无机材料学报 ›› 2018, Vol. 33 ›› Issue (3): 352-356.DOI: 10.15541/jim20170163 CSTR: 32189.14.10.15541/jim20170163
袁贝贝 1,2,3,周蓓蓓1,2,3,章跃标1,施剑林1,2
收稿日期:2017-04-07
出版日期:2018-03-20
网络出版日期:2018-03-12
作者简介:袁贝贝. E-mail: yuanbb@shanghaitech.edu.cn
YUAN Bei-Bei1, 2, 3, ZHOU Bei-Bei1, 2, 3, ZHANG Yue-Biao1, 3, SHI Jian-Lin1, 2, 3
Received:2017-04-07
Published:2018-03-20
Online:2018-03-12
About author:YUAN Bei-Bei (1991-), female, candidate of Master degree. E-mail: yuanbb@shanghaitech.edu.cn
Supported by:摘要:
通过研究具有两种轮桨状构筑基元和四种纳米笼子结构锌基金属-有机框架(Zn-MOF)的染料吸附特性和机理,发现其分子吸附的普适性, 以及尺寸和电荷的选择性。由于Zn-MOF孔道内漂浮着抗衡阴离子, 及框架上有可配位位点, 所以它能通过离子交换机理吸附阴性染料、框架上电荷转变机理吸附阳性染料、主客体相互作用吸附中性染料, 表现出优越的分子吸附多功能性。Zn-MOF内带电荷纳米笼的尺寸选择性和电荷选择性的共同作用为设计具有更高水平兼容性和识别性的优异多孔材料铺平了道路。
中图分类号:
袁贝贝,周蓓蓓,章跃标,施剑林. 具有尺寸和电荷选择性多功能分子吸附能力的电荷可转变型金属-有机框架材料[J]. 无机材料学报, 2018, 33(3): 352-356.
YUAN Bei-Bei, ZHOU Bei-Bei, ZHANG Yue-Biao, SHI Jian-Lin. Charge-switchable Metal-organic Framework for Size/Charge-selective Molecular Inclusions[J]. Journal of Inorganic Materials, 2018, 33(3): 352-356.

Fig. S2 (a) The rhombicuboctahedral cage enclosed alternatively by eight Zn2(COO)3+ and six Zn2(COO)4 clusters; (b) The cuboctahedral cage enclosed by four Zn2(COO)3+ clusters; (c) The truncated octahedral cage enclosed by six Zn2(COO)4 clusters; (d) The square-bifrustum cage enclosed alternatively by four Zn2(COO)3+ clusters and four Zn2(COO)4 clusters.The open pores size of cages in (a), (b), (c) and (d) are (e) 0.40 × 0.60 nm, (f) 0.72 × 0.76 nm, (g) 1.04 × 0.34 nm and (h) 0.70 × 0.90 nm, respectively
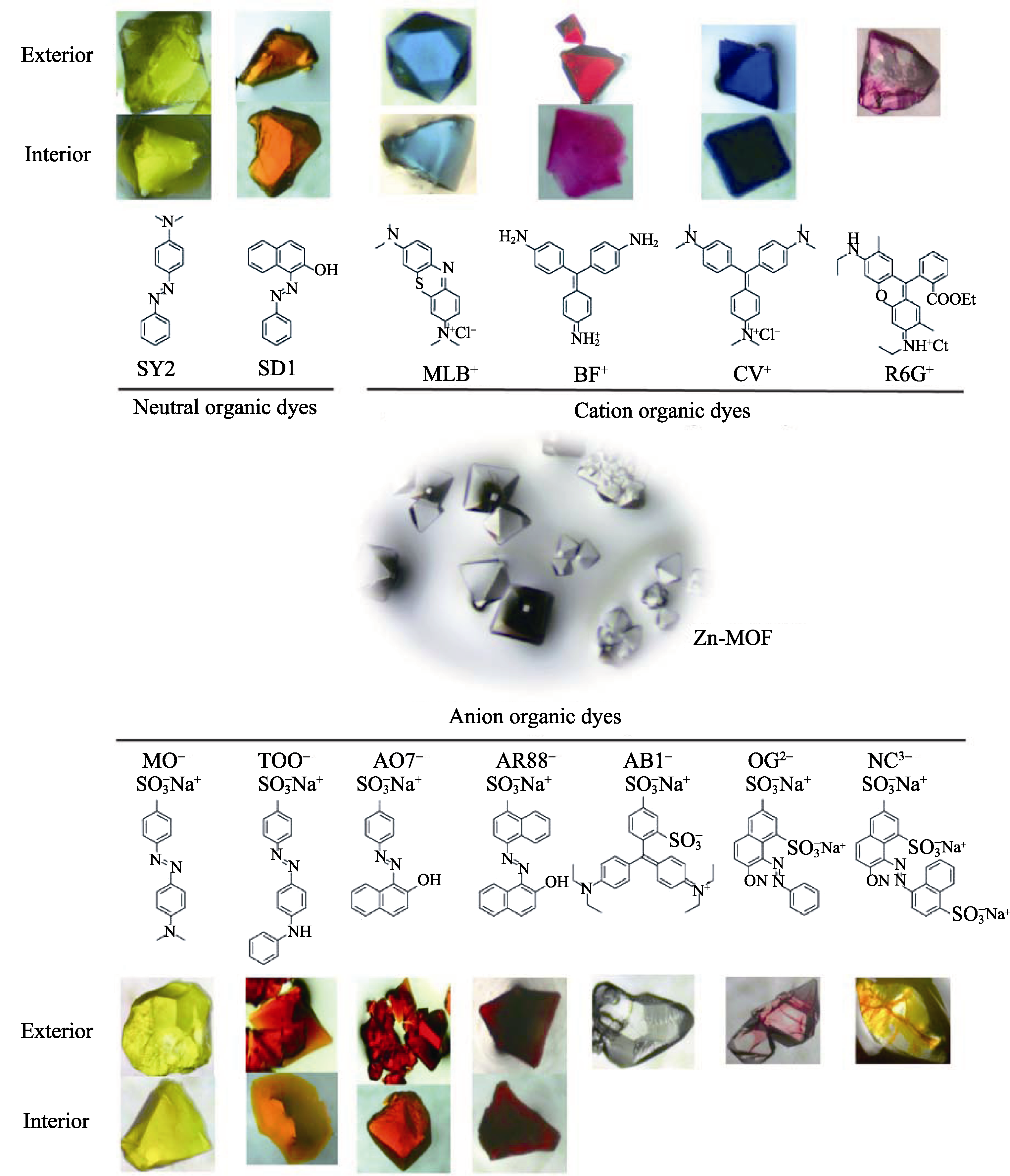
Fig. S3 The optical microscope images of the Zn-MOF crystals before (centered) and after (top and bottom) dye inclusion experiments. To confirm the uptake homogeneity of dyes, both the exterior and interior of dyes adsorbed MOF crystals were shown except those for AB-, OG2-, and NC3- with dyes penetrated only the outer part of the crystals
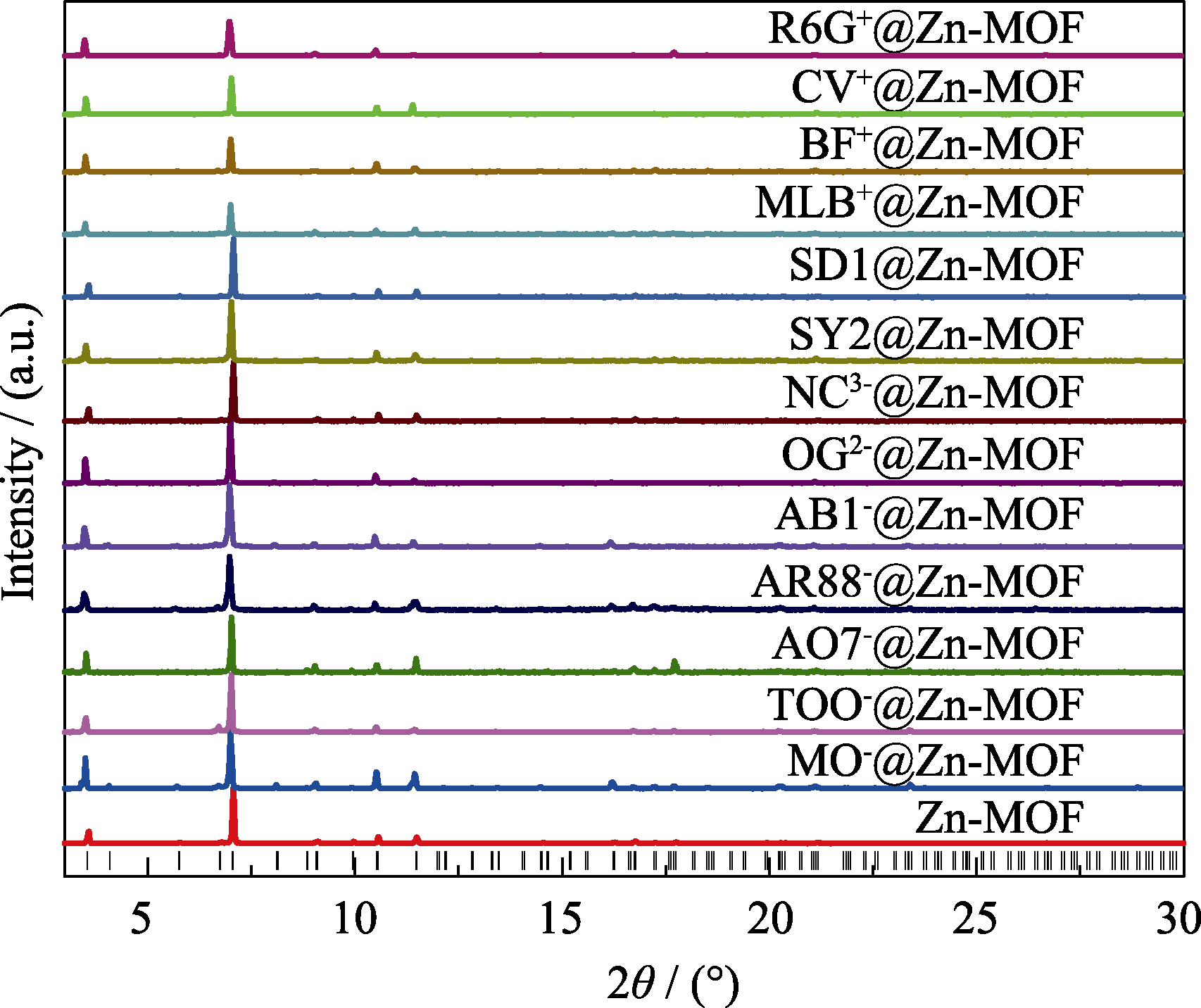
Fig. S4 PXRD patterns of Zn-MOF and dye@Zn-MOF samples compared with simulation All the PXRD were carried out with a few drop of DMF on the surface. It shows that the crystal structure is still maintained after adsorption of dyes
| MOFs | Absorption capacity/(mg•g-1) | Metal | Framework charge | Ref. | |
|---|---|---|---|---|---|
| Cationic dye | Anionic dye | ||||
| MOF-5 | Congo Red | NM | Zn | Neutral | [1] |
| MOF-5 | Pyronin Y, Azure A | NM | Zn | Neutral | [2] |
| ZIF-8 | 9.2 (MLB) | ~11.6 (MO) | Zn | Neutral | [3] |
| ZIF-8 | 5.4 (MLB) | 22 (AB40) | Zn | Neutral | [4] |
| IFMC-2 | MLB, CV | NA | Zn | Anionic | [5] |
| Compound 1 | MLB, RhB | NA | Zn | Anionic | [6] |
| NENU-505 | MLB, BR2 | NA | Zn | Anionic | [7] |
| Zn-MOF | 12.6 (MLB) | 19 (MO) | Zn | Cationic | This work |
| ITC-4 | NA | 77.4 (OG) | In | Cationic | [8] |
| Compound 1 | NA | 183.5 (OG) | In | Cationic | [9] |
| MOF-235 | 252.0 (MLB) | 477.0 (MO) | Fe | Cationic | [10] |
| MIL-100(Fe) | 736.2 (MLB) | 1045.2 (MO) | Fe | Cationic | [11] |
| MIL-100(Cr) | 643.3 (MLB) | 211.8 (MO) | Cr | Cationic | [11] |
| PCN-222 | 906 (MLB) | 589 (MO) | Zr | Neutral | [12] |
Table S2 The adsorption capacity of selected MOFs and Zn-MOF toward MLB and MO
| MOFs | Absorption capacity/(mg•g-1) | Metal | Framework charge | Ref. | |
|---|---|---|---|---|---|
| Cationic dye | Anionic dye | ||||
| MOF-5 | Congo Red | NM | Zn | Neutral | [1] |
| MOF-5 | Pyronin Y, Azure A | NM | Zn | Neutral | [2] |
| ZIF-8 | 9.2 (MLB) | ~11.6 (MO) | Zn | Neutral | [3] |
| ZIF-8 | 5.4 (MLB) | 22 (AB40) | Zn | Neutral | [4] |
| IFMC-2 | MLB, CV | NA | Zn | Anionic | [5] |
| Compound 1 | MLB, RhB | NA | Zn | Anionic | [6] |
| NENU-505 | MLB, BR2 | NA | Zn | Anionic | [7] |
| Zn-MOF | 12.6 (MLB) | 19 (MO) | Zn | Cationic | This work |
| ITC-4 | NA | 77.4 (OG) | In | Cationic | [8] |
| Compound 1 | NA | 183.5 (OG) | In | Cationic | [9] |
| MOF-235 | 252.0 (MLB) | 477.0 (MO) | Fe | Cationic | [10] |
| MIL-100(Fe) | 736.2 (MLB) | 1045.2 (MO) | Fe | Cationic | [11] |
| MIL-100(Cr) | 643.3 (MLB) | 211.8 (MO) | Cr | Cationic | [11] |
| PCN-222 | 906 (MLB) | 589 (MO) | Zr | Neutral | [12] |
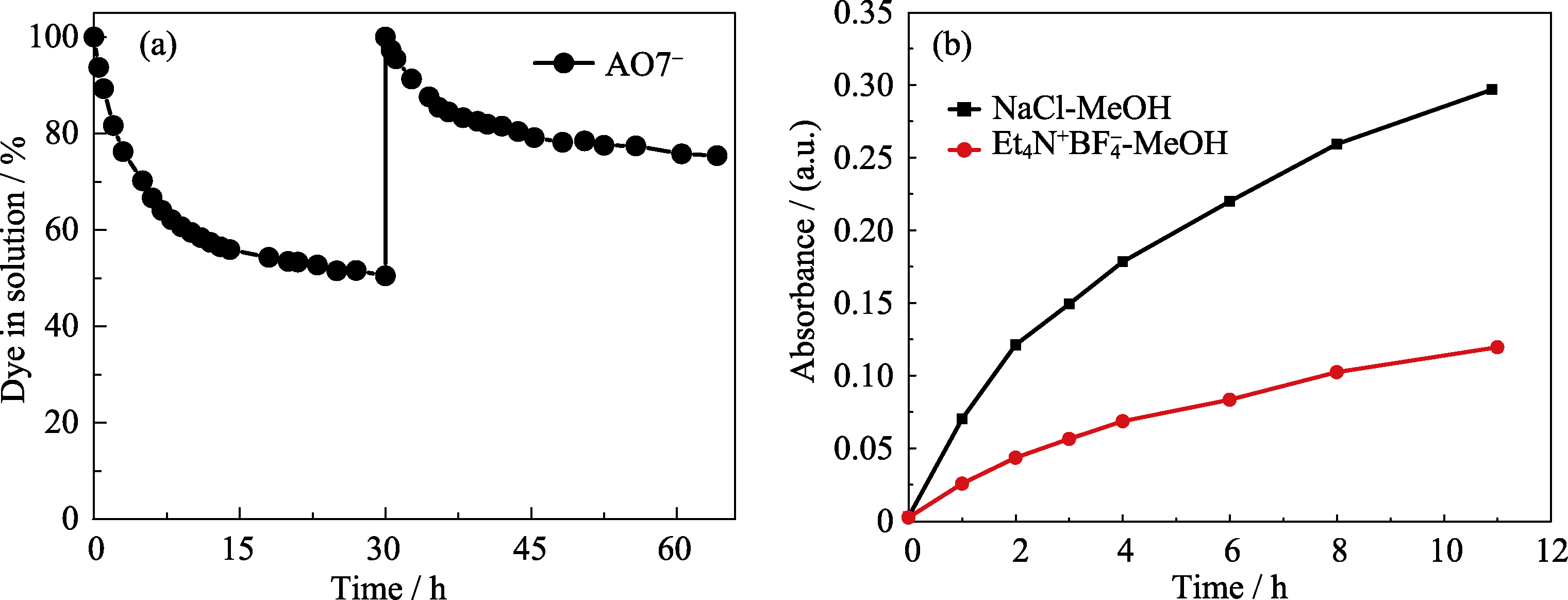
Fig. 1 (a) Dye adsorption kinetics of AO7- by Zn-MOF, which was immersed into a fresh AO7- solution at 30 h for second uptake, indicating that the concentration gradient potential served as the driving force for dye inclusion; (b) Release rates of AR88- (at λmax = 509 nm) salt out from AR88-@Zn-MOF in 0.18 mol/L methanol solution of NaCl and Et4N+BF4-, respectively
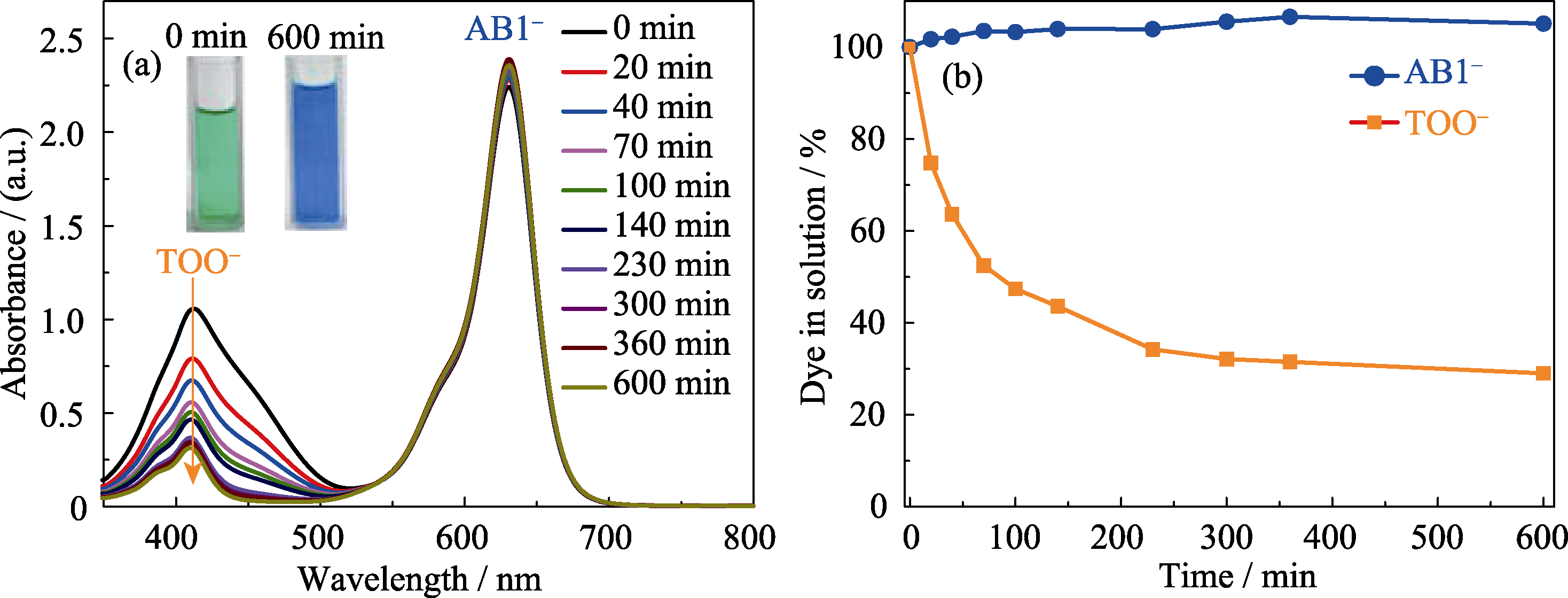
Fig. 2 (a) The time dependent UV-Vis spectra of the solutions of AB1- and TOO- in the ratio of 765∶1 for the competitive adsorption by Zn-MOF (λmax= 412 nm for TOO-, λmax= 630 nm for AB1-) with inset showing the colour of TOO-/AB1- solution before and after dye inclusion for 600 min, respectively; (b) The adsorption kinetics of AB1- and TOO- by Zn-MOF in the binary mixture solution
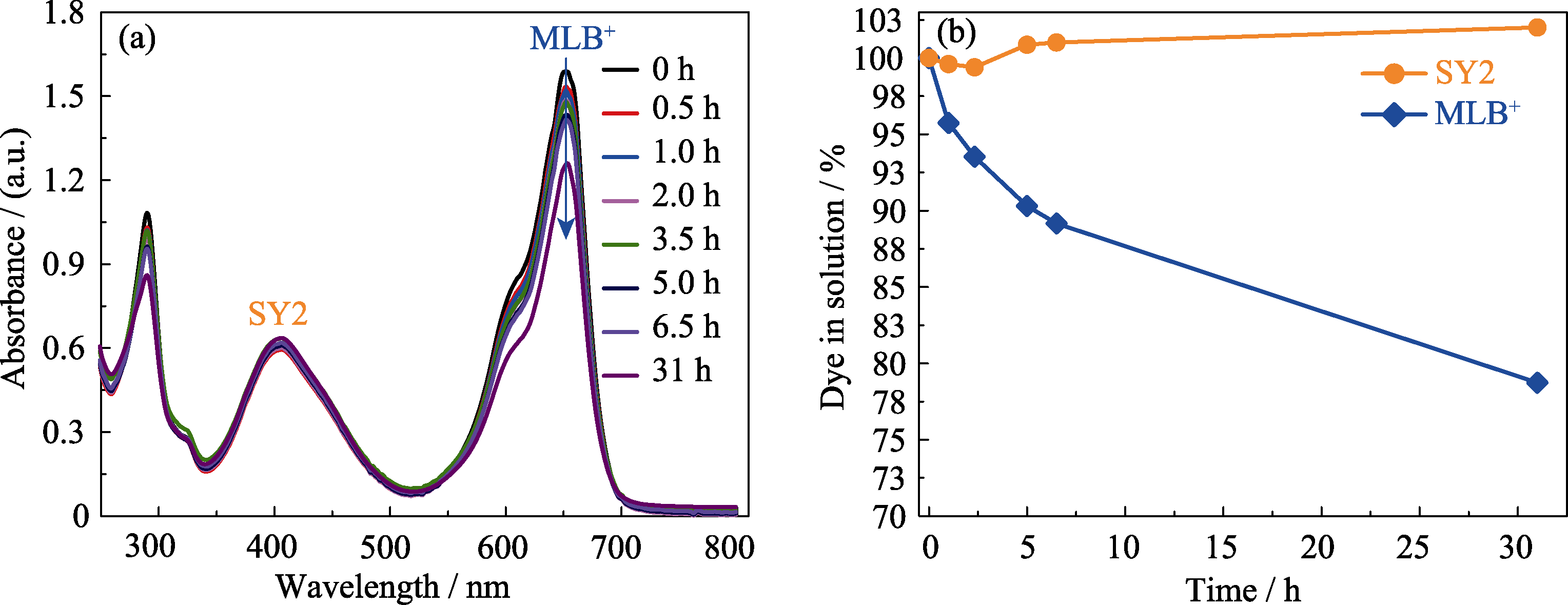
Fig. 4 (a) The time dependent UV-Vis spectra of the solutions of MLB+ and SY2 at the ratio of 1∶1 for the competitive adsorption by Zn-MOF from 0 to 31 h (λmax = 652 nm for MlB+, λmax = 406 nm for SY2); (b) The competitive adsorption rate of MIB+ and SY2 by the Zn-MOF
| [1] | CUI YUAN-JING, SONG RUI-JING, YU JIAN-CAN, et al.Dual-emitting MOF superset of dye composite for ratiometric temperature sensing. Adv. Mater., 2015, 27(8): 1420-1425. |
| [2] | XIE WEI, HE WEN-WEN, LI SHUN-LI, et al.An anionic interpenetrated zeolite-like metal-organic framework composite as a tunable dual-emission luminescent switch for detecting volatile organic molecules. Chem. Eur. J., 2016, 22(48): 17298-17304. |
| [3] | LAN YA-QIAN, JIANG HAI-LONG, LI SHUN-LI, et al.Mesoporous metal-organic frameworks with size-tunable cages: selective CO2 uptake, encapsulation of Ln3+ cations for luminescence, and column-chromatographic dye separation. Adv.Mater., 23(43): 5015-5020. |
| [4] | LI PEI-ZHOU, WANG XIAO-JUN, TAN SI-YU, et al.Clicked isoreticular metal-organic frameworks and their high performance in the selective capture and separation of large organic molecules. Angew. Chem., Int. Ed., 2015, 54(43): 12748-12752. |
| [5] | MA LI-QING, FALKOWSKI JOSEPH M, ABNEY CARTER, et al.A series of isoreticular chiral metal-organic frameworks as a tunable platform for asymmetric catalysis. Nat. Chem., 2010, 2(10): 838-846. |
| [6] | DELLA ROCCA JOSEPH, LIU DE-MIN, LIN WEN-BIN.Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res., 2011, 44(10): 957-968. |
| [7] | ZHAO XIANG, MAO CHENG-YU, LUONG KAREN TU, et al.Framework cationization by preemptive coordination of open metal sites for anion-exchange encapsulation of nucleotides and coenzymes. Angew. Chem. Int. Ed., 2016, 55(8): 2768-2772. |
| [8] | LIU FEI, CHUNG SO-YI, OH GAHEE, et al.Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. ACS Appl. Mater. Interfaces, 2012, 4(2): 922-927. |
| [9] | SIMON V, THURET A, CANDY L, et al.Recovery of hydroxycinnamic acids from renewable resources by adsorption on zeolites. Chem. Eng. J., 2015, 280: 748-754. |
| [10] | ZHAO XIANG, BU XIAN-HUI, WU TAO, et al.Selective anion exchange with nanogated isoreticular positive metal-organic frameworks. Nat. Commun., 2013, 4: 2344-2348. |
| [11] | HASAN ZUBAIR, JHUNG SUNG HWA.Removal of hazardous organics from water using metal-organic frameworks (MOFs): plausible mechanisms for selective adsorptions. J. Hazard. Mater., 2015, 283: 329-339. |
| [12] | HAN YI, SHENG SHU-NAN, YANG FAN, et al.Size-exclusive and coordination-induced selective dye adsorption in a nanotubular metal-organic framework. J. Mater. Chem. A, 2015, 3(24): 12804-12809. |
| [13] | HAQUE ENAMUL, JUN JONG WON, JHUNG SUNG HWA.Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater., 2011, 185(1): 507-511. |
| [14] | HAN SHUANG-BING, WEI YAN-HU, CORY VALENTE, et al.Chromatography in a single metal-organic framework (MOF) crystal. J. Am. Chem. Soc., 2010, 132(46): 16358-16361. |
| [15] | KANG XIAO-ZHEN, SONG ZHENG-WEI, SHI QI, et al.Utilization of zeolite imidazolate framework as an adsorbent for the removal of dye from aqueous solution. Asian [J]. Chem., 2013, 25(15): 8324-8328. |
| [16] | LI YU, ZHOU KANG, HE MING, et al.Synthesis of ZIF-8 and ZIF-67 using mixed-base and their dye adsorption. Microporous Mesoporous Mater., 2016, 234(1): 287-292. |
| [17] | QIN JUN-SHENG, ZHANG SHU-RAN, DU DONG-YING, et al.A microporous anionic metal-organic framework for sensing luminescence of lanthanide (III) ions and selective absorption of dyes by ionic exchange. Chem. Eur. J., 2014, 20(19): 5625-5630. |
| [18] | SHEN XIANG, YAN BING.Anionic metal-organic framework hybrids functionalization with lanthanide ions or cationic dyes and fluorescence sensing of small molecules. RSC Adv., 2016, 6(34): 28165-28170. |
| [19] | WU MING-YAN, JIANG FEI-LONG, WEI WEI, et al.A porous polyhedral metal-organic framework based on Zn2(COO)3 and Zn2, 2009, 9(6): 2559-2561. |
| [20] | LI HAI-CHAO, CAO XIN-YU, ZHANG CHUANG, et al.Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. RSC Adv., 2017, 7(27): 16273-16281. |
| [21] | TONG MIN-MAN, LIU DA-HUAN, YANG QING-YUAN, et al.Influence of framework metal ions on the dye capture behavior of MIL-100 (Fe, Cr) MOF type solids. J. Mater. Chem. A, 2013, 1(30): 8534-8537. |
| [22] | KHANJANI SOMAYEH, MORSALI ALI.Ultrasound-promoted coating of MOF-5 on silk fiber and study of adsorptive removal and recovery of hazardous anionic dye “congo red’’. Ultrason. Sonochem. 2014, 21(4): 1424-1429. |
| [23] | HAN SHUANG-BING, WEI YAN-HU, CORY VALENTE, et al.Chromatography in a single metal-organic framework (MOF) crystal. J. Am. Chem. Soc., 2010, 132(46): 16358-16361. |
| [24] | LI YU, ZHOU KANG, HE MING, et al.Synthesis of ZIF-8 and ZIF-67 using mixed-base and their dye adsorption. Microporous Mesoporous Mater., 2016, 234(1): 287-292. |
| [25] | KANG XIAO-ZHEN, SONG ZHENG-WEI, SHI QI, et al.Utilization of zeolite imidazolate framework as an adsorbent for the removal of dye from aqueous solution. Asian [J]. Chem., 2013, 25(15): 8324-8328. |
| [26] | QIN JUN-SHENG, ZHANG SHU-RAN, DU DONG-YING, et al.A microporous anionic metal-organic Framework for sensing luminescence of lanthanide (III) ions and selective absorption of dyes by ionic exchange. Chem. Eur. J., 2014, 20(19): 5625-5630. |
| [27] | SHEN XIANG, YAN BING.Anionic metal-organic framework hybrids functionalization with lanthanide ions or cationic dyes and fluorescence sensing of small molecules. RSC Adv., 2016, 6(34): 28165-28170. |
| [28] | ZHANG SHU-RAN, LI JING, DU DONG-YING, et al.A multifunctional microporous anionic metal-organic framework for column-chromatographicdye separation and selective detection andadsorption of Cr3+. J. Mater. Chem. A, 2015, 3(46): 23426-23434. |
| [29] | ZHAO XIANG, BU XIAN-HUI, WU TAO, et al.Selective anion exchange with nanogated isoreticular positive metal-organic frameworks. Nat.Commun., 2013(4): 2344-2348. |
| [30] | SONG BAI-QIAO, WANG XIN-LONG, ZHANG YU-TENG, et al.Periodic tiling of triangular and square nanotubes in a cationic metal-organic framework for selective anion exchange. Chem. Commun., 2015, 51(46): 9515-9518. |
| [31] | HAQUE ENAMUL, JUN JONGWON, JHUNG SUNGHWA.Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater., 2011, 185(1): 507-511. |
| [32] | TONG MIN-MAN, LIU DA-HUAN, YANG QING-YUAN, et al.Influence of framework metal ions on the dye capture behaviour of MIL-100(Fe, Cr) MOF type solids. J. Mater. Chem. A, 2013, 1(30): 8534-8537. |
| [33] | LI HAI-CHAO, CAO XIN-YU, ZHANG CHUANG, et al.Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. RSC Adv., 2017, 7(27): 16273-16281. |
| [1] | 王艳莉, 钱心怡, 沈春银, 詹亮. 石墨烯基介孔锰铈氧化物催化剂: 制备和低温催化还原NO[J]. 无机材料学报, 2024, 39(1): 81-89. |
| [2] | 凌洁, 周安宁, 王文珍, 贾忻宇, 马梦丹. Cu/Mg比对Cu/Mg-MOF-74的CO2吸附性能的影响[J]. 无机材料学报, 2023, 38(12): 1379-1386. |
| [3] | 江润璐, 吴鑫, 郭昊骋, 郑琦, 王连军, 江莞. UiO-67基导电复合材料的制备及其热电性能研究[J]. 无机材料学报, 2023, 38(11): 1338-1344. |
| [4] | 刘海方, 苏海军, 申仲琳, 姜浩, 赵迪, 刘园, 张军, 刘林, 傅恒志. 激光增材制造超高温氧化物共晶陶瓷研究进展[J]. 无机材料学报, 2022, 37(3): 255-266. |
| [5] | 吉永记, 刘东, 李强. 半透明太阳能电池的热力学极限效率研究[J]. 无机材料学报, 2022, 37(2): 204-208. |
| [6] | 郭李娜, 何雪冰, 吕琳, 吴丹, 原弘. 调控CuO表面性质选择性电催化还原CO2制HCOOH[J]. 无机材料学报, 2022, 37(1): 29-37. |
| [7] | 梁凤青, 温兆银. 固态锂电池用MOF/聚氧化乙烯复合聚合物电解质[J]. 无机材料学报, 2021, 36(3): 332-336. |
| [8] | 侯琦, 王茂槐, 刘森, 董宏斌, 郭文跃, 鲁效庆. 类石墨烯碳氮分离膜氢气提纯特性的机理研究[J]. 无机材料学报, 2020, 35(11): 1234-1238. |
| [9] | 罗清,苑青,蒋前勤,于乃森. Cu-SSZ-13/碳化硅废料复合材料的合成及其 NH3-SCR性能研究[J]. 无机材料学报, 2019, 34(9): 953-960. |
| [10] | 罗世强, 郑春满, 孙巍巍, 谢威, 柯剑煌, 刘双科, 洪晓斌, 李宇杰, 许静. ZIF-67衍生Co-NC多孔碳材料的可控制备及其在锂-硫二次电池中的应用研究[J]. 无机材料学报, 2019, 34(5): 502-508. |
| [11] | 陈 健, 郑玉婴, 张延兵, 邹海强, 卢秀恋. 氧化还原沉淀法制备MnO2/MWCNTs催化剂及其低温SCR活性[J]. 无机材料学报, 2016, 31(12): 1347-1354. |
| [12] | 李 军, 潘 磊, 王际童, 龙东辉, 乔文明, 凌立成. 球形活性炭担载MnOx-CeO2和三聚氰胺的低温脱硝行为研究[J]. 无机材料学报, 2016, 31(11): 1205-1211. |
| [13] | 华成江, 王明辉, 栾国有, 刘 岩, 吴 华. 原位晶化法在铜网上快速合成Cu3(BTC)2基膜及其催化性能研究[J]. 无机材料学报, 2015, 30(5): 529-534. |
| [14] | 杨冬花, 王新波, 石宝宝, 武正簧, 李晓峰, 窦 涛. 甲醇定向转化制二甲苯的复合分子筛ZSM-5/EU-1的合成及其应用[J]. 无机材料学报, 2014, 29(4): 357-363. |
| [15] | 吴大旺, 张秋林, 林 涛, 龚茂初, 陈耀强. Fe对Mn/CeO2-TiO2催化剂低温NH3选择性催化还原NO的影响[J]. 无机材料学报, 2012, 27(5): 495-500. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||