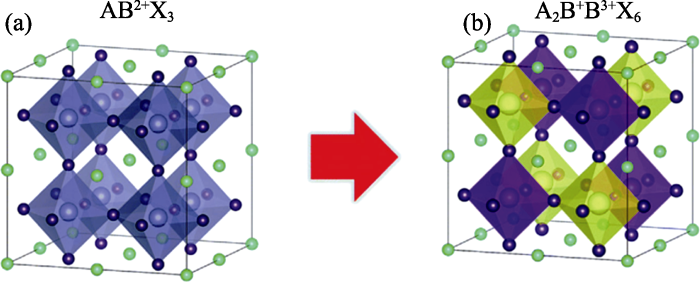
1 Cs2AgBiBr6晶体结构
卤化物钙钛矿的化学式为ABX3, 其中A(Cs+, CH3NH3+ (MA+), CH(NH2)2+ (FA+)等)为半径较大的无机或有机一价阳离子, B(Pb2+, Sn2+等)为二价阳离子, X(Cl-, Br-, I-等)为一价卤素或拟卤素阴离子。在ABX3的晶体结构中, A位阳离子位于立方体的顶角, B位阳离子位于立方晶胞体心, X位阴离子位于立方体面心(图1(a))。双钙钛矿的化学式为A2B+B3+X6, 用一对异价金属阳离子(B+/B3+)取代两个B位二价阳离子, 形成了具有交替八面体结构的晶格堆积(图1(b))。为了使A2B+B3+X6的晶体结构具有更好的稳定性和更高的缺陷容忍度, 常见的化学组分一般采用Cs+(A位), Ag+、Cu+、Au+(B+位), Bi3+、Sb3+、In3+(B3+位)和Cl-、Br- (X位)等[13⇓⇓⇓-17]。其中Cs2AgBiBr6具有环境友好性和长期稳定性, 晶格中的B+和B3+位分别被Ag+和Bi3+离子占据。由于Ag+(129 pm)和Bi3+(117 pm)半径不同, 导致Ag-Br和Bi-Br的键长略有差异, 但并没有引起晶格畸变[30]。
图1
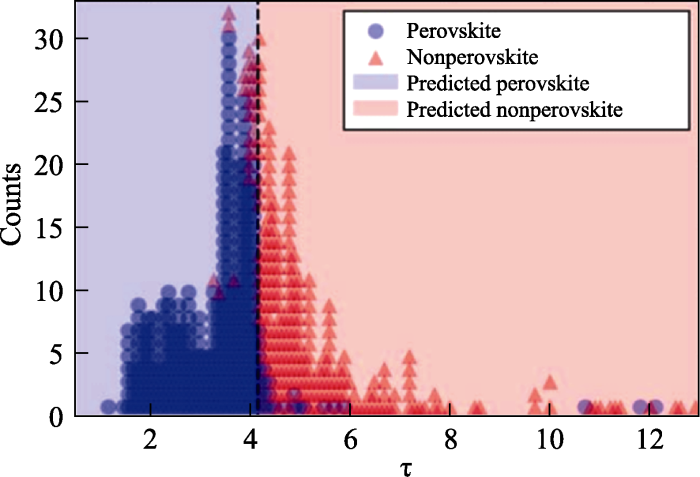
钙钛矿晶格结构的稳定性通常由两个经验参数来描述, 即八面体因子$\mu $和Goldschmidt容忍因子$t$。式(1)和式(2)中${{r}_{\text{A}}}$、${{r}_{\text{B}}}$和${{r}_{\text{x}}}$分别代表A、B和X位的离子半径。研究表明, 一般情况下, 在0.44<$\mu $< 0.90和0.81<$t$<1.11[31]时, 钙钛矿的晶体结构是稳定的。
为了更精确地描述钙钛矿的稳定性, 研究者们对上述因子参数进行了一些改进和修正, 比如Bartel等[32]引入容忍因子τ(式(3))。
图2
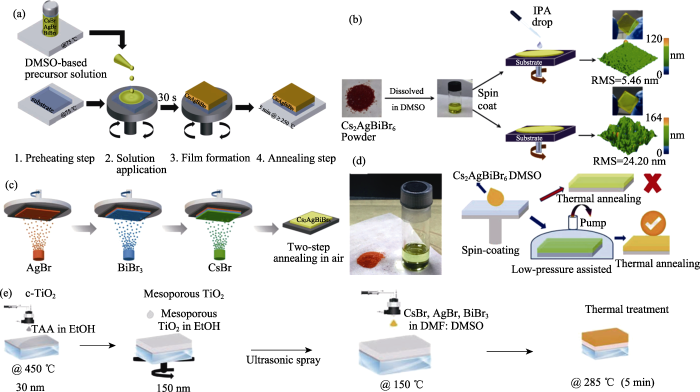
2 Cs2AgBiBr6薄膜的制备工艺
图3
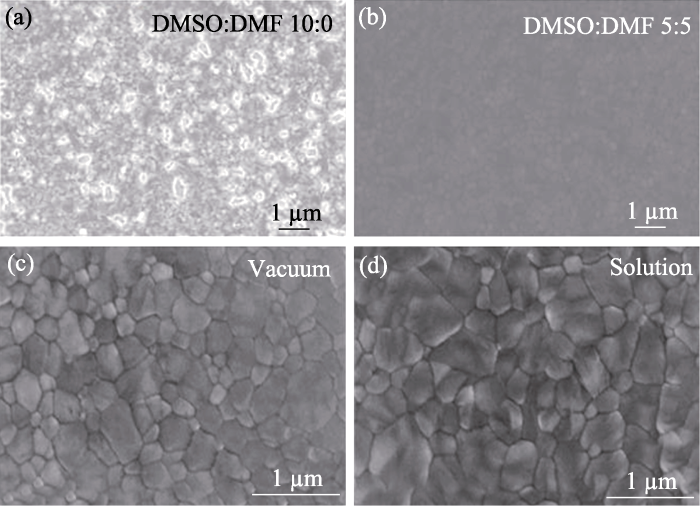
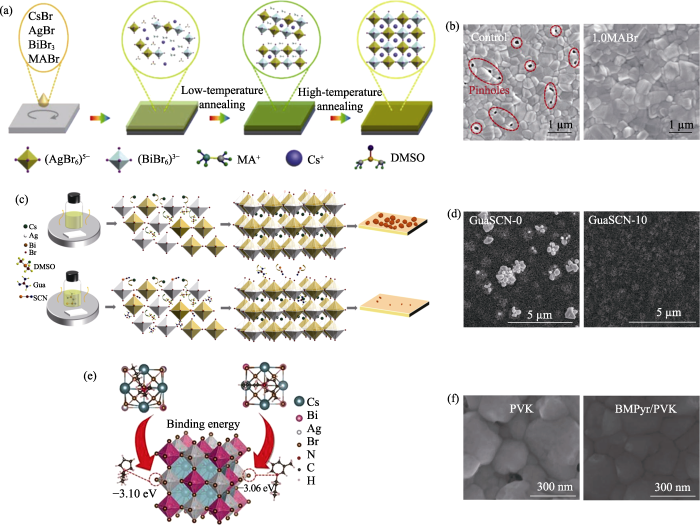
溶液法: 将钙钛矿前驱体溶液旋涂在基底上并通过退火促进晶体生长, 最后获得钙钛矿薄膜。Greul等[19]首次通过溶液法制备了Cs2AgBiBr6钙钛矿太阳能电池。由于Cs2AgBiBr6结晶速度快, 因此在薄膜表面形成了大量的团聚物, 需要对Cs2AgBiBr6薄膜的制备方法进一步优化。钙钛矿前驱体溶剂会对钙钛矿薄膜的形貌产生显著影响[39]。在配制前驱体溶液时, 通常选择具有高溶解性、低沸点和高蒸汽压的有机溶剂[40-41]。研究发现, 二甲基亚砜(DMSO)溶剂对Cs2AgBiBr6有较高的溶解度[8], 常被用作沉积Cs2AgBiBr6薄膜的前驱体溶剂[42⇓-44]。然而, DMSO的沸点高(189 ℃)、蒸气压低(20 ℃时为56 Pa), 不利于制备高质量的Cs2AgBiBr6薄膜(图4(a))[34,45⇓-47]。为了解决这一问题, 研究者向DMSO中加入了具有低沸点(153 ℃)和高蒸气压(20 ℃时为360 Pa)的N,N-二甲基甲酰胺(DMF), 通过溶液法制备了高质量的Cs2AgBiB6薄膜(图4(b))[34]。
图4
反溶剂辅助成膜法: 在旋涂钙钛矿薄膜过程中, 通过滴加非极性反溶剂快速冲洗前驱液的溶剂, 使前驱液呈现出过饱和状态并加速钙钛矿成核和结晶, 从而获得高质量钙钛矿薄膜。选择反溶剂必须考虑极性、沸点、相容性等因素。Gao等[8]选取甲苯、氯苯、异丙醇、乙醇和甲醇作为反溶剂, 研究了它们对Cs2AgBiBr6薄膜形貌的影响。由于异丙醇具有合适的极性(甲醇>乙醇>异丙醇>氯苯>甲苯)和沸点(氯苯>甲苯>异丙醇>乙醇>甲醇), 作为反溶剂的效果较好, 最终获得了表面光滑、无针孔的Cs2AgBiBr6薄膜。另外, 该方法受反溶剂滴加时间影响较大, 工艺重复性有待提升。
气相法: 将卤化物前驱物放置于蒸发热源中, 然后通过控制热源的温度调控卤化物前驱体的沉积速率, 最终获得钙钛矿薄膜。Wang等[35]利用连续气相法, 通过优化反应物的比例以及退火工艺制备出了大晶粒尺寸的Cs2AgBiBr6薄膜。研究发现, 在制备钙钛矿薄膜过程中提高BiBr3含量更有利于钙钛矿晶体生长, 其钙钛矿太阳能电池的光电转换效率达到了1.37%。Igbari等[36]对比了溶液法和气相法的差异, 发现溶液法制备的Cs2AgBiBr6薄膜具有更精确的化学计量比, 更容易形成高结晶度、大晶粒尺寸的钙钛矿薄膜(图4(c, d)), 两种方法制备的太阳能电池的光电转换效率分别为2.51%和1.41%。气相法制备钙钛矿薄膜仍需进一步优化, 例如, 通过精确调控反应物的化学计量比减少杂质相。另外,气相法对真空沉积设备的需求一定程度上限制了它的广泛应用。
3 Cs2AgBiBr6钙钛矿太阳能电池的性能优化策略
3.1 元素掺杂
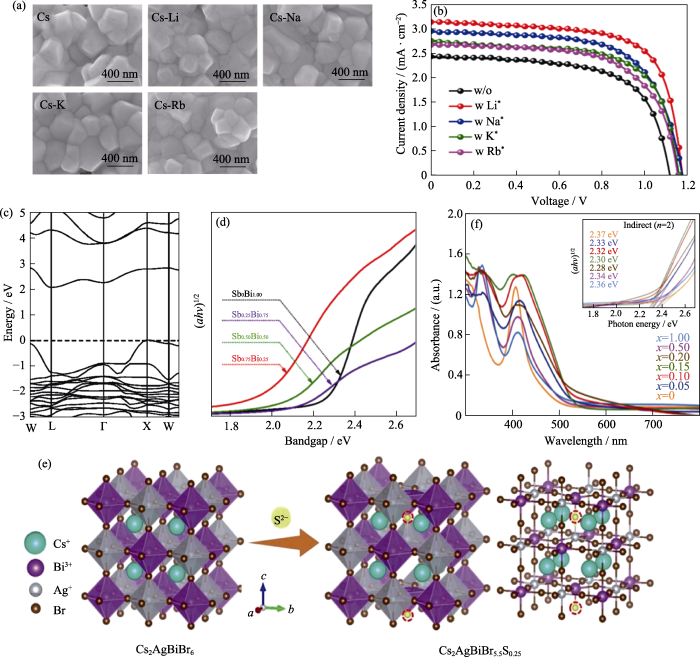
各组分的比例变化都会对钙钛矿的结构、薄膜的形貌以及光电性质产生显著影响[9,50⇓-52]。研究发现, 采用少量的一价金属离子取代A位的Cs+离子, 不仅能够改善钙钛矿薄膜的形貌, 而且能够调控钙钛矿薄膜的带隙[53-54]。Zhang等[53]首次报道了Rb+离子掺杂对Cs2AgBiBr6钙钛矿太阳能电池性能的影响, 发现采用适量的Rb+离子取代Cs+离子既可以提高Cs2AgBiBr6薄膜的质量, 又可以增强Cs2AgBiBr6薄膜的吸光能力。但Rb+浓度过量后, Cs2AgBiBr6薄膜的表面会形成针孔, 导致器件性能降低。适量Rb+离子取代将钙钛矿太阳能电池的短路电流从1.73 mA/cm2提升到1.94 mA/cm2, 光电转换效率从1.21%提升到1.39%。Li等[54]通过调控碱金属离子(Li+, Na+, K+, Rb+)的掺杂比例, 制备出表面光滑的Cs2AgBiBr6薄膜(图5(a)), 其中采用Li掺杂的Cs2AgBiBr6薄膜的带隙从1.89 eV减小到1.82 eV, 相应钙钛矿太阳能电池的短路电流从2.43 mA/cm2提升到3.15 mA/cm2, 光电转换效率从1.77%提高到2.57%, 如图5(b)所示。
图5
图5
离子掺杂优化Cs2AgBiBr6钙钛矿太阳能电池
Fig. 5
Ion doped Cs2AgBiBr6 perovskite solar cells
(a) SEM images of Cs2AgBiBr6, Cs1.99Li0.01AgBiBr6(Cs), Cs1.99Na0.01AgBiBr6(Cs-Li), Cs1.99K0.01AgBiBr6(Cs-Na), and Cs1.99Rb0.01AgBiBr6(Cs-K) films; (b) J-V curves of Cs2AgBiBr6 perovskite solar cells (w/o: Cs2AgBiBr6, w Li+: Cs1.99Li0.01AgBiBr6, w Na+: Cs1.99Na0.01AgBiBr6, w K+: Cs1.99K0.01AgBiBr6, w Rb+: Cs1.99Rb0.01AgBiBr6)[54]; (c) Band structure diagram for Cs2AgBiBr6[57]; (d) Tauc plots of Cs2AgSbxBi1-xBr6 (x=0, 0.25, 0.50, 0.75) films[58]; (e) Crystal structure diagram of Cs2AgBiBr6-2xSx; (f) UV-Vis absorption spectra with inset showing corresponding Tauc plots (right) of Cs2AgBiBr6-2xSx film[60]. Colorful figures are available on website
Cs2AgBiBr6能带(图5(c))的密度泛函理论(DFT)计算结果发现Cs2AgBiBr6具有大的间接带隙(2.06 eV)[55⇓-57], 与单结太阳能电池的理想带隙1.3 eV相差较大, 且间接带隙会导致光吸收系数不高, 不利于制备高效率的单结太阳能电池。B位上掺杂金属离子可以改变带隙。Liu等[58]在Cs2AgBiBr6晶格中掺杂Sb, 拓宽了Cs2AgBiBr6薄膜的吸光范围, 将薄膜的间接带隙从2.22 eV降低至1.97 eV(图5(d))。然而Cs2AgSbxBi1-xBr6薄膜覆盖率差且存在许多针孔, 导致钙钛矿太阳能电池的光电转换效率仅达到0.25%。并且Pantaler等[59]发现Sb掺杂虽然能够调控Cs2AgBiBr6薄膜的带隙, 但是Sb取代Bi会在Cs2AgBiBr6薄膜中产生大量空位等缺陷, 最终只获得光电转换效率为0.28%的钙钛矿太阳能电池。今后还需要进一步优化Sb掺杂策略, 以避免额外产生的空位等缺陷。
最近, Zhang等[22]采用氢化法将氢原子掺入到了Cs2AgBiBr6晶格间隙, 通过密度泛函理论计算发现氢原子的能带与Cs2AgBiBr6的价带能级和导带能级进行耦合, 价带能级从-6.20 eV上移至-5.33 eV, 导带能级从-4.06 eV上移至-3.72 eV, 氢化后Cs2AgBiBr6的带隙从2.18 eV减小到1.64 eV, 吸光范围显著拓宽, 相应器件的短路电流从1.03 mA/cm2提升到11.40 mA/cm2, 光电转换效率从0.55%提升至6.37%, 达到目前Cs2AgBiBr6钙钛矿太阳能电池的最高值。这项工作为制备低带隙的无铅双钙钛矿提供了一种有效的间位掺杂策略。
3.2 添加剂工程
有机阳离子与卤素(拟卤素)阴离子形成的有机盐是一种常见的添加剂, 可以显著提升有机-无机杂化钙钛矿太阳能电池的光电转换效率。在Cs2AgBiBr6钙钛矿太阳能电池中, Wu等[66]将甲胺溴(MABr)添加到Cs2AgBiBr6前驱体溶液中, 能够诱导Cs2AgBiBr6形成Cs2-xMAxAgBiBr6中间相(图6(a)), 延缓Cs2AgBiBr6薄膜的结晶过程, 高温退火后, 如图6(b)所示, 与对照组相比, Cs2AgBiBr6薄膜的针孔消失, 薄膜晶粒尺寸从420 nm增加到640 nm。最后器件的开路电压从0.92 V增大至0.95 V, 短路电流从2.35 mA/cm2提升到3.50 mA/cm2, 光电转换效率从1.43%提升到2.53%。Shao课题组[67]在Cs2AgBiBr6前驱体溶液中添加硫氰酸胍(GuaSCN), 发现SCN-能够与Ag+/Bi3+配位(图6(c)), 延缓Cs2AgBiBr6薄膜的结晶过程, 减少Cs2AgBiBr6薄膜表面的团簇数量(图6(d)), 相应器件的开路电压从1.01 V增大至1.04 V, 短路电流从4.81 mA/cm2提升到5.42 mA/cm2, 光电转换效率从2.55%提升到3.19%。本课题组分别采用醋酸甲脒[68]和硫脲[69]作为添加剂, 它们对于形成中间相、调控结晶过程以及钝化缺陷都有良好的效果, 使用醋酸甲脒添加剂制备钙钛矿太阳能电池的开路电压可以达到1.23 V, 是文献报道的较高水平。
图6
图6
添加剂工程优化Cs2AgBiBr6薄膜
Fig. 6
Additive engineering optimization of Cs2AgBiBr6 films
(a) Schematic illustration of MABr additive assisted Cs2AgBiBr6 crystallization process; (b) SEM images of Cs2AgBiBr6 films prepared (left) without and (right) with MABr[66]; (c) Schematic diagram of the mechanism of additive GuaSCN in the formation process of Cs2AgBiBr6 film; (d) SEM images of Cs2AgBiBr6 films prepared (left) without and (right) with GuaSCN [67]; (e) Schematic illustration of BMPyr+-Br- interaction between ionic liquid BMPyrCl and Cs2AgBiBr6 perovskite; (f) SEM images of Cs2AgBiBr6 films prepared (left) without and (right) with BMPyrCl[70]. Colorful figures are available on website
离子液体是一种近年来得到重视并引起广泛兴趣的添加剂。Shen课题组[70]在Cs2AgBiBr6前体溶液中引入离子液体1-丁基-1-甲基吡咯烷氯化铵(BMPyrCl), 发现BMPyrCl中吡咯阳离子与Cs2AgBiBr6中的溴离子之间具有较高的结合能(图6(e)), 可以有效抑制溴离子迁移, 降低薄膜的缺陷。在单电子器件的电流-电压测试中, 极限填充电压与缺陷浓度具有线性关系, 添加BMPyrCl后, 极限填充电压从1.43 V降低到1.09 V。同时引入BMPyrCl后还可以获得具有大晶粒尺寸、无针孔的Cs2AgBiBr6薄膜, 如图6(f)所示。相应器件的开路电压从1.13 V提高到1.20 V, 短路电流从2.26 mA/cm2提升到2.61 mA/cm2, 光电转换效率从1.71%提升到2.22%。
3.3 界面工程
3.3.1 界面能级匹配
界面能级匹配通过调节钙钛矿太阳能电池中吸光层的价带与空穴传输层的价带(或吸光层的导带与电子传输层的导带)之间的能级差, 为界面处的电荷转移提供足够的势能并抑制界面电荷复合。Cs2AgBiBr6钙钛矿与有机-无机杂化钙钛矿的能带位置不同, 导致双钙钛矿与电荷传输层之间的能级失配, 从而制约了器件的开路电压[72]。选择合适的电荷传输材料优化能级排列可以解决能级失配的问题。
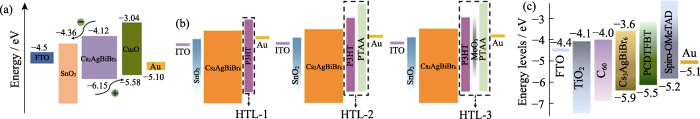
在钙钛矿/空穴传输层的界面上, Yang课题组[71]选取无机空穴传输材料Cu2O(价带: -5.58 eV, 导带: -3.04 eV)取代2,2′,7,7′-四[N,N-二(4-甲氧基苯基)氨基]-9,9′-螺二芴(Spiro-OMeTAD, HOMO能级: -5.20 eV, LUMO能级: -2.21 eV)作为Cs2AgBiBr6钙钛矿太阳能电池的空穴传输层(图7(a)), 空穴转移时间从0.63 ns缩短至 0.14 ns, 表明Cu2O空穴传输材料更有利于空穴转移。相应太阳能电池的开路电压从1.083 V提升到1.198 V, 短路电流从1.35 mA/cm2提升到1.66 mA/cm2, 光电转换效率从1.03%提升到1.52%。Zhang等[72]设计了三种空穴传输层材料: 单一的聚(3-己基噻吩)(P3HT, HTL-1), 双复合空穴传输材料P3HT/聚[双(4-苯基)(2,4,6-三甲基苯基)胺](PTAA) (HTL-2)和三复合空穴传输材料P3HT/MoO3/PTAA (HTL-3)(图7(b))。由于HTL-2和HTL-3的HOMO能级具有梯度排列的特征, 使Cs2AgBiBr6/HTL-2和Cs2AgBiBr6/HTL-3的荧光强度明显降低, 空穴转移得到加强。相应器件的短路电流从2.04 mA/cm2提升到2.29 mA/cm2和2.66 mA/cm2, 光电转换效率从1.36%提升到1.62%和1.82%。总的来说, 由于Cs2AgBiBr6的价带位置较深, 在有机-无机杂化钙钛矿太阳能电池中应用广泛的Spiro-OMeTAD和PTAA空穴传输层的价带匹配并不理想, 迫切需要开发具有较深HOMO能级的空穴传输层材料。
图7
在钙钛矿/电子传输层的界面上,Luo等[73]制备了具有双电子传输层(C60/TiO2)的Cs2AgBiBr6钙钛矿太阳能电池,由于C60的导带(-4.0 eV)介于TiO2的导带(-4.1 eV)和Cs2AgBiBr6的导带(-3.6 eV)之间(图7(c)),形成了能级梯度,因此电子转移时间从50 ns缩短至 7.9 ns,电子转移大大加快。相应器件的开路电压从0.87 V提升到1.01 V,短路电流从1.54 mA/cm2增大到2.25 mA/cm2,光电转换效率从0.93%提升到1.57%。另外,在有机-无机杂化钙钛矿太阳能电池中SnO2电子传输层已得到广泛应用,并创造了若干光电转换效率的纪录。SnO2导带位置比TiO2导带低0.4 eV左右,其与Cs2AgBiBr6的导带匹配时的电子转移等研究具有重要的意义,但目前尚无深入报道。
3.3.2 界面缺陷钝化
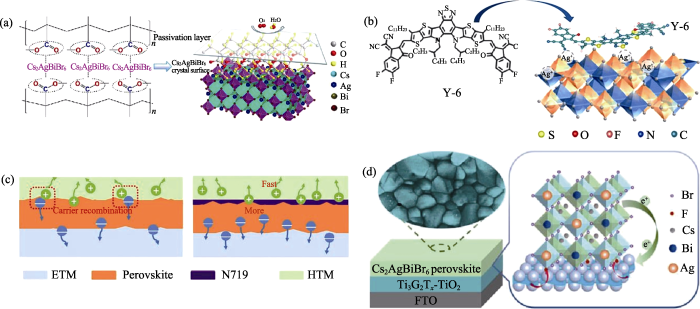
Cs2AgBiBr6薄膜表面中存在许多缺陷, 采用适当的表面钝化可以减少界面处载流子复合。例如, Li等[74]选取聚甲基丙烯酸甲酯(PMMA)作为Cs2AgBiBr6/空穴传输层的界面层, 发现PMMA分子中的-C=O官能团能够与Cs2AgBiBr6薄膜表面欠配位的Ag+发生相互作用, 钝化银空位缺陷, 如图8(a)所示。在PMMA钝化界面后, 极限填充电压从0.99 V降低到0.89 V, 器件的开路电压从1.14 V提升到1.18 V, 光电转换效率从1.78%提升到2.25%。Li等[75]将稠环分子Y-6添加在乙酸乙酯反溶剂中, 在Cs2AgBiBr6/空穴传输层界面上引入Y-6分子中间层。Y-6分子中的-C≡N和-N=C-S-官能团能够钝化Ag+迁移产生的Ag间位(Agi)和银铋反位(AgBi)缺陷(图8(b)), 缺陷密度从4.05×1016 cm-3降低到3.14×1016 cm-3, 相应器件的开路电压从1.08 V提升到1.28 V, 光电转换效率从 2.50%提升到3.31%。此外, Yang等[76]选取有机染料二(四丁基铵)顺式-双(异硫氰基)双(2,2′-联吡啶-4,4′-二羧酸)钌(II)(N719)钝化Cs2AgBiBr6/空穴传输层界面, 单电子器件的极限填充电压从1.88 V降低到0.53 V, 缺陷密度大大降低。另外, 引入N719后, 界面空穴转移时间从0.83 ns缩短到 0.59 ns, 空穴转移也得到增强(图8(c)), 相应器件的开路电压从0.998 V提升到1.06 V,光电转换效率从2.10%提升到2.84%。
图8
埋底界面是钙钛矿太阳能电池的受光面, 持续光照下易遭到破坏。此外, 在制备过程中埋底界面也会影响后续沉积的钙钛矿薄膜的质量。在有机-无机杂化钙钛矿太阳能电池中埋底界面近年来逐渐得到重视并已有一些深入研究。在Cs2AgBiBr6钙钛矿太阳能电池中, Li等[77]将MXene(Ti3C2Tx)掺入到TiO2中形成多功能电子传输层Ti3C2Tx@TiO2, 发现Ti3C2Tx中的-F能够钝化Cs2AgBiBr6薄膜的溴空位(图8(d)), 缺陷密度从1.58×1016 cm-3降低到4.23×1015 cm-3。相应器件的开路电压从0.93 V提升到0.96 V, 光电转换效率从2.00% 提升到2.81%。Wang等[78]使用羧基-叶绿素衍生物(C-Chl)敏化m-TiO2薄膜, 发现C-Chl能够抑制Cs2AgBiBr6/电子传输层的埋底界面的电子-空穴复合, 钙钛矿太阳能电池的界面转移阻抗从1731.0 Ω降低到318.5 Ω, 开路电压从1.02 V增加到1.04 V, 光电转换效率从2.28%提升到3.11%。
界面缺陷钝化的研究需要进一步深入, 首先是结合第一性原理计算明确缺陷的种类、形成能、能级位置等性质, 其次通过热导纳谱、深能级瞬态谱以及热刺激电流等方法对缺陷的位置以及密度开展深入表征, 并利用可视化平台技术揭示缺陷浓度以及空间分布等性质, 发展新型界面钝化及修复技术, 如利用有机盐、离子液体以及染料单分子层等。
4 结束语
Cs2AgBiBr6钙钛矿太阳能电池具有优异的稳定性和环境友好性, 在光电领域展现出了良好的应用前景, 但仍存在许多问题阻碍其性能提升。例如, Cs2AgBiBr6的制膜技术还不够完善, 很难制备高质量的钙钛矿薄膜; Cs2AgBiBr6具有较大的间接带隙, 不利于产生和提取光生载流子; Cs2AgBiBr6钙钛矿太阳能电池中离子迁移诱导的降解等都会影响器件性能。鉴于上述挑战, 根据材料自身特点以及研究现状, Cs2AgBiBr6钙钛矿太阳能电池的后续研究会在以下方向进一步展开。
1) Cs2AgBiBr6的前驱体溶剂工程。研究者已经开发了旋涂和喷涂等溶液工艺制备Cs2AgBiBr6薄膜。但是目前溶液工艺中溶剂工程的研究匮乏, CsBr、AgBr及BiBr3等前驱物与溶剂是否形成中间相、中间相的种类及晶体结构、中间相向钙钛矿相的演变机制都不清楚。需深入研究溶剂工程, 揭示成膜过程中的结晶动力学, 从而制备高质量的Cs2AgBiBr6薄膜。
2) Cs2AgBiBr6的带隙工程。Cs2AgBiBr6的带隙距离太阳能电池的理想带隙有很大距离, 不利于充分捕获太阳光谱。为了减小Cs2AgBiBr6的带隙, 在制备Cs2AgBiBr6的过程中可以尝试引入不同金属阳离子(Co2+、Mn2+、Ni2+、Fe3+和In3+等), 形成新的金属卤化物八面体堆积, 改变原有的能带组成和结构, 这对于拓展电池的吸收光谱、提高短路电流具有重要意义。
3) Cs2AgBiBr6钙钛矿太阳能电池的降解机理。卤化铅钙钛矿中的离子迁移是导致器件稳定性变差的重要影响因素。有关双钙钛矿离子迁移的报道却非常有限, 大多都是理论预测。双钙钛矿中B+X6和B3+X6八面体堆积方式与卤化铅钙钛矿中B2+X6八面体堆积方式不同, 具有不同的离子迁移特征, Ag+和Br-离子很容易迁移, 产生大量空位, 导致薄膜缓慢降解。因此, 在制备Cs2AgBiBr6太阳能电池的过程中, 需要选择适合的材料(如聚氨酯、离子液体等)用于抑制Ag+和Br-的迁移, 对于进一步提升Cs2AgBiBr6钙钛矿太阳能电池的稳定性具有重要意义。
参考文献
Organometal halide perovskites as visible-light sensitizers for photovoltaic cells
Two organolead halide perovskite nanocrystals, CH(3)NH(3)PbBr(3) and CH(3)NH(3)PbI(3), were found to efficiently sensitize TiO(2) for visible-light conversion in photoelectrochemical cells. When self-assembled on mesoporous TiO(2) films, the nanocrystalline perovskites exhibit strong band-gap absorptions as semiconductors. The CH(3)NH(3)PbI(3)-based photocell with spectral sensitivity of up to 800 nm yielded a solar energy conversion efficiency of 3.8%. The CH(3)NH(3)PbBr(3)-based cell showed a high photovoltage of 0.96 V with an external quantum conversion efficiency of 65%.
Metallic tin substitution of organic lead perovskite films for efficient solar cells
Thickness-dependent highly sensitive photodetection behavior of lead-free all-inorganic CsSnBr3 nanoplates
Bulk heterojunction gifts bismuth- based lead-free perovskite solar cells with record efficiency
Large-grained all-inorganic bismuth- based perovskites with narrow band gap via Lewis acid-base adduct approach
Electrically-driven violet light-emitting devices based on highly stable lead-free perovskite Cs3Sb2Br9 quantum dots
High-quality Cs2AgBiBr6 double perovskite film for lead-free inverted planar heterojunction solar cells with 2.2% efficiency
One-step synthesis of SnI2·(DMSO)x adducts for high-performance tin perovskite solar cells
Chemo-thermal surface dedoping for high-performance tin perovskite solar cells
Heterogeneous 2D/3D tin-halides perovskite solar cells with certified conversion efficiency breaking 14%
A bismuth- halide double perovskite with long carrier recombination lifetime for photovoltaic applications
Cs2AgBiBr6 single-crystal X-ray detectors with a low detection limit
Double perovskite Cs2BBiX6 (B=Ag, Cu; X=Br, Cl)/TiO2 heterojunction: an efficient Pb-free perovskite interface for charge extraction
Lead-free and stable antimony- silver-halide double perovskite (CH3NH3)2AgSbI6
Progress of lead-free halide double perovskites
Inorganic halide double perovskites with optoelectronic properties modulated by sublattice mixing
All-inorganic halide double perovskites have emerged as a promising class of materials that are potentially more stable and less toxic than lead-containing hybrid organic-inorganic perovskite optoelectronic materials. In this work, 311 cesium chloride double perovskites (Cs'Cl) were selected from a set of 903 compounds as likely being stable on the basis of a statistically learned tolerance factor (τ) for perovskite stability. First-principles calculations on these 311 double perovskites were then performed to assess their stability and identify candidates with band gaps appropriate for optoelectronic applications. We predict that 261 of the 311 Cs'Cl compounds are likely synthesizable on the basis of a thermodynamic analysis of their decomposition to competing compounds (decomposition enthalpy <0.05 eV/atom). Of these 261 likely synthesizable compounds, 47 contain no toxic elements and have direct or nearly direct (within 100 meV) band gaps between 1 and 3 eV, as computed with hybrid density functional theory (HSE06). Within this set, we identify the triple-alkali perovskites Cs[Alk][TM]Cl, where Alk is a group 1 alkali cation and TM is a transition-metal cation, as a class of Cs'Cl double perovskites with remarkable optical properties, including large and tunable exciton binding energies as computed by the -Bethe-Salpeter equation (-BSE) method. We attribute the unusual electronic structure of these compounds to the mixing of the Alk-Cl and TM-Cl sublattices, leading to materials with small band gaps, large exciton binding energies, and absorption spectra that are strongly influenced by the identity of the transition metal. The role of the double-perovskite structure in enabling these unique properties is probed through an analysis of the electronic structures and chemical bonding of these compounds in comparison with other transition-metal and alkali transition-metal halides.
Structural and functional diversity in lead-free halide perovskite materials
Lead-free halide perovskite photovoltaics: challenges, open questions, and opportunities
In recent years, lead-free metal-halide perovskite photovoltaics has attracted ever-growing attention, in view of its potential to replicate the outstanding properties of lead-halide perovskite photovoltaics, but without the toxicity burden of the latter. Despite a research effort much smaller in scale than that pursued with lead-based perovskites, considerable progress has been achieved in lead-free perovskite photovoltaics, with the highest power conversion efficiencies now being in the region of 13%. In this Perspective, we first discuss the state of the art of lead-free perovskite photovoltaics and additionally highlight promising directions and strategies that could lead to further progress in material exploration and understanding as well as in photovoltaic efficiency. Furthermore, we point out the widespread lack of experimental data on the fundamental optoelectronic properties of lead-free halide perovskite absorbers (e.g., charge carrier mobility, defect parameters, Urbach energy, and the impact of dimensionality). All of this currently hampers a rational approach to further improving their performance and points to the need for a concerted effort that could bridge this knowledge gap. Additionally, this Perspective brings to the fore the manifold photovoltaic opportunities—thus far largely unexplored with lead-free perovskite absorbers—beyond single-junction outdoor photovoltaics, which may potentially enable the realization of their full potential. The exploration of these opportunities (tandem photovoltaics, indoor photovoltaics, and building-integrated and transparent photovoltaics) could energize the investigation of existing and new classes of lead-free perovskite absorbers beyond current paradigms and toward high photovoltaic performance.
Highly stable, phase pure Cs2ABiBr6 double perovskite thin films for optoelectronic applications
Hydrogenated Cs2AgBiBr6 for significantly improved efficiency of lead-free inorganic double perovskite solar cell
Development of lead-free inorganic perovskite material, such as Cs2AgBiBr6, is of great importance to solve the toxicity and stability issues of traditional lead halide perovskite solar cells. However, due to a wide bandgap of Cs2AgBiBr6 film, its light absorption ability is largely limited and the photoelectronic conversion efficiency is normally lower than 4.23%. In this text, by using a hydrogenation method, the bandgap of Cs2AgBiBr6 films could be tunable from 2.18 eV to 1.64 eV. At the same time, the highest photoelectric conversion efficiency of hydrogenated Cs2AgBiBr6 perovskite solar cell has been improved up to 6.37% with good environmental stability. Further investigations confirmed that the interstitial doping of atomic hydrogen in Cs2AgBiBr6 lattice could not only adjust its valence and conduction band energy levels, but also optimize the carrier mobility and carrier lifetime. All these works provide an insightful strategy to fabricate high performance lead-free inorganic perovskite solar cells.
Inactive (PbI2)2RbCl stabilizes perovskite films for efficient solar cells
\n In halide perovskite solar cells the formation of secondary-phase excess lead iodide (PbI\n 2\n ) has some positive effects on power conversion efficiency (PCE) but can be detrimental to device stability and lead to large hysteresis effects in voltage sweeps. We converted PbI\n 2\n into an inactive (PbI\n 2\n )\n 2\n RbCl compound by RbCl doping, which effectively stabilizes the perovskite phase. We obtained a certified PCE of 25.6% for FAPbI\n 3\n (FA, formamidinium) perovskite solar cells on the basis of this strategy. Devices retained 96% of their original PCE values after 1000 hours of shelf storage and 80% after 500 hours of thermal stability testing at 85°C.\n
Cyclohexylammonium-based 2D/3D perovskite heterojunction with funnel-like energy band alignment for efficient solar cells (23.91%)
Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide
\n The bandgap of the black α-phase of formamidinium-based lead triiodide (FAPbI\n 3\n ) is near optimal for creating high-efficiency perovskite solar cells. However, this phase is unstable, and the additives normally used to stabilize this phase at ambient temperature—such as methylammonium, caesium, and bromine—widen its bandgap. Min\n et al.\n show that doping of the α-FAPbI\n 3\n phase with methylenediammonium dichloride enabled power conversion efficiencies of 23.7%, which were maintained after 600 hours of operation. Unencapsulated devices had high thermal stability and retained >90% efficiency even after annealing for 20 hours at 150°C in air.\n
All-perovskite tandem solar cells with 24.2% certified efficiency and area over 1 cm2 using surface- anchoring zwitterionic antioxidant
Cs2AgBiBr6 double perovskites as lead-free alternatives for perovskite solar cells
Can Pb-free halide double perovskites support high-efficiency solar cells
Properties, performance and multidimensional applications of stable lead-free Cs2AgBiBr6 double perovskite
Lead-free double perovskites for perovskite solar cells
Formability of ABX3 (X=F, Cl, Br, I) halide perovskites
In this study a total of 186 complex halide systems were collected; the formabilities of ABX\n 3 (X = F, Cl, Br and I) halide perovskites were investigated using the empirical structure map, which was constructed by Goldschmidt's tolerance factor and the octahedral factor. A model for halide perovskite formability was built up. In this model obtained, for all 186 complex halides systems, only one system (CsF–MnF2) without perovskite structure and six systems (RbF–PbF2, CsF–BeF2, KCl–FeCl2, TlI–MnI2, RbI–SnI2, TlI–PbI2) with perovskite structure were wrongly classified, so its predicting accuracy reaches 96%. It is also indicated that both the tolerance factor and the octahedral factor are a necessary but not sufficient condition for ABX\n 3 halide perovskite formability, and a lowest limit of the octahedral factor exists for halide perovskite formation. This result is consistent with our previous report for ABO3 oxide perovskite, and may be helpful to design novel halide materials with the perovskite structure.
New tolerance factor to predict the stability of perovskite oxides and halides
Simple and interpretable data-driven descriptor accurately predicts the synthesizability of single and double perovskites.
First-principles study on the structure, electronic, and optical properties of Cs2AgBiBr6-xClx mixed-halide double perovskites
Facile deposition of high-quality Cs2AgBiBr6 films for efficient double perovskite solar cells
High-quality sequential- vapor-deposited Cs2AgBiBr6 thin films for lead-free perovskite solar cells
Composition stoichiometry of Cs2AgBiBr6 films for highly efficient lead-free perovskite solar cells
The dawn of lead-free perovskite solar cell: highly stable double perovskite Cs2AgBiBr6 film
Spray-coated lead-free Cs2AgBiBr6 double perovskite solar cells with high open-circuit voltage
New insight into solvent engineering technology from evolution of intermediates via one-step spin-coating approach
Direct liquid coating of chalcopyrite light-asorbing layers for photovoltaic devices
Stable, high-sensitivity and fast-response photodetectors based on lead-free Cs2AgBiBr6 double perovskite films
MACl enhanced electron extraction in all-inorganic Cs2AgBiBr6perovskite photovoltaics
Organic-halide treatment is used to maximize electron extraction of Cs2AgBiBr6 solar cells with a PCE of 2.03% and excellent long-term stability.
Hysteresis-free lead-free double-perovskite solar cells by interface engineering
Long electron-hole diffusion length in high-quality lead-free double perovskite films
Mechanisms of nucleation and growth of nanoparticles in solution
Synthetic approaches for halide perovskite thin films
Perovskite precursor solution chemistry: from fundamentals to photovoltaic applications
Over the last several years, inorganic-organic hybrid perovskites have shown dramatic achievements in photovoltaic performance and device stability. Despite the significant progress in photovoltaic application, an in-depth understanding of the fundamentals of precursor solution chemistry is still lacking. In this review, the fundamental background knowledge of nucleation and crystal growth processes in solution including the LaMer model and Ostwald ripening process is described. This review article also highlights the recent progress in precursor-coordinating molecule interaction in solution along with the role of anti-solvent in the solvent engineering process to control nucleation and crystal growth. Moreover, chemical pathways from precursor solution to perovskite film formation are given. This represents identification of the intermediate phase induced by precursor-coordinating molecule interaction and responsible intermediate species for uniform and dense perovskite film formation. Further to the description of chemical phenomena in solution, the contemporary progress in chemical precursor composition is also provided to comprehend the current research approaches to further enhance photovoltaic performance and device stability. On the basis of the critical and comprehensive review, we provide some perspectives to further achieve high-performance perovskite solar cells with long-term device stability through precisely controlled nucleation and crystal growth in precursor solution.
Facile and scalable fabrication of highly efficient lead iodide perovskite thin-film solar cells in air using gas pump method
Fully spray-coated triple-cation perovskite solar cells
We use ultrasonic spray-coating to sequentially deposit thin films of tin oxide, a triple-cation perovskite and spiro-OMeTAD, allowing us fabricate perovskite solar cells (PSCs) with a champion reverse scan power conversion efficiency (PCE) of 19.4% on small-area substrates. We show that the use of spray-deposition permits us to rapidly (>80 mm s) coat 25 mm × 75 mm substrates that were divided into a series of devices each with an active area of 15.4 mm, yielding an average PCE of 10.3% and a peak PCE of 16.3%. By connecting seven 15.4 mm devices in parallel on a single substrate, we create a device having an effective active area of 1.08 cm and a PCE of 12.7%. This work demonstrates the possibility for spray-coating to fabricate high efficiency and low-cost perovskite solar cells at speed.
Methylammonium-free, high-performance, and stable perovskite solar cells on a planar architecture
\n Hybrid perovskite solar cells often use the more thermally stable formamidinium (FA) cation rather than methylammonium, but its larger size can create lattice distortion that results in an inactive yellow phase. Turren-Cruz\n et al.\n show that by using iodide instead of bromide as the anion (to create a redder bandgap) and an optical mix of cesium, rubidium, and FA cations, they can make solar cells with a stabilized efficiency of more than 20%. No heating steps above 100°C were needed to create the preferred black phase.\n
Insights about the absence of Rb cation from the 3D perovskite lattice: effect on the structural, morphological, and photophysical properties and photovoltaic performance
Entropic stabilization of mixed A-cation ABX3 metal halide perovskites for high performance perovskite solar cells
Improvement of Cs2AgBiBr6 double perovskite solar cell by rubidium doping
Alkali metal ion-regulated lead-free, all-inorganic double perovskites for HTM-free, carbon-based solar cells
Cs2AgBiX6 (X = Br, Cl): new visible light absorbing, lead-free halide perovskite semiconductors
Band gaps of the lead-free halide double perovskites Cs2BiAgCl6and Cs2BiAgBr6 from theory and experiment
Tuning the optical absorption of Sn-, Ge-, and Zn-substituted Cs2AgBiBr6 double perovskites: structural and electronic effects
Bandgap-tunable double-perovskite thin films by solution processing
Narrowing the bandgap of lead-free double-perovskite Cs2AgBiBr6 is required for using this material in future photovoltaics. Herein, we demonstrate a bandgap engineering of Cs2AgBiBr6 by introducing Sb to substitute up to 75% of Bi via a versatile solution-processed method in dimethyl sulfoxide at 180 degrees C. The resultant Cs2AgSbxBi1-xBr6 (x = 0, 0.25, 0.50, 0.75) thin films possess high crystallinity and good thermostability. Moreover, the Sb substitution enables an obvious bandgap reduction of 0.25 eV. The fabricated solar cell using the Cs2AgSbxBi1-xBr6 (x = 0.25) thin film obtained an increased performance than the reference Cs2AgBiBr6. The effective bandgap narrowing via a facile solution method might accelerate the development of Cs2AgBiBr6-based materials for photovoltaic applications.
Bismuth-antimony mixed double perovskites Cs2AgBi1-xSbxBr6 in solar cells
Enhancement of the intrinsic light harvesting capacity of Cs2AgBiBr6 double perovskite via modification with sulphide
Effect of alkaline earth metal chloride additives BCl2 (B = Mg, Ca, Sr and Ba) on photovoltaic performance of FAPbI3 based perovskite solar cells
Effect of additives AX (A=FA, MA, Cs, Rb, NH4, X=Cl, Br, I) in FAPbI3 on photovoltaic parameters of perovskite solar cells
Record efficiency stable flexible perovskite solar cell using effective additive assistant strategy
Additive engineering for highly efficient organic-inorganic halide perovskite solar cells: recent advances and perspectives
Crystallization kinetics of organic-inorganic trihalide perovskites and the role of the lead anion in crystal growth
Methylammonium lead halide perovskite solar cells continue to excite the research community due to their rapidly increasing performance which, in large part, is due to improvements in film morphology. The next step in this progression is control of the crystal morphology which requires a better fundamental understanding of the crystal growth. In this study we use in situ X-ray scattering data to study isothermal transformations of perovskite films derived from chloride, iodide, nitrate, and acetate lead salts. Using established models we determine the activation energy for crystallization and find that it changes as a function of the lead salt. Further analysis enabled determination of the precursor composition and showed that the primary step in perovskite formation is removal of excess organic salt from the precursor. This understanding suggests that careful choice of the lead salt will aid in controlling crystal growth, leading to superior films and better performing solar cells.
Methylammonium bromide assisted crystallization for enhanced lead-free double perovskite photovoltaic performance
First investigation of additive engineering for highly efficient Cs2AgBiBr6-based lead-free inorganic perovskite solar cells
Inorganic lead (Pb)-free Cs2AgBiBr6 double perovskite is one of the most promising light absorbers in perovskite solar cells (PSCs) to solve the instability and Pb toxicity problems of organic–inorganic perovskites. However, the intrinsic and extrinsic defects of Cs2AgBiBr6 films strongly limit the power conversion efficiencies (PCEs) of Cs2AgBiBr6-based PSCs. Herein, the first investigation of additive engineering in Cs2AgBiBr6-based PSCs is reported to achieve an outstanding efficiency. The introduction of guanidinium thiocyanate (GuaSCN) additive effectively controls the nucleation of Cs2AgBiBr6 crystals during the film formation process, improves the perovskite film quality, suppresses the charge carrier recombination, and accelerates the charge extraction simultaneously. Consequently, after optimizing the GuaSCN amount, the device shows a stable PCE of 3.02% under maximum power point tracking (MPPT) condition. Furthermore, the introduction of GuaSCN additive remarkably improves the ambient stability of the devices. This work provides insights on additive engineering for enhancing the efficiency and stability of inorganic Pb-free Cs2AgBiBr6-based PSCs toward future industrialization of this technology.
VOC over 1.2 V for Cs2AgBiBr6 solar cells based on formamidinium acetate additive
Regulating the film crystallization kinetics with thiourea additive in Cs2AgBiBr6 solar cells
Cs2AgBiBr6 solar cells have the advantages of non-toxicity and high stability and are regarded as one of the most promising novel solar cells. The crystallization kinetics of the films play a crucial role on the film microstructure and the optoelectronic properties. Herein, thiourea is introduced into the Cs2AgBiBr6 precursor solution as an additive. Fourier transform infrared spectroscopy characterization confirms that thiourea acts as a Lewis base to form an adduct with Ag+, Bi3+. The modified Cs2AgBiBr6 film is used to fabricate solar cells. As a result, the power conversion efficiency and the open-circuit voltage of the optimized device are 1.65% and 1.07 V, significantly higher than the control device (1.04% and 0.89 V). Dark current–voltage, electrochemical impedance spectroscopy, etc, reveal that defects and recombination in the solar cells are inhibited. This work provides an effective method to regulate the crystallization kinetics of Cs2AgBiBr6 film and is helpful for further enhancement of the photovoltaic performance of Cs2AgBiBr6 solar cells.
Pinning bromide ion with ionic liquid in lead-free Cs2AgBiBr6 double perovskite solar cells
Band matching strategy for all-inorganic Cs2AgBiBr6 double perovskite solar cells with high photovoltage
Efficient nonlead double perovskite solar cell with multiple hole transport layers
Dual interfacial engineering for efficient Cs2AgBiBr6 based solar cells
Suppressing interfacial shunt loss via functional polymer for performance improvement of lead-free Cs2AgBiBr6 double perovskite solar cells
Efficient and stable Cs2AgBiBr6 double perovskite solar cells through in-situ surface modulation
Simultaneous power conversion efficiency and stability enhancement of Cs2AgBiBr6 lead-free inorganic perovskite solar cell through adopting a multifunctional dye interlayer
Single-layered MXene nanosheets doping TiO2 for efficient and stable double perovskite solar cells