A critical review of high entropy alloys and related concepts
1
2017
... 高熵合金(High entropy alloys, HEAs)一般是将多种近原子比的合金元素固溶在一起, 每种元素的含量在5%~35%, 最终形成一种单相结构[1].与传统的二主元合金相比, 高熵合金具有优异的物理化学性能, 如高强度[2]、耐腐蚀[3]和良好的热稳定性[4],主要得益于高熵材料的四大效应: 高熵效应, 迟滞扩散效应, 晶格畸变效应以及鸡尾酒效应.作为一个相对成熟的体系, 高熵合金为其他高熵材料的发展奠定了坚实的基础. ...
Synthesis and properties of multiprincipal component AlCoCrFeNiSix alloys
1
2010
... 高熵合金(High entropy alloys, HEAs)一般是将多种近原子比的合金元素固溶在一起, 每种元素的含量在5%~35%, 最终形成一种单相结构[1].与传统的二主元合金相比, 高熵合金具有优异的物理化学性能, 如高强度[2]、耐腐蚀[3]和良好的热稳定性[4],主要得益于高熵材料的四大效应: 高熵效应, 迟滞扩散效应, 晶格畸变效应以及鸡尾酒效应.作为一个相对成熟的体系, 高熵合金为其他高熵材料的发展奠定了坚实的基础. ...
The effect of SiC nanoparticles addition on the electrochemical response of mechanically alloyed CoCrFeMnNi high entropy alloy
1
2018
... 高熵合金(High entropy alloys, HEAs)一般是将多种近原子比的合金元素固溶在一起, 每种元素的含量在5%~35%, 最终形成一种单相结构[1].与传统的二主元合金相比, 高熵合金具有优异的物理化学性能, 如高强度[2]、耐腐蚀[3]和良好的热稳定性[4],主要得益于高熵材料的四大效应: 高熵效应, 迟滞扩散效应, 晶格畸变效应以及鸡尾酒效应.作为一个相对成熟的体系, 高熵合金为其他高熵材料的发展奠定了坚实的基础. ...
Thermal stability and performance of NbSiTaTiZr high-entropy alloy barrier for copper metallization
1
2011
... 高熵合金(High entropy alloys, HEAs)一般是将多种近原子比的合金元素固溶在一起, 每种元素的含量在5%~35%, 最终形成一种单相结构[1].与传统的二主元合金相比, 高熵合金具有优异的物理化学性能, 如高强度[2]、耐腐蚀[3]和良好的热稳定性[4],主要得益于高熵材料的四大效应: 高熵效应, 迟滞扩散效应, 晶格畸变效应以及鸡尾酒效应.作为一个相对成熟的体系, 高熵合金为其他高熵材料的发展奠定了坚实的基础. ...
Entropy-stabilized oxides
1
2015
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
High entropy carbide ceramics from different starting materials
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Mechanochemical-assisted synthesis of high-entropy metal nitride via a soft urea strategy
2
2018
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
... [7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Mechanical properties of hot-pressed high-entropy diboride-based ceramics
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
A high entropy silicide by reactive spark plasma sintering
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
High-entropy transparent fluoride laser ceramics
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Data-driven design of ecofriendly thermoelectric high-entropy sulfides
1
2018
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Multicomponent equiatomic rare earth oxides
1
2017
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Solution combustion synthesis and magnetic property of rock-salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high-entropy oxide nanocrystalline powder
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Mechanical properties and thermal stability of (NbTiAlSiZr)Nx high-entropy ceramic films at high temperatures
1
2018
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Research progress of transition metal non-oxide high-entropy ceramics
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Tunable pseudocapacitive contribution by dimension control in nanocrystalline-constructed (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O solid solutions to achieve superior lithium-storage properties
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Colossal dielectric constant in high entropy oxides
1
2016
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Long-range antiferromagnetic order in a rocksalt high entropy oxide
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Room temperature lithium superionic conductivity in high entropy oxides
1
2016
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Entropy-stabilized metal oxide solid solutions as CO oxidation catalysts with high-temperature stability
1
2018
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Mechanochemical synthesis of high entropy oxide materials under ambient conditions: dispersion of catalysts via entropy maximization
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Rare earth and transition metal based entropy stabilised perovskite type oxides
1
2018
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Dielectric properties and electrocaloric effect of high-entropy (Na0.2Bi0.2Ba0.2Sr0.2Ca0.2)TiO3 ceramic
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Dielectric and energy storage properties of flash-sintered high-entropy (Bi0.2Na0.2K0.2Ba0.2Ca0.2)TiO3 ceramic
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Photocatalytic hydrogen evolution on a high-entropy oxide
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
High-entropy perovskite fluorides: a new platform for oxygen evolution catalysis
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
A new eight-cation inverse high entropy spinel with large configurational entropy in both tetrahedral and octahedral sites: Synthesis and cation distribution by X-ray absorption spectroscopy
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Facile synthesis and ferrimagnetic property of spinel (CoCrFeMnNi)3O4 high-entropy oxide nanocrystalline powder
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Spinel to rock-salt transformation in high entropy oxides with Li incorporation
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
High entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12: a novel high temperature stable thermal barrier material
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
(La0.2Ce0.2Nd0.2Sm0.2Eu0.2)PO4: a high-entropy rare-earth phosphate monazite ceramic with low thermal conductivity and good compatibility with Al2O3
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
High entropy (Yb0.25Y0.25Lu0.25Er0.25)2SiO5 with strong anisotropy in thermal expansion
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
The new extremely substituted high entropy (Ba,Sr,Ca,La)Fe6-x (Al,Ti,Cr,Ga,In,Cu,W)xO19 microcrystals with magnetoplumbite structure
1
2020
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Extremely polysubstituted magnetic material based on magnetoplumbite with a hexagonal structure: synthesis, structure, properties, prospects
1
2019
... 2015年, Rost等[5]首次将高熵概念引入到高熵氧化物(High entropy oxides, HEOs)领域, 在1000 ℃成功合成了具有单一氯化钠结构的(Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O陶瓷.此后, 相继研发了各种高熵陶瓷, 如高熵碳化物[6]、氮化物[7]、硼化物[8]、硅化物[9]、氟化物[10], 还有硫化物[11].高熵陶瓷的合成并不局限于传统的固相法, 先进的合成方法如机械化学法[7]、喷雾热分解法[12]、溶液燃烧法[13]以及磁控溅射法[14]均适用于高熵陶瓷, 从而促进高熵陶瓷的迅猛发展[15].作为高熵陶瓷的分支, 高熵氧化物陶瓷演绎出丰富的晶体结构, 以Mg、Co、Ni为主要元素的氯化钠结构高熵氧化物是发展最成熟的体系, 具有卓越的储锂性能[16]、巨大的介电常数[17]、反铁磁性[18]、锂离子超导[19], 还可用作催化剂载体[20,21], 具有十分广泛的用途.钙钛矿结构(ABO3)的高熵陶瓷可以在A-site和B-site同时掺杂, 形成十组分单相氧化物[22].这类陶瓷具有电热效应[23]、铁电极化储能效应[24], 可用作析氢催化[25]和析氧催化[26]载体.高熵陶瓷还可制备成尖晶石和反尖晶石结构[27], 它们具有优异的铁磁性、储锂性能和电催化性能[28,29,30].另外, 一系列的高熵烧绿石结构Re2Zr2O7[31]、石榴石结构Re3Al5O12[32]、含磷氧化物[33]和含硅氧化物[34]都具有较低的热导率, 可用作热绝缘材料.磁铅矿结构高熵陶瓷具有铁磁性、稳定的介电常数和磁导率等性质[35,36], 目前关于它们的报道比较少. ...
Oxygen ion conductors
1
2003
... 萤石结构是一种特殊的面心立方结构, 阳离子位于晶胞的八个角顶和六个面心, 阴离子填充于八个小立方体的中心.立方氧化锆和氧化铈都具有萤石结构, 晶胞中含有大量的空隙, 是经典的氧离子导体[37].近几年, 萤石结构的高熵氧化物也得到了迅速发展, 主要集中于新成分和新方法的探索[38,39], 它具有窄带隙, 高电导和低热导等特点[40,41], 有很大的发展潜能和应用前景. ...
High entropy oxide systems based on rare earth elements
1
2019
... 萤石结构是一种特殊的面心立方结构, 阳离子位于晶胞的八个角顶和六个面心, 阴离子填充于八个小立方体的中心.立方氧化锆和氧化铈都具有萤石结构, 晶胞中含有大量的空隙, 是经典的氧离子导体[37].近几年, 萤石结构的高熵氧化物也得到了迅速发展, 主要集中于新成分和新方法的探索[38,39], 它具有窄带隙, 高电导和低热导等特点[40,41], 有很大的发展潜能和应用前景. ...
Solid-state synthesis of multicomponent equiatomic rare-earth oxides
2
2020
... 萤石结构是一种特殊的面心立方结构, 阳离子位于晶胞的八个角顶和六个面心, 阴离子填充于八个小立方体的中心.立方氧化锆和氧化铈都具有萤石结构, 晶胞中含有大量的空隙, 是经典的氧离子导体[37].近几年, 萤石结构的高熵氧化物也得到了迅速发展, 主要集中于新成分和新方法的探索[38,39], 它具有窄带隙, 高电导和低热导等特点[40,41], 有很大的发展潜能和应用前景. ...
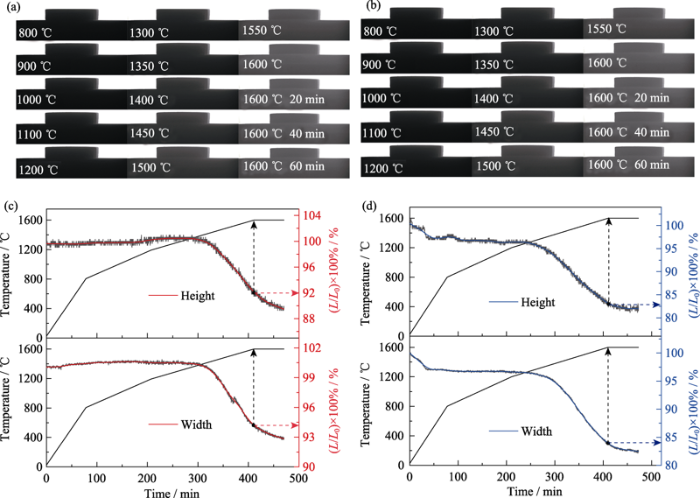
... 根据陶瓷坯体的尺寸收缩情况可以推测坯体B的致密度比A高得多.利用Rietveld精修的晶胞参数计算ZHCYLO的理论密度ρth约为6.4188 g/cm3, 随后通过阿基米德排水法测试坯体A和B的致密度分别为82.25%和93.23%.总之, 高熵陶瓷的烧结受众多因素的影响, 除了元素组成、烧结气氛和冷却速率外[39], 球磨工艺也是重要影响因素之一. ...
Multicomponent equiatomic rare earth oxides with a narrow band gap and associated praseodymium multivalency
1
2017
... 萤石结构是一种特殊的面心立方结构, 阳离子位于晶胞的八个角顶和六个面心, 阴离子填充于八个小立方体的中心.立方氧化锆和氧化铈都具有萤石结构, 晶胞中含有大量的空隙, 是经典的氧离子导体[37].近几年, 萤石结构的高熵氧化物也得到了迅速发展, 主要集中于新成分和新方法的探索[38,39], 它具有窄带隙, 高电导和低热导等特点[40,41], 有很大的发展潜能和应用前景. ...
High-entropy fluorite oxides
2
2018
... 萤石结构是一种特殊的面心立方结构, 阳离子位于晶胞的八个角顶和六个面心, 阴离子填充于八个小立方体的中心.立方氧化锆和氧化铈都具有萤石结构, 晶胞中含有大量的空隙, 是经典的氧离子导体[37].近几年, 萤石结构的高熵氧化物也得到了迅速发展, 主要集中于新成分和新方法的探索[38,39], 它具有窄带隙, 高电导和低热导等特点[40,41], 有很大的发展潜能和应用前景. ...
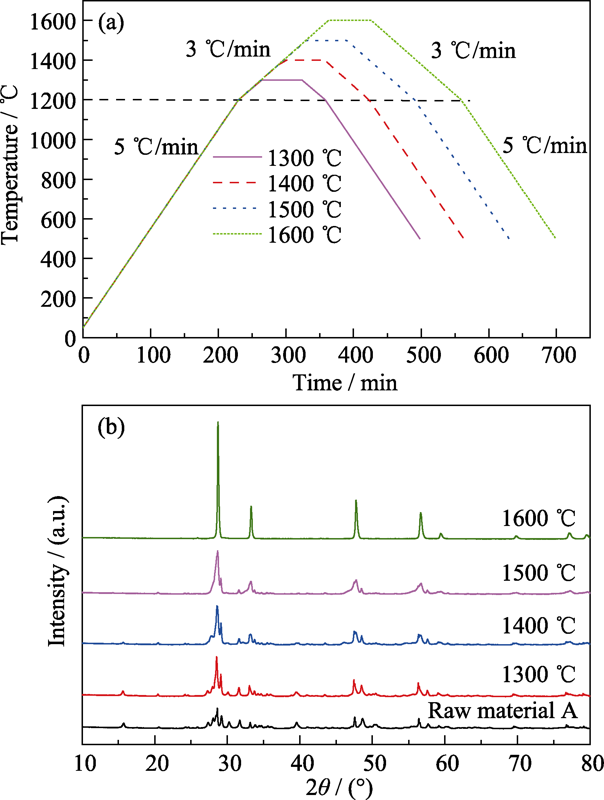
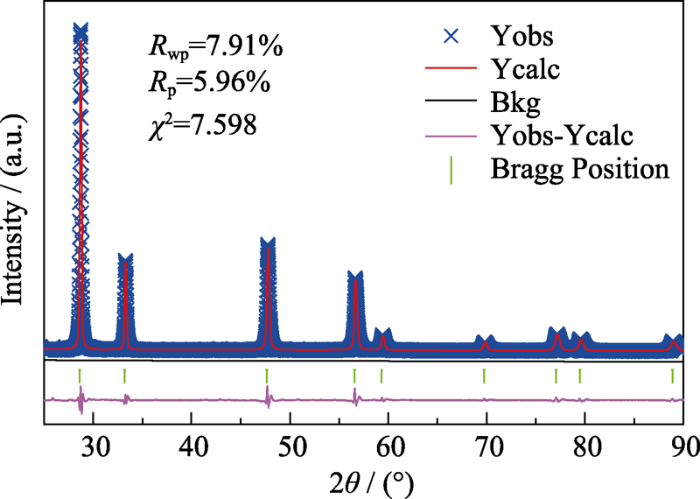
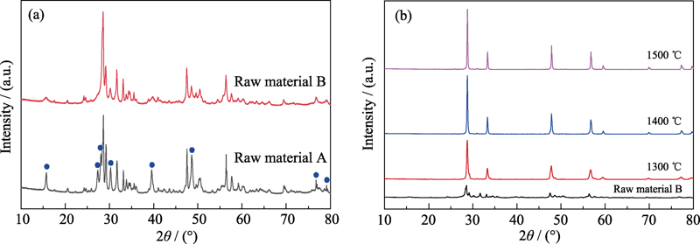
... 为了探究升温过程ZHCYLO的物相转变过程, 按照一定的工艺(图1(a))对粉末A进行煅烧, 并对煅烧后的样品进行XRD分析.测试结果(图1(b))表明, 1300 ℃煅烧的样品与煅烧前相比, 物相基本保持一致; 1400 ℃煅烧时, 样品各角度范围内(如2θ=48°和57°左右)的衍射峰开始合并[43]; 随着煅烧温度进一步升高, 主峰/杂峰强度逐渐增强/减弱, 最终形成单相萤石结构.Gild等[41]采用(Zr0.25Hf0.25Ce0.25Y0.125La0.125)O2-δ组分合成高熵陶瓷, 但并未获得单一物相.本研究通过改变元素比例得到单相萤石结构, 这是因为阳离子的配比直接影响组分的混合焓, 而混合焓又是影响固溶体形成的重要因素.过正的混合焓会减小元素之间的混溶隙, 容易产生元素偏析现象; 而过负的混合焓会诱导元素之间相互结合, 更容易形成二元化合物.恰当的阳离子配比会产生接近于0的混合焓, 各元素混乱分布, 陶瓷更容易形成单相结构, 这为非等摩尔高熵陶瓷提供了更为广阔的发展空间[43,44].对单相陶瓷的XRD衍射峰进行Rietveld精修[45,46], 精修结果如图2所示.ZHCYLO的晶胞参数约为0.5387 nm, 该值处于CeO2晶胞参数0.5411 nm和(Zr0.2Hf0.2Ce0.2Ti0.2Sn0.2)O2晶胞参数0.53512 nm之间[47], 这与组分的平均离子半径有一定的关系. ...
Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides
2
1976
... 氧化物的晶体结构、空间群及对应阳离子配位数和半径[42] ...
... Crystal structures, space groups (number), cation coordination numbers (CN) and corresponding cationic radii (rc) of selected oxides[42] ...
From high-entropy ceramics to compositionally-complex ceramics: a case study of fluorite oxides
2
2020
... 为了探究升温过程ZHCYLO的物相转变过程, 按照一定的工艺(图1(a))对粉末A进行煅烧, 并对煅烧后的样品进行XRD分析.测试结果(图1(b))表明, 1300 ℃煅烧的样品与煅烧前相比, 物相基本保持一致; 1400 ℃煅烧时, 样品各角度范围内(如2θ=48°和57°左右)的衍射峰开始合并[43]; 随着煅烧温度进一步升高, 主峰/杂峰强度逐渐增强/减弱, 最终形成单相萤石结构.Gild等[41]采用(Zr0.25Hf0.25Ce0.25Y0.125La0.125)O2-δ组分合成高熵陶瓷, 但并未获得单一物相.本研究通过改变元素比例得到单相萤石结构, 这是因为阳离子的配比直接影响组分的混合焓, 而混合焓又是影响固溶体形成的重要因素.过正的混合焓会减小元素之间的混溶隙, 容易产生元素偏析现象; 而过负的混合焓会诱导元素之间相互结合, 更容易形成二元化合物.恰当的阳离子配比会产生接近于0的混合焓, 各元素混乱分布, 陶瓷更容易形成单相结构, 这为非等摩尔高熵陶瓷提供了更为广阔的发展空间[43,44].对单相陶瓷的XRD衍射峰进行Rietveld精修[45,46], 精修结果如图2所示.ZHCYLO的晶胞参数约为0.5387 nm, 该值处于CeO2晶胞参数0.5411 nm和(Zr0.2Hf0.2Ce0.2Ti0.2Sn0.2)O2晶胞参数0.53512 nm之间[47], 这与组分的平均离子半径有一定的关系. ...
... [43,44].对单相陶瓷的XRD衍射峰进行Rietveld精修[45,46], 精修结果如图2所示.ZHCYLO的晶胞参数约为0.5387 nm, 该值处于CeO2晶胞参数0.5411 nm和(Zr0.2Hf0.2Ce0.2Ti0.2Sn0.2)O2晶胞参数0.53512 nm之间[47], 这与组分的平均离子半径有一定的关系. ...
Zn0.1Ca0.1Sr0.4Ba0.4ZrO3: a non-equimolar multicomponent perovskite ceramic with low thermal conductivity
1
2020
... 为了探究升温过程ZHCYLO的物相转变过程, 按照一定的工艺(图1(a))对粉末A进行煅烧, 并对煅烧后的样品进行XRD分析.测试结果(图1(b))表明, 1300 ℃煅烧的样品与煅烧前相比, 物相基本保持一致; 1400 ℃煅烧时, 样品各角度范围内(如2θ=48°和57°左右)的衍射峰开始合并[43]; 随着煅烧温度进一步升高, 主峰/杂峰强度逐渐增强/减弱, 最终形成单相萤石结构.Gild等[41]采用(Zr0.25Hf0.25Ce0.25Y0.125La0.125)O2-δ组分合成高熵陶瓷, 但并未获得单一物相.本研究通过改变元素比例得到单相萤石结构, 这是因为阳离子的配比直接影响组分的混合焓, 而混合焓又是影响固溶体形成的重要因素.过正的混合焓会减小元素之间的混溶隙, 容易产生元素偏析现象; 而过负的混合焓会诱导元素之间相互结合, 更容易形成二元化合物.恰当的阳离子配比会产生接近于0的混合焓, 各元素混乱分布, 陶瓷更容易形成单相结构, 这为非等摩尔高熵陶瓷提供了更为广阔的发展空间[43,44].对单相陶瓷的XRD衍射峰进行Rietveld精修[45,46], 精修结果如图2所示.ZHCYLO的晶胞参数约为0.5387 nm, 该值处于CeO2晶胞参数0.5411 nm和(Zr0.2Hf0.2Ce0.2Ti0.2Sn0.2)O2晶胞参数0.53512 nm之间[47], 这与组分的平均离子半径有一定的关系. ...
Structural features of Sm- and Gd-doped ceria studied by synchrotron X-ray diffraction and μ-Raman spectroscopy
1
2015
... 为了探究升温过程ZHCYLO的物相转变过程, 按照一定的工艺(图1(a))对粉末A进行煅烧, 并对煅烧后的样品进行XRD分析.测试结果(图1(b))表明, 1300 ℃煅烧的样品与煅烧前相比, 物相基本保持一致; 1400 ℃煅烧时, 样品各角度范围内(如2θ=48°和57°左右)的衍射峰开始合并[43]; 随着煅烧温度进一步升高, 主峰/杂峰强度逐渐增强/减弱, 最终形成单相萤石结构.Gild等[41]采用(Zr0.25Hf0.25Ce0.25Y0.125La0.125)O2-δ组分合成高熵陶瓷, 但并未获得单一物相.本研究通过改变元素比例得到单相萤石结构, 这是因为阳离子的配比直接影响组分的混合焓, 而混合焓又是影响固溶体形成的重要因素.过正的混合焓会减小元素之间的混溶隙, 容易产生元素偏析现象; 而过负的混合焓会诱导元素之间相互结合, 更容易形成二元化合物.恰当的阳离子配比会产生接近于0的混合焓, 各元素混乱分布, 陶瓷更容易形成单相结构, 这为非等摩尔高熵陶瓷提供了更为广阔的发展空间[43,44].对单相陶瓷的XRD衍射峰进行Rietveld精修[45,46], 精修结果如图2所示.ZHCYLO的晶胞参数约为0.5387 nm, 该值处于CeO2晶胞参数0.5411 nm和(Zr0.2Hf0.2Ce0.2Ti0.2Sn0.2)O2晶胞参数0.53512 nm之间[47], 这与组分的平均离子半径有一定的关系. ...
EXPGUI, a graphical user interface for GSAS
1
2001
... 为了探究升温过程ZHCYLO的物相转变过程, 按照一定的工艺(图1(a))对粉末A进行煅烧, 并对煅烧后的样品进行XRD分析.测试结果(图1(b))表明, 1300 ℃煅烧的样品与煅烧前相比, 物相基本保持一致; 1400 ℃煅烧时, 样品各角度范围内(如2θ=48°和57°左右)的衍射峰开始合并[43]; 随着煅烧温度进一步升高, 主峰/杂峰强度逐渐增强/减弱, 最终形成单相萤石结构.Gild等[41]采用(Zr0.25Hf0.25Ce0.25Y0.125La0.125)O2-δ组分合成高熵陶瓷, 但并未获得单一物相.本研究通过改变元素比例得到单相萤石结构, 这是因为阳离子的配比直接影响组分的混合焓, 而混合焓又是影响固溶体形成的重要因素.过正的混合焓会减小元素之间的混溶隙, 容易产生元素偏析现象; 而过负的混合焓会诱导元素之间相互结合, 更容易形成二元化合物.恰当的阳离子配比会产生接近于0的混合焓, 各元素混乱分布, 陶瓷更容易形成单相结构, 这为非等摩尔高熵陶瓷提供了更为广阔的发展空间[43,44].对单相陶瓷的XRD衍射峰进行Rietveld精修[45,46], 精修结果如图2所示.ZHCYLO的晶胞参数约为0.5387 nm, 该值处于CeO2晶胞参数0.5411 nm和(Zr0.2Hf0.2Ce0.2Ti0.2Sn0.2)O2晶胞参数0.53512 nm之间[47], 这与组分的平均离子半径有一定的关系. ...
A five-component entropy-stabilized fluorite oxide
3
2018
... 为了探究升温过程ZHCYLO的物相转变过程, 按照一定的工艺(图1(a))对粉末A进行煅烧, 并对煅烧后的样品进行XRD分析.测试结果(图1(b))表明, 1300 ℃煅烧的样品与煅烧前相比, 物相基本保持一致; 1400 ℃煅烧时, 样品各角度范围内(如2θ=48°和57°左右)的衍射峰开始合并[43]; 随着煅烧温度进一步升高, 主峰/杂峰强度逐渐增强/减弱, 最终形成单相萤石结构.Gild等[41]采用(Zr0.25Hf0.25Ce0.25Y0.125La0.125)O2-δ组分合成高熵陶瓷, 但并未获得单一物相.本研究通过改变元素比例得到单相萤石结构, 这是因为阳离子的配比直接影响组分的混合焓, 而混合焓又是影响固溶体形成的重要因素.过正的混合焓会减小元素之间的混溶隙, 容易产生元素偏析现象; 而过负的混合焓会诱导元素之间相互结合, 更容易形成二元化合物.恰当的阳离子配比会产生接近于0的混合焓, 各元素混乱分布, 陶瓷更容易形成单相结构, 这为非等摩尔高熵陶瓷提供了更为广阔的发展空间[43,44].对单相陶瓷的XRD衍射峰进行Rietveld精修[45,46], 精修结果如图2所示.ZHCYLO的晶胞参数约为0.5387 nm, 该值处于CeO2晶胞参数0.5411 nm和(Zr0.2Hf0.2Ce0.2Ti0.2Sn0.2)O2晶胞参数0.53512 nm之间[47], 这与组分的平均离子半径有一定的关系. ...
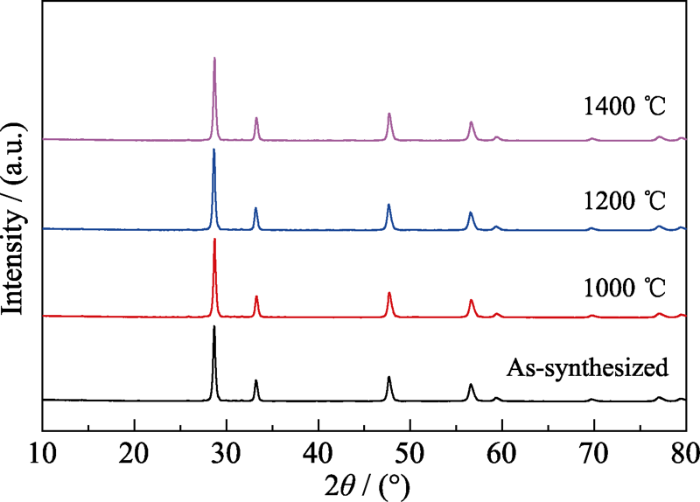
... 为了探索上述ZHCYLO是“高熵”或“熵稳定”的, 将预制的单相陶瓷低温退火4 h, 随炉冷却后对样品进行XRD测试.从图3可以看出, 1000、1200和1400 ℃退火后的ZHCYLO依旧保持着单相萤石结构, 并没有产生第二相, 证明这种陶瓷是“高熵”陶瓷[47,48].在以往的高熵氧化物陶瓷中, (Mg0.2Cu0.2Ni0.2Co0.2Zn0.2)O和(Zr0.2Hf0.2Ce0.2Ti0.2Sn0.2)O2都是“熵稳定”的, 它们会在某一温度范围内发生可逆的单相-多相转变, 只有在淬冷的条件下, 单相才得以保存至室温.而本研究采用程序冷却的方式制备单相ZHCYLO, 有效避免了淬冷工艺在陶瓷表面和内部产生裂纹, 进而影响材料性能. ...
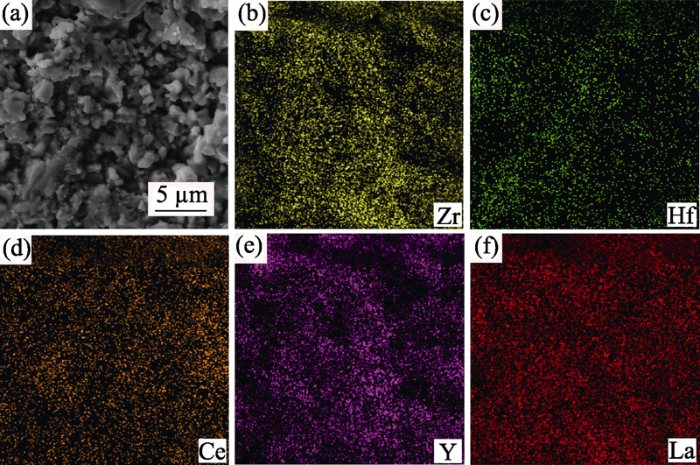
... 图4展现了ZHCYLO陶瓷的表面形貌和元素分布, 可以观察到样品表面含有许多圆柱形隧道状的孔洞.在高熵陶瓷中, 阳离子尺寸/质量无序, 增加了声子散射, 而无压烧结多孔萤石结构进一步说明该陶瓷可能具有隔热材料的潜力[49,50].另外该陶瓷的元素分布比较均匀, 没有出现明显的偏析团聚现象[47]. ...
Order emerging from disorder
1
2019
... 为了探索上述ZHCYLO是“高熵”或“熵稳定”的, 将预制的单相陶瓷低温退火4 h, 随炉冷却后对样品进行XRD测试.从图3可以看出, 1000、1200和1400 ℃退火后的ZHCYLO依旧保持着单相萤石结构, 并没有产生第二相, 证明这种陶瓷是“高熵”陶瓷[47,48].在以往的高熵氧化物陶瓷中, (Mg0.2Cu0.2Ni0.2Co0.2Zn0.2)O和(Zr0.2Hf0.2Ce0.2Ti0.2Sn0.2)O2都是“熵稳定”的, 它们会在某一温度范围内发生可逆的单相-多相转变, 只有在淬冷的条件下, 单相才得以保存至室温.而本研究采用程序冷却的方式制备单相ZHCYLO, 有效避免了淬冷工艺在陶瓷表面和内部产生裂纹, 进而影响材料性能. ...
High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C
1
2019
... 图4展现了ZHCYLO陶瓷的表面形貌和元素分布, 可以观察到样品表面含有许多圆柱形隧道状的孔洞.在高熵陶瓷中, 阳离子尺寸/质量无序, 增加了声子散射, 而无压烧结多孔萤石结构进一步说明该陶瓷可能具有隔热材料的潜力[49,50].另外该陶瓷的元素分布比较均匀, 没有出现明显的偏析团聚现象[47]. ...
Size disorder as a descriptor for predicting reduced thermal conductivity in medium- and high-entropy pyrochlore oxides
1
2020
... 图4展现了ZHCYLO陶瓷的表面形貌和元素分布, 可以观察到样品表面含有许多圆柱形隧道状的孔洞.在高熵陶瓷中, 阳离子尺寸/质量无序, 增加了声子散射, 而无压烧结多孔萤石结构进一步说明该陶瓷可能具有隔热材料的潜力[49,50].另外该陶瓷的元素分布比较均匀, 没有出现明显的偏析团聚现象[47]. ...
Specific adsorption behavior of water on a Y2O3 surface
1
2000
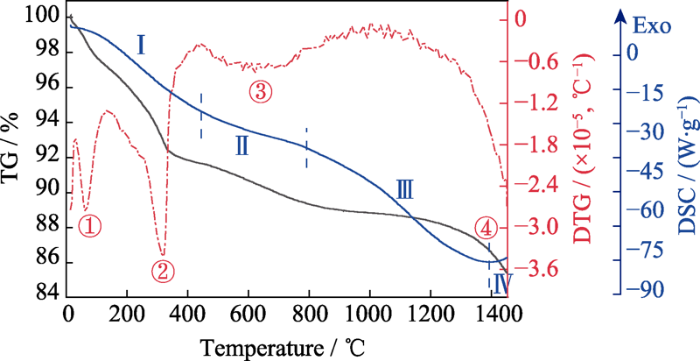
... 图5是生料粉末A的TG-DSC曲线, 从图中可以清晰地看到四个失重峰, 分别位于80、300、600和1400 ℃附近.1号失重峰对应粉体中吸附水的蒸发.此外, 在球磨制备生料粉末过程中, Y2O3和La2O3可能发生水化生成Y(OH)3和La(OH)3[51,52,53,54], 因此2号和3号失重峰对应于结晶水的蒸发.在图1(b)中, 1300 ℃煅烧粉末的物相结构基本与生料保持一致, 1400 ℃以上煅烧粉末的物相结构逐渐向单相结构演变, 而4号失重峰正好在1400 ℃, 综合XRD和TG-DSC结果, 可以推测4号失重峰与单相固溶体的形成有着密切联系.DSC曲线主要分为个四个阶段: 阶段Ⅰ吸附水脱附和部分结晶水蒸发吸收大量的热; 阶段Ⅱ与粉体中剩余结晶水蒸发吸热有关; 阶段Ⅲ主要是因为原料中某些氧化物之间发生固溶, 吸收热量; 阶段Ⅳ有放热的微弱趋势, 这可能是由于陶瓷发生再结晶而逐渐形成萤石结构. ...
Entropy-stabilized oxides owning fluorite structure obtained by hydrothermal treatment
1
2000
... 图5是生料粉末A的TG-DSC曲线, 从图中可以清晰地看到四个失重峰, 分别位于80、300、600和1400 ℃附近.1号失重峰对应粉体中吸附水的蒸发.此外, 在球磨制备生料粉末过程中, Y2O3和La2O3可能发生水化生成Y(OH)3和La(OH)3[51,52,53,54], 因此2号和3号失重峰对应于结晶水的蒸发.在图1(b)中, 1300 ℃煅烧粉末的物相结构基本与生料保持一致, 1400 ℃以上煅烧粉末的物相结构逐渐向单相结构演变, 而4号失重峰正好在1400 ℃, 综合XRD和TG-DSC结果, 可以推测4号失重峰与单相固溶体的形成有着密切联系.DSC曲线主要分为个四个阶段: 阶段Ⅰ吸附水脱附和部分结晶水蒸发吸收大量的热; 阶段Ⅱ与粉体中剩余结晶水蒸发吸热有关; 阶段Ⅲ主要是因为原料中某些氧化物之间发生固溶, 吸收热量; 阶段Ⅳ有放热的微弱趋势, 这可能是由于陶瓷发生再结晶而逐渐形成萤石结构. ...
Effect of reaction routes on the porosity and permeability of porous high entropy (Y0.2Yb0.2Sm0.2Nd0.2Eu0.2)B6 for transpiration cooling
1
2020
... 图5是生料粉末A的TG-DSC曲线, 从图中可以清晰地看到四个失重峰, 分别位于80、300、600和1400 ℃附近.1号失重峰对应粉体中吸附水的蒸发.此外, 在球磨制备生料粉末过程中, Y2O3和La2O3可能发生水化生成Y(OH)3和La(OH)3[51,52,53,54], 因此2号和3号失重峰对应于结晶水的蒸发.在图1(b)中, 1300 ℃煅烧粉末的物相结构基本与生料保持一致, 1400 ℃以上煅烧粉末的物相结构逐渐向单相结构演变, 而4号失重峰正好在1400 ℃, 综合XRD和TG-DSC结果, 可以推测4号失重峰与单相固溶体的形成有着密切联系.DSC曲线主要分为个四个阶段: 阶段Ⅰ吸附水脱附和部分结晶水蒸发吸收大量的热; 阶段Ⅱ与粉体中剩余结晶水蒸发吸热有关; 阶段Ⅲ主要是因为原料中某些氧化物之间发生固溶, 吸收热量; 阶段Ⅳ有放热的微弱趋势, 这可能是由于陶瓷发生再结晶而逐渐形成萤石结构. ...
Research thermal decomposition fo lanthanum hydroxide by thermogravimetry
1
1987
... 图5是生料粉末A的TG-DSC曲线, 从图中可以清晰地看到四个失重峰, 分别位于80、300、600和1400 ℃附近.1号失重峰对应粉体中吸附水的蒸发.此外, 在球磨制备生料粉末过程中, Y2O3和La2O3可能发生水化生成Y(OH)3和La(OH)3[51,52,53,54], 因此2号和3号失重峰对应于结晶水的蒸发.在图1(b)中, 1300 ℃煅烧粉末的物相结构基本与生料保持一致, 1400 ℃以上煅烧粉末的物相结构逐渐向单相结构演变, 而4号失重峰正好在1400 ℃, 综合XRD和TG-DSC结果, 可以推测4号失重峰与单相固溶体的形成有着密切联系.DSC曲线主要分为个四个阶段: 阶段Ⅰ吸附水脱附和部分结晶水蒸发吸收大量的热; 阶段Ⅱ与粉体中剩余结晶水蒸发吸热有关; 阶段Ⅲ主要是因为原料中某些氧化物之间发生固溶, 吸收热量; 阶段Ⅳ有放热的微弱趋势, 这可能是由于陶瓷发生再结晶而逐渐形成萤石结构. ...
Mechanical alloying and milling
1
2001
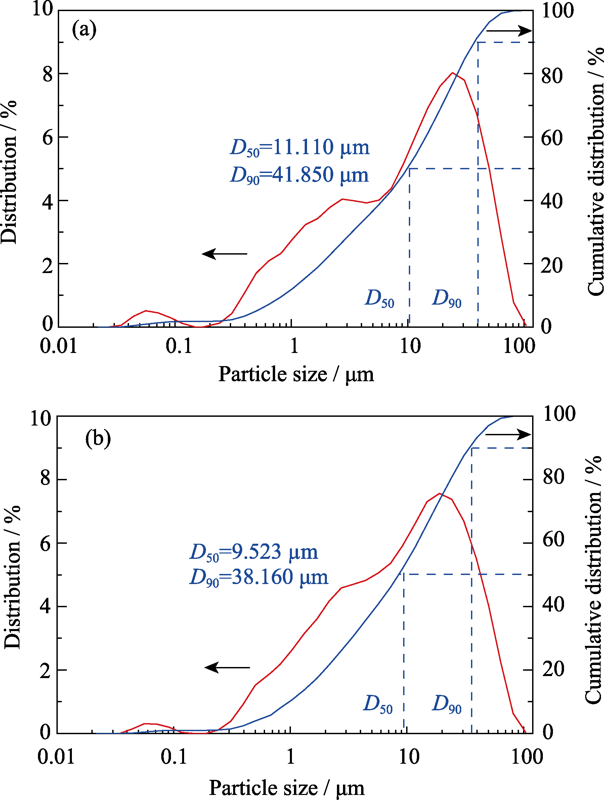
... 图6为粉末A和B的激光粒度分布曲线, 两者峰形相近, 均没有表现出单峰趋势, 这是因为各氧化物原料粒径存在差异.干法球磨工艺作用于粉体的能量比湿法球磨大得多, 大量机械能转化为颗粒表面能并产生缺陷, 因此粉末B的粒径整体略小于粉末A[55].对比两种生料粉末的XRD图谱(图7(a)) 发现: 与A粉末相比, B粉末在“·”标记处的衍射峰有不同程度的角度合并和强度降低现象, 这可能是由于粉末内部产生少量固态扩散和机械合金化造成的[56]. ...
Phase stability and mechanical properties of novel high entropy transition metal carbides
1
2019
... 图6为粉末A和B的激光粒度分布曲线, 两者峰形相近, 均没有表现出单峰趋势, 这是因为各氧化物原料粒径存在差异.干法球磨工艺作用于粉体的能量比湿法球磨大得多, 大量机械能转化为颗粒表面能并产生缺陷, 因此粉末B的粒径整体略小于粉末A[55].对比两种生料粉末的XRD图谱(图7(a)) 发现: 与A粉末相比, B粉末在“·”标记处的衍射峰有不同程度的角度合并和强度降低现象, 这可能是由于粉末内部产生少量固态扩散和机械合金化造成的[56]. ...