DOI URL |
| [10] |
RAZIQ F, SUN L Q, WANG Y Y, et al. Synthesis of large surface-area g-C3N4 comodified with MnOx and Au-TiO2 as efficient visible-light photocatalysts for fuel production. Advanced Energy Materials, 2018,8(3):1701580.
DOI URL |
| [11] |
PELAEZ M, NOLAN N T, PILLAI S C, et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Applied Catalysis B-Environmental, 2012,125:331-349.
DOI URL |
| [12] |
WEI K, LIE K X, YAN L S, et al. One-step fabrication of g-C3N4 nanosheets/TiO2 hollow microspheres heterojunctions with atomic level hybridization and their application in the multi-component synergistic photocatalytic systems. Applied Catalysis B-Environmental, 2018,222:88-98.
DOI URL |
| [13] |
LI Z J, HUANG Z W, GUO W L, et al. Enhanced photocatalytic removal of Uranium(VI) from aqueous solution by magnetic TiO2/Fe3O4 and its graphene composite. Environmental Science & Technology, 2017,51(10):5666-5674.
DOI URL PMID |
| [14] |
TIAN C, ZHAO H, MEI J, et al. Cost-efficient graphitic carbon nitride as an effective photocatalyst for antibiotic degradation: an insight into the effects of different precursors and coexisting ions, and photocatalytic mechanism. Chemistry - An Asian Journal, 2019,14(1):162-169.
DOI URL |
| [15] |
WANG X, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nature Materials, 2009,8(1):76-80.
DOI URL PMID |
| [16] |
ONG W, TAN L, NG Y H, et al. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chemical Reviews, 2016,116(12):7159-7329.
DOI URL PMID |
| [17] |
DONG H, GUO X, YANG C, et al. Synthesis of g-C3N4 by different precursors under burning explosion effect and its photocatalytic degradation for tylosin. Applied Catalysis B: Environmental, 2018,230:65-76.
DOI URL |
| [18] |
PHAM T T, SHIN E W. Influence of g-C3N4 precursors in g-C3N4/NiTiO3 composites on photocatalytic behavior and the interconnection between g-C3N4 and NiTiO3. Langmuir, 2018,34(44):13144-13154.
DOI URL PMID |
| [19] |
HOLST J R, GILLAN E G. From triazines to heptazines: deciphering the local structure of amorphous nitrogen-rich carbon nitride materials. Journal of the American Chemical Society, 2008,130(23):7373-7379.
DOI URL PMID |
| [20] |
DONG F, WANG Z, SUN Y, et al. Engineering the nanoarchitecture and texture of polymeric carbon nitride semiconductor for enhanced visible light photocatalytic activity. Journal of Colloid and Interface Science, 2013,401:70-79.
DOI URL |
| [21] |
YAN S C, LI Z S, ZOU Z G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir, 2009,25(17):10397-10401.
DOI URL PMID |
| [22] |
ZHANG G, ZHANG J, ZHANG M, et al. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. Journal of Materials Chemistry, 2012,22(16):8083-8091.
DOI URL |
| [23] |
TONG Z W, YANG D, XIAO T X, et al. Biomimetic fabrication of g-C3N4/TiO2 nanosheets with enhanced photocatalytic activity toward organic pollutant degradation. Chemical Engineering Journal, 2015,260:117-125.
DOI URL |
| [24] | LI J, ZHANG M, LI X, et al. Effect of the calcination temperature on the visible light photocatalytic activity of direct contact Z-scheme g-C3N4-TiO2 heterojunction. Applied Catalysis B-Environmental, 2017,212:106-114. |
| [25] |
LIU J, ZHANG T, WANG Z, et al. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. Journal of Materials Chemistry, 2011,21(38):14398-14401.
DOI URL |
| [26] |
YANG W, LI B. A novel liquid template corrosion approach for layered silica with various morphologies and different nanolayer thicknesses. Nanoscale, 2014,6(4):2292-2298.
DOI URL |
| [27] |
XIA P, ZHU B, YU J, et al. Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction. Journal of Materials Chemistry A, 2017,5(7):3230-3238.
DOI URL |
| [28] |
LI Y, SASAKI T, SHIMIZU Y, et al. Hexagonal-close-packed, hierarchical amorphous TiO2 nanocolumn arrays: transferability, enhanced photocatalytic activity, and superamphiphilicity without UV irradiation. Journal of the American Chemical Society, 2008,130(44):14755-14762.
DOI URL PMID |
| [29] |
KONSTANTINOU I K, ALBANIS T A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Applied Catalysis B: Environmental, 2004,49(1):1-14.
DOI URL |
| [30] |
HUO L, XIE W, QIAN T, et al. Reductive immobilization of pertechnetate in soil and groundwater using synthetic pyrite nanoparticles. Chemosphere, 2017,174:456-465.
DOI URL PMID |
| [31] |
FU H, ZHANG S, XU T, et al. Photocatalytic degradation of RhB by fluorinated Bi2WO6 and distributions of the intermediate products. Environmental Science & Technology, 2008,42(6):2085-2091.
DOI URL PMID |
| [32] |
LEI P, CHEN C, YANG J, et al. Degradation of dye pollutants by immobilized polyoxometalate with H2O2 under visible-light irradiation. Environmental Science & Technology, 2005,39(21):8466-8474.
DOI URL PMID |
| [33] |
CHEN C, ZHAO W, LI J, et al. Formation and identification of intermediates in the visible-light-assisted photodegradation of Sulforhodamine-B dye in aqueous TiO2 dispersion. Environmental Science & Technology, 2002,36(16):3604-3611.
DOI URL PMID |
| [34] |
JIANG Z, WAN W, LI H, et al. A hierarchical Z-Scheme α-Fe2O3/ g-C3N4 hybrid for enhanced photocatalytic CO2 reduction. Advanced Materials, 2018,30(10):1706108.
DOI URL |
| [35] | LIU H, CHEN D, WANG Z, et al. Microwave-assisted molten-salt rapid synthesis of isotype triazine-/heptazine based g-C3N4 heterojunctions with highly enhanced photocatalytic hydrogen evolution performance. Applied Catalysis B-Environmental, 2017,203:300-313. |
| [36] |
ZHANG H, CHEN B, BANFIELD J F, et al. Atomic structure of nanometer-sized amorphous TiO2. Physical Review B, 2008,78(21):214106.
DOI URL |
| [1] | 贾鑫, 李晋宇, 丁世豪, 申倩倩, 贾虎生, 薛晋波. Pd纳米颗粒协同氧空位增强TiO2光催化CO2还原性能[J]. 无机材料学报, 2023, 38(11): 1301-1308. |
| [2] | 洪佳辉, 马冉, 仵云超, 文涛, 艾玥洁. MOFs自牺牲模板法制备CoNx/g-C3N4纳米材料用作高效光催化还原U(VI)[J]. 无机材料学报, 2022, 37(7): 741-749. |
| [3] | 蒋丽, 高慧慧, 曹茹雅, 张守伟, 李家星. 三维大孔g-C3N4吸附和光催化还原U(VI)性能研究[J]. 无机材料学报, 2020, 35(3): 359-366. |
| [4] | 张 丽, 张秀秀, 戴超华1, 欧阳杰, 阎建辉. 硫化物负载ZnO/ZnAl2O4复合空心球及其光催化还原CO2[J]. 无机材料学报, 2016, 31(7): 731-738. |
| [5] | 周民杰, 张 娜, 侯朝辉. 石墨烯-ZnIn2S4纳米复合微球的制备及光催化产氢活性[J]. 无机材料学报, 2015, 30(7): 713-718. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||