自2020年“双碳”目标提出以来, 我国正面临一场空前的能源体系改革, 中国需要在未来30年内完成深度脱碳, 绿色能源将逐步强化直至取代传统化石能源的“垄断”地位。作为可再生能源体系的重要组成部分, 锂离子电池技术一经问世便吸引了广泛关注[1-2]。目前, 除了作为3C电子产品的主要能源器件, 锂电池在汽车工业、航空航天和智慧电网等领域中的应用也逐渐增加, 对其性能提出了更高的要求[3-4]。为了获得更持久的续航里程, 动力锂电池需要开发能量密度更高的电池系统。其中, 具有超高比容量(3860 mAh·g-1)和低还原电位(-3.04 V (vs SHE))的锂金属有望成为下一代高能量密度电池的负极材料[5-6]。然而, 锂金属与有机液态电解质接触时, 界面处持续发生副反应, 导致锂枝晶不可控生长, 刺穿电池内部隔膜, 引发短路甚至安全问题。针对这一缺陷, 通常考虑使用固态电解质替代液态电解质, 提升电池的安全性[7⇓-9]。但是, 其过低的室温离子电导率和较差的界面相容性严重阻碍了进一步发展与应用[10]。
在此背景下, 能够平衡界面接触和离子电导率的凝胶态电解质受到了越来越多的关注。谭双杰等[11]将阻燃有机磷酸盐固定在具有高机械强度的聚碳酸亚乙烯酯的聚合物基质中, 开发了一种不可燃凝胶态电解质, 该电解质同时具有高离子电导率和锂离子转移数、不可燃性、高机械强度和良好的电化学相容性等优点。此外, 利用原位聚合在电池内部形成的凝胶态电解质具备的低黏度、易处理、润湿能力强等特性, 能够充分浸润活性材料, 产生理想的界面接触, 从而获得良好的离子迁移路径[12⇓-14]。例如, 郭玉国课题组[15]将传统的醚基单体1,3-二氧戊环(1,3-Dioxolane, DOL)与1,2-二甲氧基乙烷(1,2-Dimethoxyethane, DME)混合, 商用六氟磷酸锂(Lithium Hexafluorophosphate, LiPF6)作为锂盐的同时可引发DOL的开环聚合, 获得了一种可在锂硫电池和商用正极电池中稳定运行的新型凝胶态电解质。虽然聚醚基聚合物是一种对锂金属负极最稳定的电解质, 但其电化学窗口狭窄, 室温下离子电导率较低, 限制了在高能量密度电池中的应用[16⇓⇓⇓-20]。LiPF6虽然具有理想的离子电导率和优异的电极相容性, 但存在电化学稳定性差的问题[14,21⇓ -23]。因此, 开发综合性能优异的凝胶态电解质, 需要选取合适的聚合物主体、锂盐等组分。
为了平衡电导率、高压稳定性及电极相容性之间的关系, 本研究开发了一种固液复合的高性能GCE。采用热引发原位聚合的方法, 以聚乙二醇二甲基丙烯酸酯(Poly(ethylene glycol) Diacrylate, PEGDA)为单体, 添加碳酸乙烯酯(Ethylene Carbonate, EC)和碳酸二乙酯(Diethyl Carbonate, DEC)的混合溶剂, 并引入双三氟甲磺酰亚胺锂(Lithium Bis(trifluoromethane)sulfonimide, LiTFSI)和二氟草酸硼酸锂(Lithium Difluoro(oxalato)borate, LiDFOB)作为双锂盐体系与聚合物组分共同作用, 在提升电化学性能的同时, 进一步增强电解质与锂金属负极间的界面稳定性。
1 实验方法
1.1 GCE的制备
LiTFSI、LiDFOB、EC和DEC(苏州多多化学科技有限公司)均为无水级电池材料。将EC和DEC试剂按混合, 分别称取LiTFSI和LiDFOB溶于溶剂中, 制备的双盐体系电解液(Liquid Electrolyte, LE)为1 mol/L LiTFSI和0.2 mol/L LiDFOB的EC/DEC (体积比1 : 1)溶液。PEGDA(≥99%, Mn=400)和偶氮二异丁腈(Azodiisobutyronitrile, AIBN, 98%)购于上海阿拉丁试剂有限公司。将PEGDA与LE混合后配制GCE的前驱体溶液, PEGDA的质量分数为10%、20%、30%。添加质量分数1%的热引发剂AIBN, 充分搅拌。PEGDA前驱体溶液于70 ℃下加热2 h, 获得聚合完全的PEGDA基凝胶复合电解质, 制得的电解质分别命名为GCE-x(x=10, 20, 30)。上述实验均在无水无氧的手套箱中进行。
1.2 电池的组装
CR2025型纽扣电池的电池组件(316不锈钢, SS)、锂金属片(14 mm×0.45 mm, Li)、铝箔(电池级)均购于深圳市科晶智达有限公司。根据不同的测试需求, 在手套箱中分别组装了SS||SS电池、Li||SS电池、Li||Li电池、Li||LiFePO4电池, 正极材料中的LiFePO4、科琴黑、聚偏二氟乙烯(Polyvinylidene Difluoride, PVDF)的投料质量比为90 : 5 : 5, 面容量为0.67 mAh·cm-2。正极极片制备工艺及电池组装流程详见补充材料S1。
1.3 材料的表征方法
采用美国赛默飞世尔科技公司的Thermo NiColet iS50型傅里叶变换红外光谱仪(Fourier Transform Infrared Spectrometer, FT-IR)分析PEGDA单体及其聚合物的官能团与化学结构, 波长范围为400~ 4000 cm-1。采用德国布鲁克AXS公司的D2 Phaser型X射线衍射仪(X-Ray Diffractometer, XRD)表征不同聚合物含量的凝胶态电解质的结晶度, 扫描范围2θ=5°~80°。采用北京东方德菲仪器有限公司的OCA40Micro接触角测量仪测试不同PEGDA含量的前驱体溶液在LiFePO4电极片表面的接触角。通过日本电子JSM-7500F型场发射扫描电子显微镜(Field Emission Scanning Electron Microscope, FESEM)观察锂金属片截面及表面的微观形貌。使用美国赛默飞世尔科技公司Escalab 250Xi 型X射线光电子能谱仪(X-ray Photoelectron Spectroscopy, XPS)分析锂金属片表面的元素信息。
1.4 电化学测试
采用线性扫描伏安法(Linear Sweep Voltammetry, LSV)测试电解质的电化学稳定窗口, 电压范围为开路电压(Open Circuit Voltage, OCV)~6 V, 扫速1 mV·s-1。电化学阻抗谱(Electrochemical Impedance Spectroscopy, EIS)的频率范围为10-2~106 Hz, 扰动电压为10 mV。采用计时电流法(Chronoamperometry)测试电解质的锂离子迁移数, 设置电势差为10 mV, 时间800 s, 根据公式(1)得到锂离子迁移数:
其中, tLi+为锂离子转移数, ΔV为电势差, R0和RS分别为测试前后电极与电解质的界面阻抗值, I0和IS分别为初态电流和稳态电流。上述测试均在美国阿美特克公司的PARSTAT MC多通道电化学工作站上进行。
采用武汉蓝电电子股份有限公司的LAND CT3001A-1U电池测试平台测试电池的充放电循环性能。
2 结果与讨论
2.1 GCE的制备及结构分析
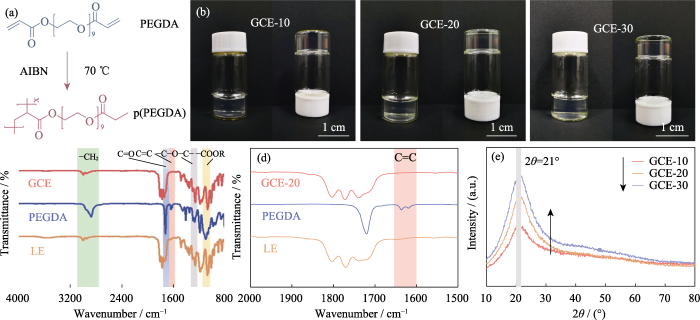
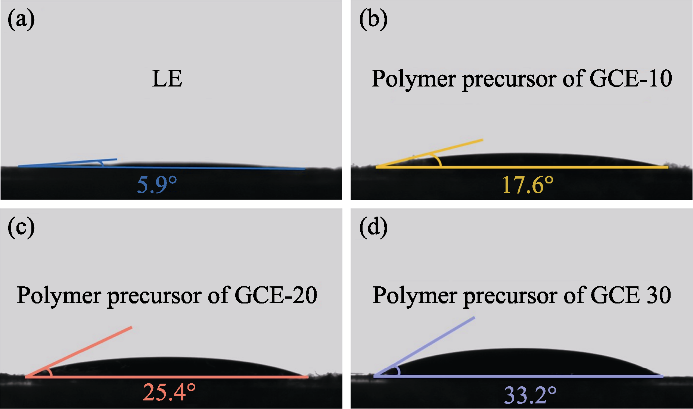
本研究以PEGDA为单体, AIBN作聚合反应的引发剂, 同时引入EC和DEC作为增塑剂, 在70 ℃合成以交联聚合的聚乙二醇二甲基丙烯酸酯(p(PEGDA))为骨架结构的凝胶态电解质。聚合反应反应式如图1(a)所示, 带有两个活性末端C=C基团的PEGDA在加入热引发剂AIBN后, 加热至70 ℃时迅速发生分子间均聚。AIBN的活性链使分子链之间发生相互或内部连接, 最终得到p(PEGDA)网络骨架结构, 成功利用原位聚合手段在电池内部获得了凝胶态电解质。如图S1所示, PEGDA含量不同的三种前驱体溶液在LiFePO4正极极片上均表现出良好的浸润性, 这是电解质与电极之间获得良好界面接触的关键。
图1
图1
GCE的制备及结构分析
Fig. 1
Preparation and structural analysis of GCE
(a) Polymerization reaction of PEGDA; (b) Optical photographs of GCE-x; (c, d) FT-IR spectra of GCE-20, PEGDA and LE; (e) XRD patterns of GCE-x; Colorful figures are available on website
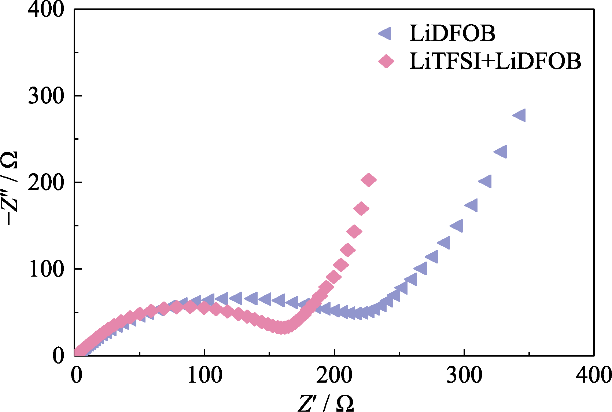
为了获得离子电导率较高的GCE, 使用在聚合物中具有高解离度的LiTFSI作为锂盐, 同时引入0.2 mol/L LiDFOB构筑双盐体系的凝胶网络。LiDFOB具有良好的溶解度及热稳定性, 特别是在成膜性方面具有突出的优势。碳酸酯类溶剂与锂金属负极接触时, 锂金属表面容易产生大量疏松多孔或树枝状的锂沉积层, 引入LiDFOB可以辅助形成不含HF成分的固态电解质界面(Solid Electrolyte Interphase, SEI)层, 改善与锂金属负极的相容性。同时, LiTFSI对金属集流体具有腐蚀作用, 而LiDFOB可以钝化铝金属, 缓解LiTFSI对集流体的腐蚀作用[24]。然而, LiDFOB的单盐电解质表现出比同等浓度的LiTFSI-LiDFOB双盐电解质更高的阻抗。如图S2所示, 分别使用1.2 mol/L的LiDFOB和LiTFSI- LiDFOB的凝胶电解质组装Li||LiFePO4电池, LiTFSI-LiDFOB电池的阻抗明显较小。
现有研究结果显示, LiTFSI与LiDFOB可产生共同作用, 有效提升电解质与锂金属负极的兼容性。焦淑红等[25]利用XPS和FT-IR等手段发现LiTFSI与LiDFOB的双盐电解质能够钝化正极的铝集流体, 并且在锂金属负极表面形成稳定的SEI层, 以实现锂金属电池的长期稳定循环。以此为基础, 刘越等[26]采用混合分子动力学模拟研究了LiTFSI与LiDFOB在锂金属电池中的共同作用机制, 阐释了LiTFSI对于LiDFOB的保护作用。研究表明, LiDFOB的B-O键相对最弱, 易发生断裂。LiDFOB会在自由基的作用下迅速分解并与锂金属反应, 产生游离的Li0和硼原子。硼原子插入反应使电解质中的溶剂分子发生分解, 产生的分子碎片会与锂盐分子碎片及硼原子持续反应。而在双锂盐体系中, LiTFSI优先发生分解, 依靠“牺牲机制”保护LiDFOB, LiDFOB的分解速度明显降低。从而减少游离Li0和硼原子的数量, 起到优化SEI层、保护锂金属负极的作用。
2.2 GCE的电化学性能及锂金属相容性分析
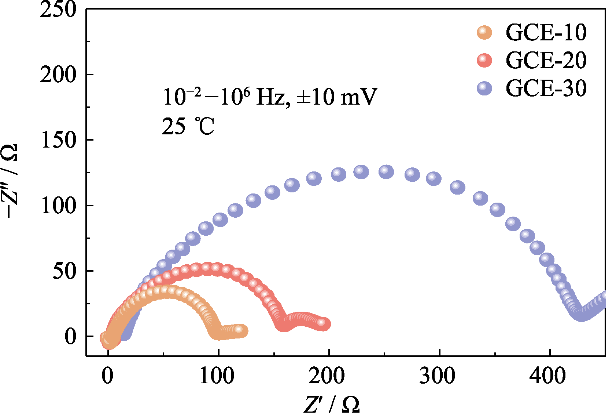
为研究不同聚合物含量的电解质在电池中与锂金属负极的相容性, 分析GCE-x电解质的Li||Li对称电池在初始状态下的阻抗谱图(如图S3所示)。图中GCE-10和GCE-20电池的界面阻抗值均较小, 分别为93和152 Ω, GCE-30电池达到了409 Ω, 表明锂离子在聚合物含量较高的GCE中迁移需要克服更大的迁移势垒, 不利于锂离子在界面快速传导。
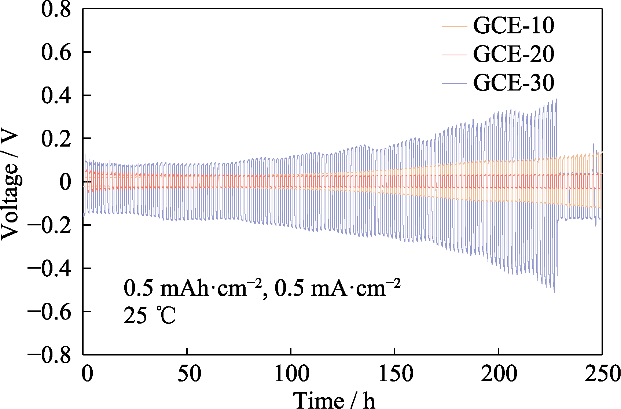
观察Li||Li对称电池在充放电循环测试中的过电势情况, 可知在此过程中离子对向迁移产生的电势差, 进而评估锂的沉积/剥离行为。图S4为GCE-x的Li||Li对称电池的电压-时间曲线, 测试温度为25 ℃, 电池在比容量0.5 mAh·cm-2, 电流密度0.5 mA·cm-2的恒定电流下进行充放电循环, Li|GCE-10|Li电池的初始过电势为22 mV, 250 h后电压升高至137 mV。GCE-30对称电池在初始阶段的过电势为104 mV, 在后续的循环中过电势快速升高, 227 h达到509 mV的峰值后骤降, 表明电池已经发生内部短路。相比之下, GCE-20电池可以在30 mV附近的低过电势下运行, 具有最稳定的电化学性能, 下文将对GCE-20电解质进行重点研究。
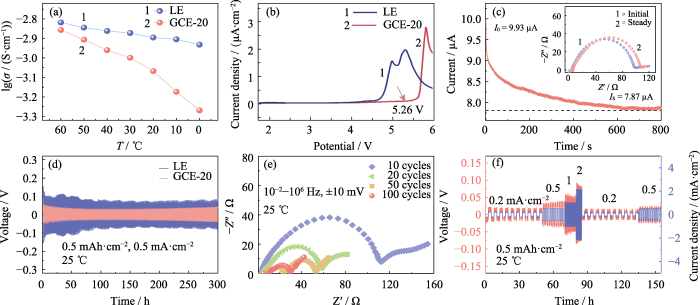
电解质的离子电导率直接反映了离子在电场中进行迁移的能力。分别在60、50、40、30、20、10和0 ℃环境中, 测试LE和GCE-20的离子电导率。如图2(a)所示, GCE-20在30 ℃下的离子电导率为1.00 mS·cm-1, 当测试温度升高至60 ℃时, 电导率达到1.39 mS·cm-1。这是由于活化能随着测试温度升高而下降, 聚合物链段及锂离子的运动活性增强所导致的, 而聚合物链段的运动速度明显受到温度影响, 因此GCE-20凝胶电解质的电导率变化较电解液幅度更大。
图2
图2
GCE-20的电化学性能
Fig. 2
Electrochemical performance of GCE-20
(a) Ionic conductivities of LE and GCE-20; (b) LSV curves of LE and GCE-20; (c) Current-time profile of Li|GCE-20|Li cell with inset showing corresponding Nyquist plots; (d) Voltage-time curves of symmetric Li||Li cells assembled with LE and GCE-20; (e) Nyquist plots of Li|GCE-20|Li cell after cycling; (f) Voltage-time and current density-time curves of Li|GCE-20|Li cell; Colorful figures are available on website
除此之外, 电解质的锂离子迁移数也是衡量锂离子传导能力的指标之一, 定义为单位时间内通过电解质某一垂直于锂离子迁移方向的截面的锂离子数量与通过该截面阴阳离子总和之比。数值越高, 锂离子在离子迁移过程中所占的比例越大, 迁移效率越高。图2(c)为Li|GCE-20|Li电池的计时电流测试曲线, 其中, 插图为该电池测试前后的电化学阻抗的对比。根据公式(1), GCE-20的锂离子迁移数为0.21。引入添加剂或者使用无机填料掺杂可以获得更高的锂离子迁移速率, 这既有利于提高电池的充放电速率, 又可以增强其循环稳定性。
Li||Li对称电池在充放电过程中, 电解质中的阴阳离子发生对向迁移。充电时, 锂离子向负极迁移, 阴离子向正极迁移, 放电时则相反。因此, 在充放电过程中正负极间的离子浓度梯度与内建电场逐渐升高, 阻碍阴阳离子的对向移动, 导致电池内部出现浓差极化, 造成过电势变化。如图2(d)所示, Li|GCE-20|Li电池稳定循环300 h后过电势为46 mV。而Li|LE|Li电池在测试过程中产生的过电势明显高于Li|GCE-20|Li电池(65~118 mV), 这是因为生长的锂枝晶使内部某些位点发生了软短路。结果表明GCE-20电池内部的电化学行为更为理想。图2(e)为对称电池经历了10、20、50及100圈循环的EIS测试。随着充放电循环次数增加, 电池阻抗出现下降趋势。在此过程中, 电解质与锂金属界面间构筑了稳定的SEI层, 界面接触得到优化, 从而使界面阻抗明显下降。
25 ℃时, 分别在0.2、0.5、1、2、0.2和0.5 mA·cm-2的电流密度下对Li|GCE-20|Li电池进行10次充放电循环。图2(f)反映了该过程中对称电池的过电势随时间变化的趋势。低电流密度下的过电势很小且能够保持相对稳定, 在流密度升高后, 过电势随之提升, 过程中未出现电压骤增/骤减的现象。
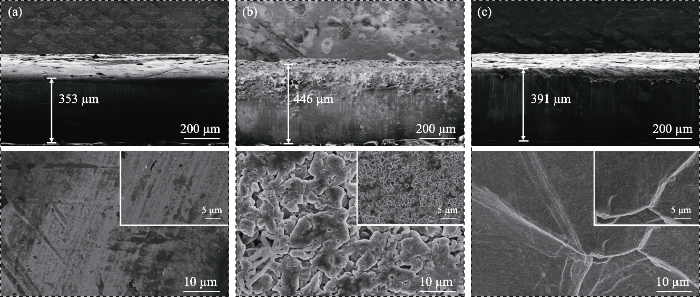
循环后的锂片镀层形貌可以直观地表征电池内部锂的沉积/剥离行为情况。Li||Li对称电池在容量0.5 mAh·cm-2, 电流密度0.5 mA·cm-2的电流下充放电循环100 h后进行拆解, 通过FESEM观察锂金属片截面及表面的微观形貌。如图3(a, b)所示, 未经处理的原始锂片厚度为353 µm, 且表面平整光滑。液态电解质与锂金属相互作用导致Li|LE|Li电池的锂片表面沉积了大量疏松多孔的锂沉积层, 多呈细碎不均匀的苔藓状, 锂金属片的厚度增至446 µm, 出现明显的体积膨胀效应, 并产生了大量枝晶。相比之下, Li|GCE-20|Li电池内的锂片的厚度为391 µm, 表面沉积层致密均匀, 未出现细碎锂镀层(图3(c)), 表明该凝胶态电解质可以有效抑制锂金属负极的体积膨胀。GCE-20中的LiDFOB可以辅助形成稳定的SEI层以平衡电池内部电势, 通过诱导锂均匀沉积来延缓锂枝晶生长, 从而在一定程度上起到优化锂沉积/剥离行为、保护锂金属负极的作用。
图3
图3
锂金属的SEM照片
Fig. 3
SEM images of metallic Li
Cross-sectional (up) and top-view (down) SEM images of (a) fresh metallic Li and lithium deposition morphology in symmetric Li||Li cells with (b) LE and (c) GCE-20
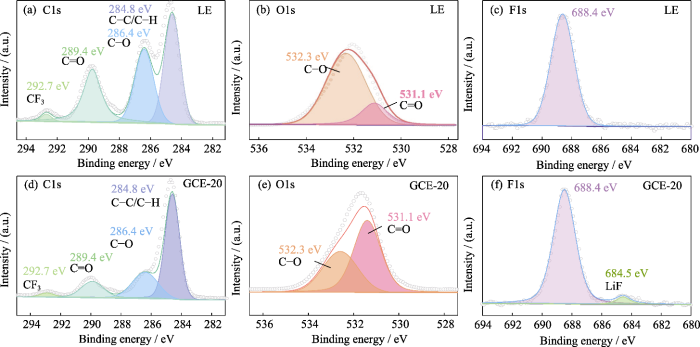
随后, 使用XPS表面元素分析探究在LiTFSI-LiDFOB双盐体系GCE作用下的锂金属负极表面SEI层的成分。图S5为使用LE与GCE-20的锂金属负极表面XPS图谱。C1s谱图(图S5(a, d))主要有4个信号峰, 284.8 eV处对应C-C/C-H。286.4和289.4 eV的两个峰分别对应C-O和C=O, 它们主要来源于碳酸酯类溶剂的分解产物(如ROCO2-, ROC-等)。292.7 eV处的峰对应CF3, 主要来源于锂盐的分解产物。在O1s谱图(图S5(b, e))中, 531.1和532.3 eV位置的峰分别对应C=O和C-O, C-O的相对含量明显降低, 这主要与分解产物的含量下降有关。在LiTFSI与LiDFOB的共同作用下, LiOCH3、Li2O2C2H4等副产物的形成受到限制。此外, 与LE不同(图S5(e)), 在GCE-20的F1s谱图(图S5(f))中, 684.5 eV处为LiF的信号峰, LiF可辅助形成致密稳定的SEI层。
2.3 Li||LiFePO4电池的电化学性能分析
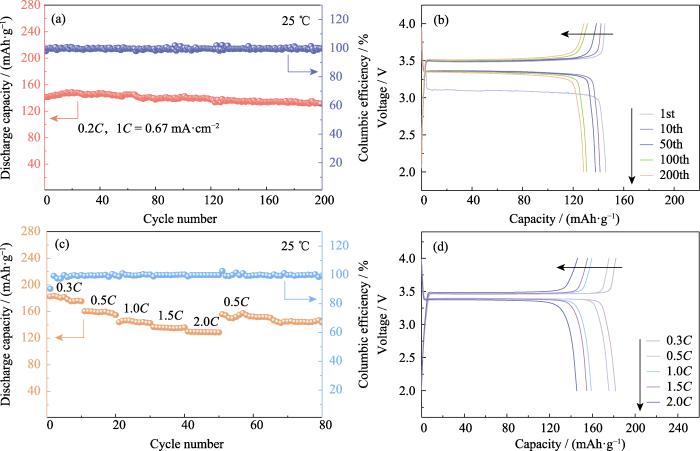
LiFePO4具有容量高、循环寿命长、安全性出众等优势, 是一种主流的正极活性材料, 其理论比容量为170 mAh·g-1。25 ℃时, 以0.2C(1C=0.67 mA·cm-2)的恒定电流对Li|GCE-20|LiFePO4电池进行200次充放电循环。如图4(a, b)所示, 首次循环的放电比容量为141.4 mAh·g-1, 第200圈的放电比容量为131.4 mAh·g-1, 容量保持率达到92.95%, 单圈容量衰减不足0.04%。平台电压稳定, 符合LiFePO4电池的特征。库仑效率作为评估电池循环稳定性能的重要指标, 指电池放电容量与同循环过程中充电容量之比。Li|GCE-20|LiFePO4电池的首圈库仑效率为97.8%, 由于首圈放电过程会形成SEI层, 产生部分不可逆容量, 导致首圈库伦效率较低。
图4
图4
Li|GCE-20|LiFePO4电池的电化学性能
Fig. 4
Electrochemical performance of the Li|GCE-20|LiFePO4 cells
(a) Cycling performance and (b) corresponding voltage-capacity curves at 0.2C; (c) Rate performance and (d) corresponding voltage-capacity curves; Colorful figures are available on website
3 结论
研究采用热引发原位聚合的方法, 开发了PEGDA基GCE。对GCE进行FT-IR、XRD表征分析, 结合电化学测试, 筛选出最优的GCE配方。进一步组装电池研究电解质的电化学性能, 并通过观察锂金属的微观形貌及表面元素表征, 分析电解质对于锂金属负极的保护作用, 说明:
1) 原位聚合法制备的GCE-x(x=10, 20, 30)可以良好地浸润电极片, PEGDA质量分数为20%时, 电解质具有最优的电化学稳定性。
2) 引入LiTFSI和LiDFOB的双锂盐体系, 可以与聚合物组分形成良好的相互作用, 使电解质获得了宽电化学窗口(5.26 V)以及高离子电导率(30 ℃, 1×10-3 S·cm-1)。同时, 该双锂盐体系可以用于构筑稳定的SEI层, 有效保护锂金属负极。
3) 使用GCE-20匹配商业LiFePO4正极材料, 组装的全电池能够在0.2C的电流下稳定充放电循环200圈, 容量保持率达到92.95%, 并且表现出良好的倍率性能。
综上所述, 本工作获得了一种安全且电化学性能优异的GCE, 为开发安全稳定的高能量密度锂金属电池提供了一种有效的解决方案。
补充材料:
本文相关补充材料可登录
双锂盐凝胶复合电解质的制备及其在锂金属电池中的应用
郭宇翔1, 黄立强2, 王 刚1, 王宏志1
(1. 东华大学 材料科学与工程学院 纤维材料改性国家重点实验室, 上海 201620; 2. 同济大学 材料科学与工程学院 车用新能源研究院, 上海 201804)
S1 电池制备工艺
将LiFePO4、科琴黑与PVDF按目标比例混合研磨, 加入溶剂N-甲基吡咯烷酮(N-Methylpyrrolidone, NMP), 充分搅拌分散, 获得均匀且黏稠的活性物质浆料。使用平板涂敷机在铝箔上进行浆料刮涂, 随后转移至真空干燥箱中, 在80 ℃下烘干12 h, 将电极片裁切后再次进行干燥, 转移至无水无氧的手套箱内。
依次在负极壳中央放置垫片、弹片和锂金属片, 锂金属片的厚度为0.35 mm。随后, 使用移液枪将GCE的前驱体溶液滴加在负极片表面中央(添加量为50 µL), 再依次放置Celgard 2500型电池隔膜与正极极片(Celgard 2500型电池隔膜购自美国Celgard公司)。组装Li||LiFePO4电池前, 对正极极片进行称重, 记录活性物质负载量, LiFePO4正极的活性物质面载量为3.94 mg·cm-2。最后, 在电池封口机上加压封口, 转移至70 ℃环境中加热2 h, 引发聚合从而获得凝胶态电解质。为了保证电解质充分浸润LiFePO4极片, 电池组装完毕后需要先静置1 h。
图S1
图S1
聚合物前驱体溶液与正极片的接触角
Fig. S1
Contact angles between polymer precursor solution and cathodes
(a) LE; (b) GCE-10; (c) GCE-20; (d) GCE-30
图S2
图S2
不同锂盐GCE的Li||LiFePO4电池的Nyquist谱图
Fig. S2
Nyquist plots of GCE assembled Li||LiFePO4 cells with different lithium salt
图S3
图S3
GCE-x电解质Li||Li对称电池的Nyquist谱图
Fig. S3
Nyquist plots of symmetric Li||Li cells assembled with GCE-x electrolytes
图S4
图S4
GCE-x电解质Li||Li对称电池的电压-时间曲线
Fig. S4
Voltage-time profiles of symmetric Li||Li cells assembled with GCE-x electrolytes
图S5
图S5
Li||Li对称电池中的锂金属负极表面XPS谱图
Fig. S5
XPS spectra of metallic Li anode in symmetric Li||Li cells
(a, d) C1s, (b, e) O1s, (c, f) F1s XPS spectra of metallic Li anode with (a-c) LE and (d-f) GCE-20
参考文献
Challenges for rechargeable Li batteries
Surface fluorination of reactive battery anode materials for enhanced stability
Significant increases in the energy density of batteries must be achieved by exploring new materials and cell configurations. Lithium metal and lithiated silicon are two promising high-capacity anode materials. Unfortunately, both of these anodes require a reliable passivating layer to survive the serious environmental corrosion during handling and cycling. Here we developed a surface fluorination process to form a homogeneous and dense LiF coating on reactive anode materials, with in situ generated fluorine gas, by using a fluoropolymer, CYTOP, as the precursor. The process is effectively a "reaction in the beaker", avoiding direct handling of highly toxic fluorine gas. For lithium metal, this LiF coating serves as a chemically stable and mechanically strong interphase, which minimizes the corrosion reaction with carbonate electrolytes and suppresses dendrite formation, enabling dendrite-free and stable cycling over 300 cycles with current densities up to 5 mA/cm. Lithiated silicon can serve as either a pre-lithiation additive for existing lithium-ion batteries or a replacement for lithium metal in Li-O and Li-S batteries. However, lithiated silicon reacts vigorously with the standard slurry solvent N-methyl-2-pyrrolidinone (NMP), indicating it is not compatible with the real battery fabrication process. With the protection of crystalline and dense LiF coating, LiSi can be processed in anhydrous NMP with a high capacity of 2504 mAh/g. With low solubility of LiF in water, this protection layer also allows LiSi to be stable in humid air (∼40% relative humidity). Therefore, this facile surface fluorination process brings huge benefit to both the existing lithium-ion batteries and next-generation lithium metal batteries.
Issues and challenges facing rechargeable lithium batteries
Artificial solid electrolyte interphase for aqueous lithium energy storage systems
An ultrathin graphene artificial interphase stabilizes active material and conductive carbon in aqueous energy storage systems.
Lithium superionic conductors with corner-sharing frameworks
Pathways for practical high-energy long-cycling lithium metal batteries
State-of-the-art lithium (Li)-ion batteries are approaching their specific energy limits yet are challenged by the ever-increasing demand of today's energy storage and power applications, especially for electric vehicles. Li metal is considered an ultimate anode material for future high-energy rechargeable batteries when combined with existing or emerging high-capacity cathode materials. However, much current research focuses on the battery materials level, and there have been very few accounts of cell design principles. Here we discuss crucial conditions needed to achieve a specific energy higher than 350 Wh kg(-1), up to 500 Wh kg(-1), for rechargeable Li metal batteries using high-nickel-content lithium nickel manganese cobalt oxides as cathode materials. We also provide an analysis of key factors such as cathode loading, electrolyte amount and Li foil thickness that impact the cell-level cycle life. Furthermore, we identify several important strategies to reduce electrolyte-Li reaction, protect Li surfaces and stabilize anode architectures for long-cycling high-specific-energy cells.
Electrical energy storage for the grid: a battery of choices
The increasing interest in energy storage for the grid can be attributed to multiple factors, including the capital costs of managing peak demands, the investments needed for grid reliability, and the integration of renewable energy sources. Although existing energy storage is dominated by pumped hydroelectric, there is the recognition that battery systems can offer a number of high-value opportunities, provided that lower costs can be obtained. The battery systems reviewed here include sodium-sulfur batteries that are commercially available for grid applications, redox-flow batteries that offer low cost, and lithium-ion batteries whose development for commercial electronics and electric vehicles is being applied to grid storage.
Building better batteries in the solid state: a review
Most of the current commercialized lithium batteries employ liquid electrolytes, despite their vulnerability to battery fire hazards, because they avoid the formation of dendrites on the anode side, which is commonly encountered in solid-state batteries. In a review two years ago, we focused on the challenges and issues facing lithium metal for solid-state rechargeable batteries, pointed to the progress made in addressing this drawback, and concluded that a situation could be envisioned where solid-state batteries would again win over liquid batteries for different applications in the near future. However, an additional drawback of solid-state batteries is the lower ionic conductivity of the electrolyte. Therefore, extensive research efforts have been invested in the last few years to overcome this problem, the reward of which has been significant progress. It is the purpose of this review to report these recent works and the state of the art on solid electrolytes. In addition to solid electrolytes stricto sensu, there are other electrolytes that are mainly solids, but with some added liquid. In some cases, the amount of liquid added is only on the microliter scale; the addition of liquid is aimed at only improving the contact between a solid-state electrolyte and an electrode, for instance. In some other cases, the amount of liquid is larger, as in the case of gel polymers. It is also an acceptable solution if the amount of liquid is small enough to maintain the safety of the cell; such cases are also considered in this review. Different chemistries are examined, including not only Li-air, Li–O2, and Li–S, but also sodium-ion batteries, which are also subject to intensive research. The challenges toward commercialization are also considered.
Lithium battery chemistries enabled by solid-state electrolytes
Polymer electrolytes for lithium-based batteries: advances and prospects
Polymer electrolytes have attracted great interest for next-generation lithium (Li)-based batteries in terms of high energy density and safety. In this review, we summarize the ion-transport mechanisms, fundamental properties, and preparation techniques of various classes of polymer electrolytes, such as solvent-free polymer electrolytes (SPEs), gel polymer electrolytes (GPEs), and composite polymer electrolytes (CPEs). We also introduce the recent advances of non-aqueous Li-based battery systems, in which their performances can be intrinsically enhanced by polymer electrolytes. Those include high-voltage Li-ion batteries, flexible Li-ion batteries, Li-metal batteries, lithium-sulfur (Li-S) batteries, lithium-oxygen (Li-O-2) batteries, and smart Li-ion batteries. Especially, the advantages of polymer electrolytes beyond safety improvement are highlighted. Finally, the remaining challenges and future perspectives are outlined to provide strategies to develop novel polymer electrolytes for high-performance Li-based batteries.
In-situ encapsulating flame- retardant phosphate into robust polymer matrix for safe and stable quasi-solid-state lithium metal batteries
Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries
A robust, highly stretchable ion-conducive skin for stable lithium metal batteries
Initial stages of thermal decomposition of LiPF6-based lithium ion battery electrolytes by detailed Raman and NMR spectroscopy
Upgrading traditional liquid electrolyte via in situ gelation for future lithium metal batteries
In situ gelation adds fresh potential for conventional liquid electrolytes in applications to lithium metal batteries.
Interface layer formation in solid polymer electrolyte lithium batteries: an XPS study
Superior lithium ion conduction of polymer electrolyte with comb-like structure via solvent-free copolymerization for bipolar all-solid-state lithium battery
Polymer electrolytes: present, past and future
Poly(ethylene oxide)-based electrolytes for lithium-ion batteries
Beyond PEO-Alternative host materials for Li+-conducting solid polymer electrolytes
Lithium-ion conducting electrolyte salts for lithium batteries
Electrolytes and interphases in Li-ion batteries and beyond
Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6
Low-temperature electrolyte design for lithium-ion batteries: prospect and challenges
Stable cycling of high-voltage lithium metal batteries in ether electrolytes
Predicted operando polymerization at lithium anode via boron insertion
Organic-inorganic composite SEI for a stable Li metal anode by in-situ polymerization
How does nanoscale crystalline structure affect ion transport in solid polymer electrolytes?
First principles modelling of amorphous polymer electrolytes: Li+-PEO, Li+-PEI, and Li+-PES complexes
Polycarbonate- based solid polymer electrolytes for Li-ion batteries
Characterization of solid polymer electrolytes based on poly (trimethylenecarbonate) and lithium tetrafluoroborate