无机材料学报 ›› 2017, Vol. 32 ›› Issue (4): 372-378.DOI: 10.15541/jim20160333 CSTR: 32189.14.10.15541/jim20160333
祁星耀, 周清锋, 崔芒伟, 杨永珍, 蒋海伟, 梁 伟, 康利涛
收稿日期:2016-05-24
修回日期:2016-07-10
出版日期:2017-04-20
网络出版日期:2017-03-24
作者简介:祁星耀(1979–), 男, 硕士研究生. E-mail: kangltxy@163.com
基金资助:QI Xing-Yao, ZHOU Qing-Feng, CUI Mang-Wei, YANG Yong-Zhen, JIANG Hai-Wei, LIANG Wei, KANG Li-Tao
Received:2016-05-24
Revised:2016-07-10
Published:2017-04-20
Online:2017-03-24
About author:QI Xing-Yao. E-mail: kangltxy@163.com
Supported by:摘要:
通过简单、低成本的化学浴沉积法在泡沫镍上原位生成了Zn-Ni 氢氧化物(Zn-Ni double hydroxides)纳米片。SEM观察结果表明, Zn-Ni 氢氧化物纳米片均匀附着在泡沫镍表面, 形成均一的多孔纳米片阵列层。此外, 还有大量的Zn-Ni 氢氧化物纳米片聚集成多孔团聚体, 分布于泡沫镍骨架的空隙处, 从而获得较高的活性物质负载量(4.27 mg/cm2)。CV、CP和电化学阻抗测试表明, Zn-Ni 氢氧化物纳米片在2 mol/L KOH电解液中充放电电流密度1 A/g时, 比电容为746.2 F/g(面积电容为3.18 F/cm2); 3000次充放电循环后, 仍保持70.9%的初始比电容。
中图分类号:
祁星耀, 周清锋, 崔芒伟, 杨永珍, 蒋海伟, 梁 伟, 康利涛. 原位氧化法制备Zn-Ni氢氧化物纳米片及其电荷存储特性研究[J]. 无机材料学报, 2017, 32(4): 372-378.
QI Xing-Yao, ZHOU Qing-Feng, CUI Mang-Wei, YANG Yong-Zhen, JIANG Hai-Wei, LIANG Wei, KANG Li-Tao. Capacitive Performance of Zn-Ni Hydroxide Nano-sheet Arrays on Nickel Foams via a Mild Chemical-bath Deposition Process[J]. Journal of Inorganic Materials, 2017, 32(4): 372-378.
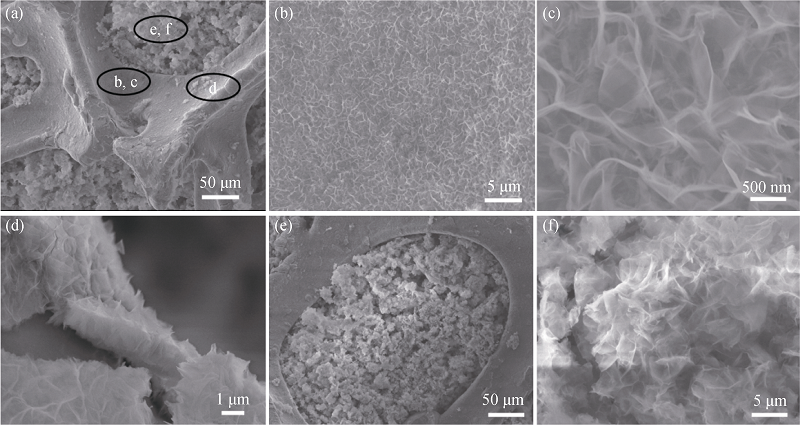
图3 Ni@Zn-Ni氢氧化物(a), 在泡沫镍骨架表面的Zn-Ni 氢氧化物纳米片(b~d)和在泡沫镍中空位置的Zn-Ni 氢氧化物纳米片(e~f)的SEM照片
Fig. 3 SEM images of the Ni@Zn-Ni electrode (a), Zn-Ni on the surface of the nickel foam skeletons(b-d), Zn-Ni in the void space of the nickel (foam e-f)
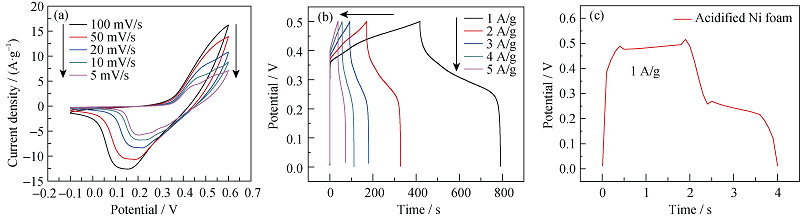
图5 Ni@Zn-Ni DHs电极在不同扫描速率下的CV曲线(a)和在不同电流密度下的恒电流充放电曲线(b), 酸化泡沫镍在1 A/g下的恒电流充放电曲线(c)
Fig. 5 (a) Cyclic voltammetric and (b) galvanostatic charge-discharge curves of Ni@Zn-Ni DHs at various scan rates and current densities, (c) GCD curve of acidified Ni foam at current density of 1 A/g
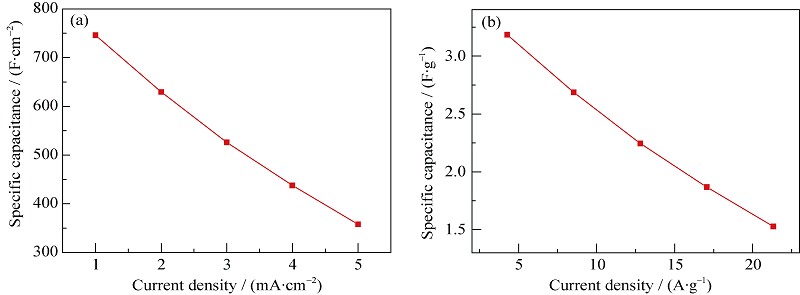
图6 Ni@Zn-Ni DHs电极在不同放电电流密度下的面积电容(a)和比电容(b)
Fig. 6 (a) Areal capacitance and (b) specific capacitance of Ni@Zn-Ni DHs electrodes at different discharge current densities
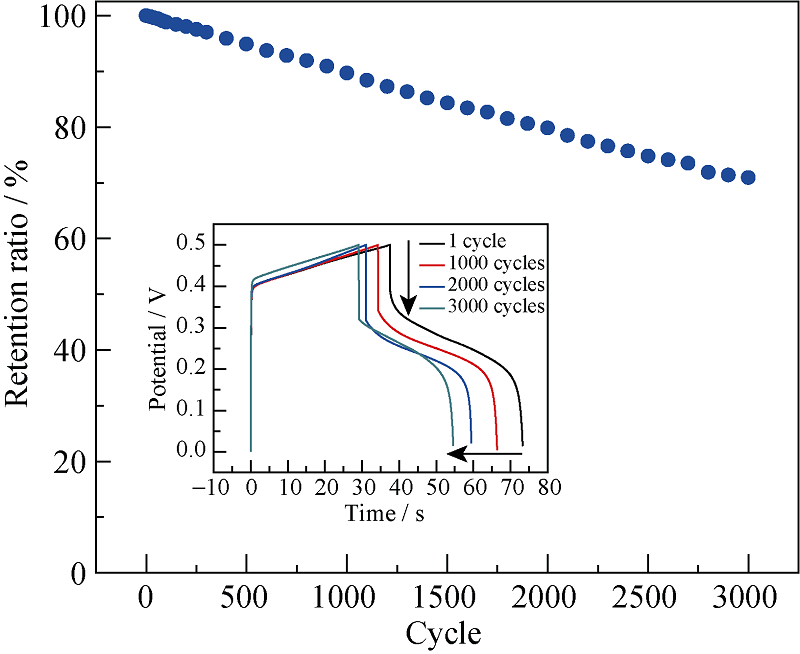
图7 Zn-Ni 氢氧化物样品在5 A/g下3000次循环测试中比电容变化和每1000次循环过程中的充放电曲线
Fig. 7 Stability test in terms of specific capacitance and galvanostatic charge-discharge curves after every 1000 cycle at a current density of 5 A/g (insert) for Ni@Zn-Ni DHs
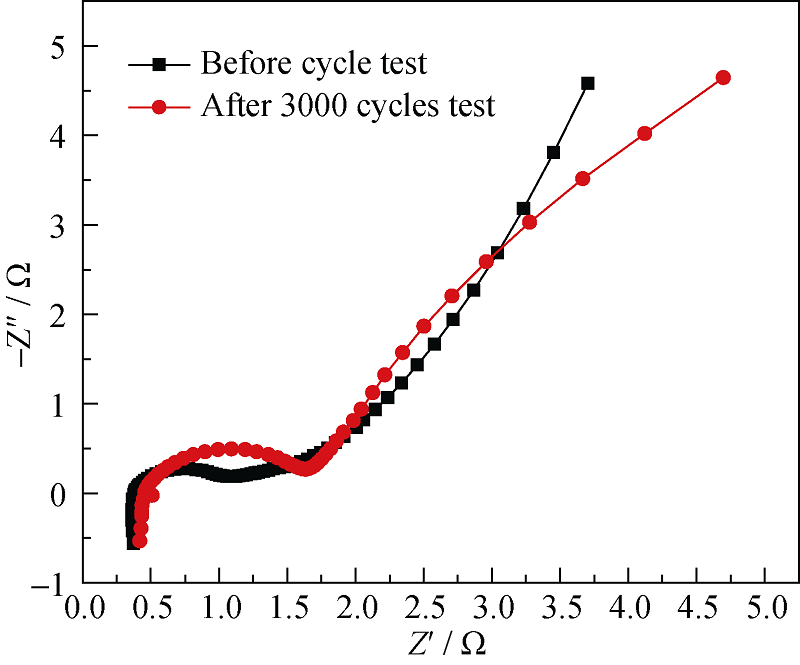
图8 3000次充放电循环前后的Ni@Zn-Ni DHs样品在2 mol/L KOH电解液中的阻抗谱图
Fig. 8 Nyquist plots of Ni@Zn-Ni DHs before and after 3000 charge-discharge cycles in 2 mol/L KOH aqueous electrolyte.
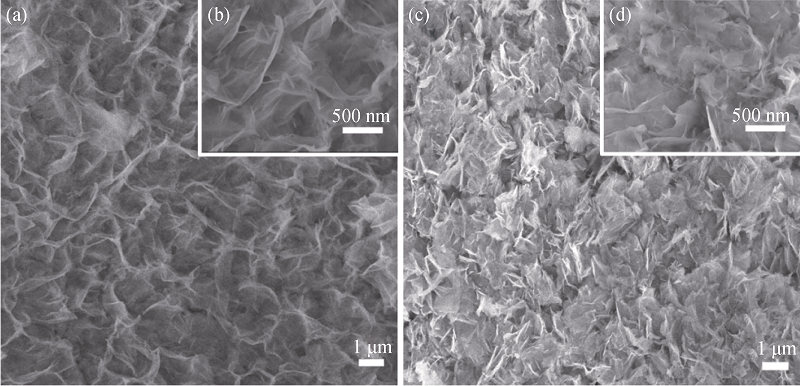
图10 Ni@Zn-Ni DHs电极在3000次充放电循环后的SEM照片
Fig. 10 SEM images of the Ni@Zn-Ni DHs electrode after 3000 charge-discharge cyclesSEM images of Zn-Ni nano-sheets grown on the surface of the Ni foam skeletons (a, b); Zn-Ni nano-sheets accommodated in the voids between skeletons of the Ni foam (c, d)
| [1] | SIMON P, GOGOTSI Y.Materials for electrochemical capacitors. Nature Materials, 2008, 7(11): 845-854. |
| [2] | WANG G, ZHANG L, ZHANG J.A review of electrode materials for electrochemical supercapacitors. Chemical Society Reviews, 2012, 41(2): 797-828. |
| [3] | CHEN X, CHEN C, ZHANG Z, et al.Nitrogen-doped porous carbon for supercapacitor with long-term electrochemical stability. Journal of Power Sources, 2013, 230: 50-58. |
| [4] | KANG J, HIRATA A, KANG L, et al.Enhanced supercapacitor performance of MnO2 by atomic doping. AngewandteChemie, 2013, 125(6): 1708-1711. |
| [5] | SU Z, YANG C, XU C, et al.Co-electro-deposition of the MnO2- PEDOT: PSS nanostructured composite for high areal mass, flexible asymmetric supercapacitor devices. Journal of MaterialsChemistry A, 2013, 1(40): 12432-12440. |
| [6] | HUANG L, CHEN D, DING Y, et al.Nickel-cobalt hydroxide nanosheets coated on NiCo2O4 nanowires grown on carbon fiber paper for high-performance pseudocapacitors. Nano Letters, 2013, 13(7): 3135-3139. |
| [7] | GAMBY J, TABERNA P L, SIMON P, et al.Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. Journal of Power Sources, 2001, 101(1): 109-116. |
| [8] | FUERTES A B, LOTA G, CENTENO T A, et al.Templated mesoporous carbons for supercapacitor application. ElectrochimicaActa, 2005, 50(14): 2799-2805. |
| [9] | CHMIOLA J, YUSHIN G, DASH R, et al.Effect of pore size and surface area of carbide derived carbons on specific capacitance. Journal of Power Sources, 2006, 158(1): 765-772. |
| [10] | FUTABA D, HATA K, YAMADA T, et al.Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nature Materials, 2006, 5(12): 987-994. |
| [11] | TAO K, LI P, KANG L, et al.Facile and low-cost combustion-synthesized amorphous mesoporous NiO/carbon as high mass-loading pseudocapacitor materials. Journal of Power Sources, 2015, 293: 23-32. |
| [12] | LI W, CHEN J, ZHAO J, et al.Application of ultrasonic irradiation in preparing conducting polymer as active materials for supercapacitor. Materials Letters, 2005, 59(7): 800-803. |
| [13] | HU C, CHANG K, LIN M, et al.Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Letters, 2006, 6(12): 2690-2695. |
| [14] | WANG G, HUANG J, CHEN S, et al.Preparation and supercapacitance of CuO nanosheet arrays grown on nickel foam. Journal of Power Sources, 2011, 196(13): 5756-5760. |
| [15] | LI Y, CHANG S, LIU X, et al.Nanostructured CuO directly grown on copper foam and their supercapacitance performance. ElectrochimicaActa, 2012, 85: 393-398. |
| [16] | HUANG J, WU H, CAO D, et al.Influence of Ag doped CuOnanosheet arrays on electrochemical behaviors for supercapacitors. Electrochimica Acta, 2012, 75: 208-212. |
| [17] | XIE K, LI J, LAI Y, et al.Polyaniline nanowire array encapsulated in titania nanotubes as a superior electrode for supercapacitors. Nanoscale, 2011, 3(5): 2202-2207. |
| [18] | KULAL P, DUBAL D, LOKHANDE C, et al.Chemical synthesis of Fe2O3 thin films for supercapacitor application. Journal of Alloys and Compounds, 2011, 509(5): 2567-2571. |
| [19] | WU M, LEE R, JOW J, et al.Nanostructured iron oxide films prepared by electrochemical method for electrochemical capacitors. Electrochemical and Solid-State Letters, 2009, 12(1): A1-A4. |
| [20] | DUBAL D, FULARI V, LOKHANDE C.Effect of morphology on supercapacitive properties of chemically grown β-Ni(OH)2 thin films. Microporous and Mesoporous Materials, 2012, 151: 511-516. |
| [21] | HSU H, CHANG K, SALUNKHE R, et al.Synthesis and characterization of mesoporous Ni-Co oxy-hydroxides for pseudocapacitor application. Electrochimica Acta, 2013, 94: 104-112. |
| [22] | ZHOU Q, CUI M, TAO K, et al.High areal capacitance three-dimensional Ni@Ni(OH)2 foams via in situ oxidizing Ni foams in mild aqueous solution. Applied Surface Science, 2016, 365: 125-130. |
| [23] | ZHOU C, ZHANG Y, LI Y, et al.Construction of high-capacitance 3D CoO@polypyrrole nanowire array electrode for aqueous asymmetric supercapacitor. Nano letters, 2013, 13(5): 2078-2085. |
| [24] | ZHANG G, WU H, HOSTER H, et al.Single-crystalline NiCo2O4nanoneedle arrays grown on conductive substrates as binder-free electrodes for high-performance supercapacitors. Energy & Environmental Science, 2012, 5(11): 9453-9456. |
| [25] | JIANG J, LI Y, LIU J, et al.Building one-dimensional oxide nanostructure arrays on conductive metal substrates for lithium-ion battery anodes. Nanoscale, 2011, 3(1): 45-58. |
| [26] | JIANG J, LI Y, LIU J, et al.Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Advanced Materials, 2012, 24(38): 5166-5180. |
| [27] | LIU Y, LIU Z, LU N, et al.Facile synthesis of polypyrrole coated copper nanowires: a new concept to engineered core-shell structures. Chemical Communications, 2012, 48(20): 2621-2623. |
| [28] | ZHONG J, WANG A, LI G, et al.Co3O4/Ni(OH)2 composite mesoporousnanosheet networks as a promising electrode for supercapacitor applications. Journal of Materials Chemistry, 2012, 22(12): 5656-5665. |
| [29] | MING B, LI J, KANG F, et al.Microwave-hydrothermal synthesis of birnessite-type MnO2nanospheres as supercapacitor electrode materials. Journal of Power Sources, 2012, 198: 428-431. |
| [30] | TOUPIN M, BROUSSE T, BÉLANGER D. Influence of microstucture on the charge storage properties of chemically synthesized manganese dioxide. Chemistry of Materials, 2002, 14(9): 3946-3952. |
| [31] | YU M, ZENG Y, ZHANG C, et al.Titanium dioxide@polypyrrole core-shell nanowires for all solid-state flexible supercapacitors. Nanoscale, 2013, 5(22): 10806-10810. |
| [32] | ZHENG H, ZHAI T, YU M, et al.TiO2@C core-shell nanowires for high-performance and flexible solid-state supercapacitors. Journal of Materials Chemistry C, 2013, 1(2): 225-229. |
| [33] | LI J, YANG M, WEI J, et al.Preparation and electrochemical performances of doughnut-like Ni(OH)2-Co(OH)2 composites as pseudocapacitor materials. Nanoscale, 2012, 4(15): 4498-4503. |
| [34] | LIU M, MIAO Y, ZHANG C, et al.Hierarchical composites of polyaniline-graphene nanoribbons-carbon nanotubes as electrode materials in all-solid-state supercapacitors. Nanoscale, 2013, 5(16): 7312-7320. |
| [35] | WU X, XING W, ZHANG L, et al.Nickel nanoparticles prepared by hydrazine hydrate reduction and their application in supercapacitor. Powder Technology, 2012, 224: 162-167. |
| [36] | FAN Z, YAN J, WEI T, et al.Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Advanced Functional Materials, 2011, 21(12): 2366-2375. |
| [37] | GUAN C, LIU J, CHENG C, et al.Hybrid structure of cobalt monoxide nanowire@nickelhydroxidenitratenanoflake aligned on nickel foam for high-rate supercapacitor. Energy & Environmental Science, 2011, 4(11): 4496-4499. |
| [38] | ZHU Y, JI X, PAN C, et al.A carbon quantum dot decorated RuO2 network: outstanding supercapacitances under ultrafast charge and discharge. Energy & Environmental Science, 2013, 6(12): 3665-3675. |
| [39] | KONG L, LANG J, LIU M, et al.Facile approach to prepare loose-packed cobalt hydroxide nano-flakes materials for electrochemical capacitors. Journal of Power Sources, 2009, 194(2): 1194-1201. |
| [40] | DENG J, KANG L, BAI G, et al.Solution combustion synthesis of cobalt oxides (Co3O4 and Co3O4/CoO) nanoparticles as supercapacitor electrode materials. ElectrochimicaActa, 2014, 132: 127-135. |
| [1] | 杨恩东, 李宝乐, 张珂, 谭鲁, 娄永兵. ZnCo2O4-ZnO@C@CoS核壳复合材料的制备及其在超级电容器中的应用[J]. 无机材料学报, 2024, 39(5): 485-493. |
| [2] | 景欣欣, 陈必清, 翟佳鑫, 袁美玲. Ni-Co-B-RE(Sm、Dy、Tb)复合电极: 化学沉积法制备及电催化析氢性能研究[J]. 无机材料学报, 2024, 39(5): 467-476. |
| [3] | 晁少飞, 薛艳辉, 吴琼, 伍复发, MUHAMMAD Sufyan Javed, 张伟. MXene异质结Ti-O-H-O电子快速通道促进高效率储钾[J]. 无机材料学报, 2024, 39(11): 1212-1220. |
| [4] | 丁玲, 蒋瑞, 唐子龙, 杨运琼. MXene材料的纳米工程及其作为超级电容器电极材料的研究进展[J]. 无机材料学报, 2023, 38(6): 619-633. |
| [5] | 孙鹏, 张绍宁, 毕辉, 董武杰, 黄富强. 基于结构调节碳材料的掺氮种类和含量及其超级电容器储能应用[J]. 无机材料学报, 2021, 36(7): 766-772. |
| [6] | 刘芳芳, 传秀云, 杨扬, 李爱军. 氮/硫共掺杂对纤水镁石模板碳纳米管电化学性能的影响[J]. 无机材料学报, 2021, 36(7): 711-717. |
| [7] | 朱云娜, 陈必清, 程天舒, 杜婵, 张士民, 赵静. 非晶Nd-Ni-B/NF稀土复合电极材料的制备及其析氢性能[J]. 无机材料学报, 2021, 36(6): 637-644. |
| [8] | 李泽晖,谭美娟,郑元昊,骆雨阳,经求是,蒋靖坤,李明杰. 导电金属有机骨架材料在超级电容器中的应用[J]. 无机材料学报, 2020, 35(7): 769-780. |
| [9] | 季邦, 赵文锋, 段洁利, 马立哲, 付兰慧, 杨洲. 泡沫镍网负载TiO2/WO3薄膜对乙烯的光催化降解[J]. 无机材料学报, 2020, 35(5): 581-588. |
| [10] | 陈钧,马培华,张诚,劳伦·鲁尔曼,吕耀康. 新型多功能无机/有机复合薄膜的制备及电化学性能研究[J]. 无机材料学报, 2020, 35(2): 217-223. |
| [11] | 费明婕, 张任平, 朱归胜, 俞兆喆, 颜东亮. 磷酸根掺杂MnFe2O4及其赝电容特性[J]. 无机材料学报, 2020, 35(10): 1137-1141. |
| [12] | 丁卓峰, 杨永强, 李在均. 组氨酸功能化碳点/石墨烯气凝胶的制备及超级电容器性能[J]. 无机材料学报, 2020, 35(10): 1130-1136. |
| [13] | 马亚楠, 刘宇飞, 余晨旭, 张传坤, 罗时军, 高义华. 不同横向尺寸单层Ti3C2Tx纳米片的制备及其电化学性能研究[J]. 无机材料学报, 2020, 35(1): 93-98. |
| [14] | 李腾飞, 黄璐君, 闫旭东, 刘庆雷, 顾佳俊. 碳化钛/椴木多孔碳复合材料用于超级电容器性能的研究[J]. 无机材料学报, 2020, 35(1): 126-130. |
| [15] | 张天宇, 崔聪, 程仁飞, 胡敏敏, 王晓辉. 同步氨化/碳化法制备MXene/C平面多孔复合电极[J]. 无机材料学报, 2020, 35(1): 112-118. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||