锂-硫电池是以金属锂为负极, 单质硫为正极的新一代二次电池体系, 其理论能量密度达到2600 Wh∙kg-1, 是传统锂离子电池的3~5倍, 理论比容量高达1675 mAh∙g-1, 因此被认为是目前最具发展潜力的二次电池系统之一[1,2]。不仅如此, 单质硫的储量丰富、价格低廉、环境友好等特点使其更具商业竞争力。尽管锂-硫电池具备目前商业锂离子 电池无法企及的优势, 但其实际应用依然道阻且 长[3,4]。首先, 单质硫及其最终还原产物Li2S的电子、离子电导率较差, 导致锂-硫电池的活性物质利用率低, 初始容量不佳; 其次, 充放电过程中由于反应物与最终放电产物之间的密度差异带来巨大的体积变化, 使正极结构坍塌, 导致电池循环寿命短、循环性能差。此外, 存在对电池性能最为致命的“飞梭效应”, 即放电中间产物(Li2Sn, 4≤n≤8)溶解于醚类电解液中, 并在电场作用下穿过隔膜迁移至锂 负极区域直接与锂负极反应, 造成负极锂片腐蚀破坏[5,6]。聚硫锂不断地在正负极之间来回飞梭造成正极活性物质持续流失, 最终导致电池失效。
通过上述分析, 若能将聚硫锂限制在正极结构内部, 最大程度避免其扩散, 同时解决好单质硫导电性差和体积膨胀等问题, 将能最大程度地解决锂-硫电池存在的问题[7,8,9]。因此, 国内外科研工作者提出了一系列研究思路, 尤其是近十年, 在此方面的报道更是层出不穷且效果显著。其中, 以具有较高比表面积的碳材料为代表的锂-硫电池正极载硫基体的相关研究更是引起了广泛关注[10,11]。碳材料不仅可以提高复合正极的导电性以及单质硫的电荷传输效率, 而且其复杂的孔结构可构筑大量有效的离子通道, 有助于改善电池的循环性能和倍率性能[12]。此外, 多孔结构可以提供额外的缓冲空间从而有效抑制单质硫在充放电过程中的体积膨胀, 维持电极结构的稳定性。更重要的是, 碳材料较大的比表面积能够对聚硫锂起到物理吸附的作用, 从而在一定程度上限制了聚硫锂的溶出, 抑制“飞梭效应”[13]。
近十年来, 一类被称为“金属有机框架(Metal- Organic Frameworks, MOFs)材料”的有机-无机杂化纳米多孔材料受到了广泛关注, 成为新材料领域的研究热点与前沿方向之一[14]。凭借MOFs材料自身的优势, 如具有较大的比表面积、较高的孔隙率、种类的多样性以及结构可设计等特点, MOFs在储能领域的潜在可能性也引起了广大科研人员的兴趣, 且取得了一大批优秀成果[15,16]。MOFs可以直接作为模板负载参与能量转换反应的活性物质, 也可以作为牺牲模板, 将其自身组分转换为活性物质参与能量转换反应。ZIF-67是由Co2+离子和2-甲基咪唑配位而成, 将其在惰性气氛下退火处理, 有机组分可转换为氮掺杂的多孔碳, 同时可将Co2+还原为金属钴。据相关研究报道, 氮掺杂可以改善碳基材料的导电性、表面极性以及对聚硫锂较强的吸附性能, 而金属Co的嵌入对锂-硫电池氧化还原反应动力学具有一定促进作用[17,18]。
本研究针对锂-硫电池目前存在的主要问题, 以改善电池循环性能和倍率性能为目标, 以ZIF-67为模板, 通过后续的高温烧结以及刻蚀处理获得一系列具备不同Co负载量的氮掺杂多孔碳材料, 系统研究Co负载量对锂-硫电池电化学性能的影响, 从而获得使各类材料呈现最佳电化学性能的优化工艺。这种基于MOFs衍生材料的可控负载研究方法可以给其他能源存储转换领域的相关研究起到一定的借鉴作用。
1 实验方法
1.1 材料制备
ZIF-67的合成: 称取2.328 g Co(NO3)2∙6H2O和2.627 g 2-甲基咪唑并分别溶解于100 mL甲醇溶液中, 搅拌至澄清后将2-甲基咪唑的甲醇溶液缓慢倒入含有Co(NO3)2∙6H2O的甲醇溶液中得到蓝紫色透明溶液。磁力搅拌2 h后于室温下静置24 h, 所得紫色沉淀用乙醇清洗数次后离心分离并置于真空干燥箱中于80 ℃下真空干燥12 h。所得蓝紫色粉末即为ZIF-67前驱体。
Co-NC复合碳材料制备: 将ZIF-67前驱体置于管式炉中, 在氩气保护下以5 ℃∙min-1升温至650 ℃保温3 h, 随炉冷却至室温收集产物并命名为Co-NC。随后将Co-NC在2 mol/L的H2SO4溶液中分别处理6、24和48 h, 收集产物并分别命名为Co-NC(6 h)、Co-NC(24 h)、Co-NC(48 h), 未做硫酸处理的样品命名为Co-NC(0 h)。
S/Co-NC(0~48 h)正极材料制备: 采用融熔法将硫渗入到Co-NC(0~48 h)多孔碳结构中, 按质量比3 : 1分别称取升华硫和Co-NC并置于研钵中研磨30 min, 随后将混合物置于管式炉中, 在氮气保护下155 ℃处理8 h, 冷却后收集产物。
1.2 材料表征
采用Hitachi S-4800场发射扫描电子显微镜对样品进行形貌观察; 采用FEI Tecnai 2100透射电子显微镜观察样品内部结构及高分辨晶格条纹; 采用RIGAKU TTR-3 X射线衍射仪对所有样品进行晶体结构分析, 扫描速率为0.02(°)∙s-1; 采用V-Sorb 2800型BET测试仪分析样品比表面积及孔分布状况; 采用TGA-600热分析仪分析复合正极材料中的Co含量和S含量; 采用Thermo Fisher K-Alpha 1063 X射线光电子能谱仪分析样品元素组成及价态。
1.3 电化学性能表征
本研究采用传统的浆料涂覆工艺制备正极片, 将原料按质量比即S/Co-NC : LA133 : 乙炔黑=80 : 10 : 10, 投置于球磨罐中并加入一定量的去离子水作为溶剂, 高能球磨1 h后将所得浆料均匀涂覆于铝箔上, 经充分干燥后裁成ϕ15 mm的正极极片装配电池, 单个极片上硫面密度控制为~1.6 mg∙cm-2。所有电池组装过程都在充满氩气的手套箱中完成, 以锂片为对电极, 以ϕ19 mm的Celgard 2400为隔膜, 以0.5 mol/L LiTFSI+0.2 mol/L LiNO3的DOL/DME (1,3-二氧戊环/乙二醇二甲醚, 体积比1 : 1)为电解液, 装配成CR2016纽扣电池。室温静置12 h后采用LAND CT2001A型多通道充放电测试系统对纽扣电池进行充放电测试, 充放电电压范围为1.7~ 2.8 V。采用Prinston Versa STAT电化学工作站进行循环伏安测试。所有电化学测试都在恒温25 ℃下 进行。
2 结果与讨论
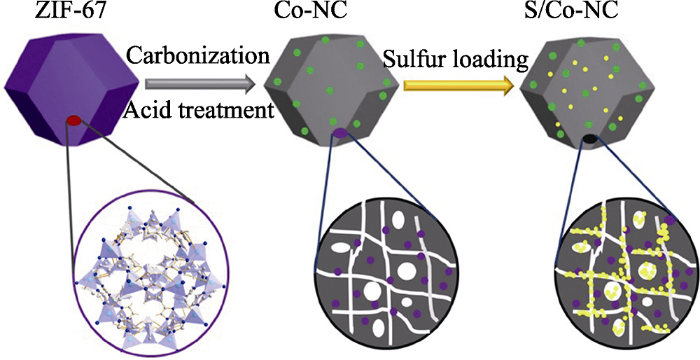
图1为S/Co-NC(0~48 h)复合材料合成示意图。首先, 以预先合成的ZIF-67前驱体为自牺牲模板, 通过在惰性气氛下高温烧结及后续的酸处理过程形成具有金属Co颗粒嵌入的氮掺杂多孔碳材料, 其孔隙结构是由ZIF-67中有机骨架2-甲基咪唑高温裂解产生的, 同时, 2-甲基咪唑富含吡啶型氮, 烧结后可形成具有原位氮掺杂的碳材料, Co2+被还原为Co金属。随后, 采用熔融法将单质硫注入到Co-NC(0~ 48 h)的孔隙结构中形成S/Co-NC(0~48 h)复合硫正极材料。多孔结构可以有效地缓冲硫在充放电过程中的体积膨胀, 同时在一定程度上能够限制聚硫锂的溶出, 从而保证较高的活性物质利用率。
图1
图1
S/Co-NC(0~48 h)复合材料合成示意图
Fig. 1
Schematic illustration of the preparation process of S/Co-NC(0~48 h) composites
2.1 材料微观形貌表征
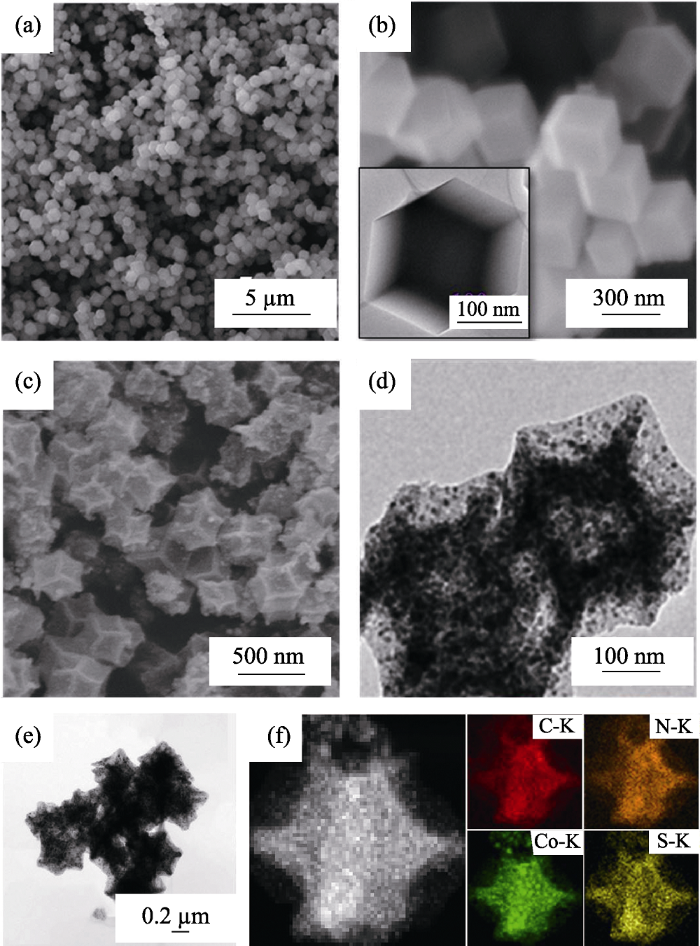
ZIF-67前驱体和Co-NC(48 h)、S/Co-NC(48 h)复合材料的SEM、TEM照片和EDS元素面扫描图如图2所示。ZIF-67前驱体具有较为均匀的十二面体形貌, 颗粒尺寸约为300~400 nm (图2(a~b))。经650 ℃高温烧结及酸处理48 h后, 得到的Co-NC (48 h)多面体基本维持了前驱体的多面体形貌, 伴随着有机骨架的裂解, 颗粒尺寸略有收缩, 表面轻微凹陷(图2(c))。从图2(d)所示的TEM照片中清晰可见, Co-NC(48 h)呈现出明显的多孔结构, 图中黑点为均匀镶嵌在碳基体里面的金属Co纳米颗粒[19]。随后, 采用熔融法将单质硫灌注到Co-NC的孔道结构中, 制得S/Co-NC复合硫正极材料。在图2(e)所示S/Co-NC的TEM照片中并未观察到大块的单质硫存在, 且其孔道结构基本已被单质硫填满, 说明S被成功注入到多孔碳的孔道结构中。结合图2(f)中的EDS 元素面扫描图可知, C、N、Co、S元素均匀分布于S/Co-NC材料中, 进一步说明了单质硫的成功注入。此外, 图2(f)中N元素与Co元素的存在印证了多孔碳材料中的原位N掺杂以及均匀镶嵌在碳基体中的金属Co纳米颗粒。
图2
图2
(a~b) ZIF-67前驱体和(c~d) Co-NC(48 h)的(a,c) SEM、(b,d) TEM照片; S/Co-NC复合材料的(e) TEM照片和(f)EDS元素面扫描图
Fig. 2
(a,c) SEM and (b,d) TEM images of (a-b) ZIF-67 precursors and (c-d) Co-NC (48 h); (e) TEM image and (f) EDS elemental mappings of S/Co-NC (48 h) composite
2.2 晶体结构分析
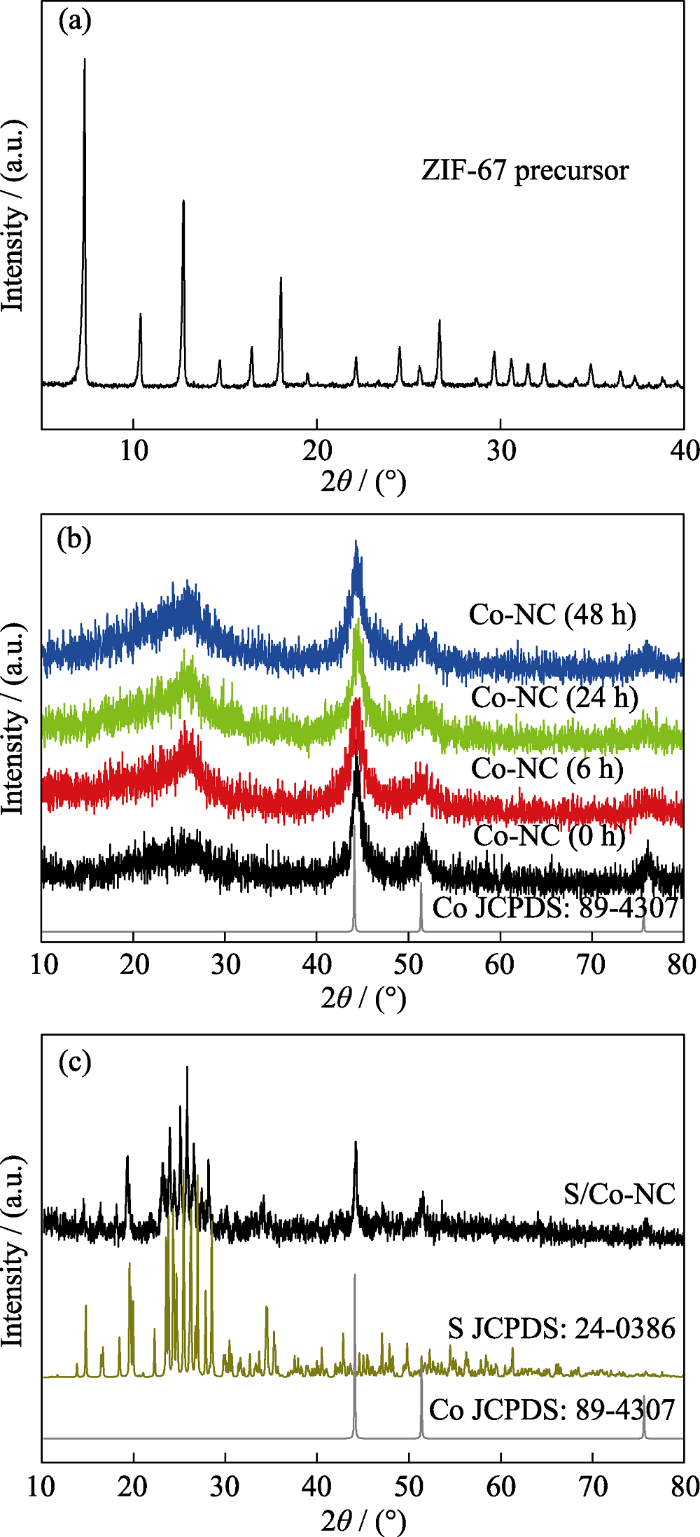
图3为ZIF-67前驱体, Co-NC(0~48 h)以及S/Co-NC(48 h)复合材料的X射线衍射图谱。在ZIF-67的XRD图谱中(图3(a)), 7.2°处尖锐的衍射峰表明合成的ZIF-67具有非常好的结晶性[20]。经高温退火处理后, ZIF-67的衍射峰完全被金属Co (PDF#89-4307)(图3(b))的三个特征峰代替, 其半峰宽较大, 说明形成的Co金属尺寸较小。位于26°较宽的衍射峰对应于部分石墨化的碳, 表明金属Co的存在有助于促进无定型碳向石墨化碳的转变[21]。图3(c)为S/Co-NC(48 h)复合材料的XRD图谱, 其衍射峰与单质硫的特征峰对应良好, 说明S已成功与多孔碳材料复合。
图3
图3
(a) ZIF-67前驱体、(b) Co-NC(0~48 h)以及(c) S/Co-NC (48 h)复合材料的XRD图谱
Fig. 3
XRD patterns of (a) ZIF-67 precursor, (b) Co-NC(0~ 48 h) and (c) S/Co-NC(48 h) composites
2.3 比表面积及孔径分析
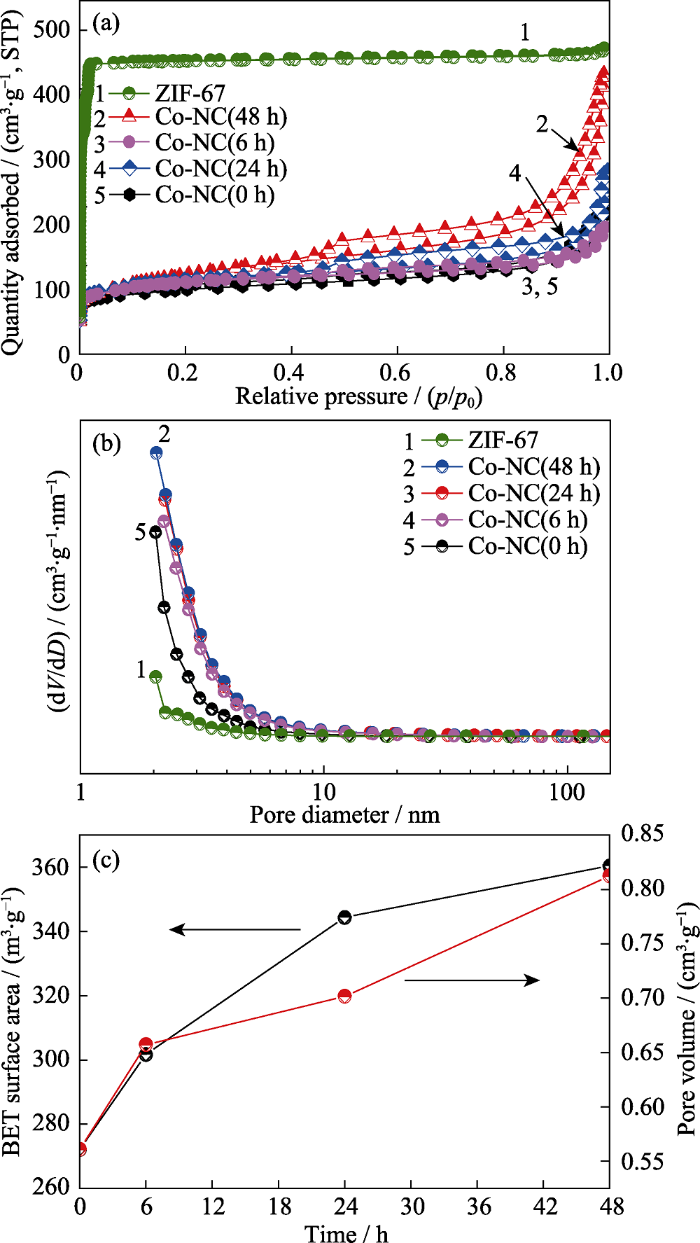
表1列出了ZIF-67前驱体、Co-NC(0~48 h)以及不同刻蚀时间后所得多孔碳材料的 BET 比表 面积、平均孔径以及孔体积数据。ZIF-67前驱体 具有1481.22 m2∙g-1的高比表面积且富含大量微孔结构, 平均孔径和孔体积分别为1.9919 nm和0.7252 cm3∙g-1。如图4(a~b)所示, 经过高温烧结之后, ZIF-67中的微孔消失并形成大量介孔, 比表面积降至272.15 m2∙g-1, 孔径增至8.2433 nm。随着刻蚀时间的不断延长, 比表面积与孔体积也逐渐增大, 其变化趋势图如图4(c)所示。本研究通过刻蚀处理一方面间接控制Co-NC(0~48 h)复合材料中的Co含量, 另一方面可产生更多的孔隙, 进而存储更多的硫, 且在充放电循环过程中有利于电解液的渗透和离子传导。
表1 ZIF-67和Co-NC复合材料的BET结果
Table 1
| Sample | BET surface area/(m2∙g-1) | Pore diameter /nm | Pore volume /(cm3∙g-1) |
|---|---|---|---|
| ZIF-67 | 1481.22 | 1.9919 | 0.7252 |
| Co-NC(0 h) | 272.15 | 8.2433 | 0.5608 |
| Co-NC(6 h) | 301.73 | 8.7121 | 0.6571 |
| Co-NC(24 h) | 344.41 | 9.6929 | 0.7016 |
| Co-NC(48 h) | 360.50 | 9.7984 | 0.8124 |
图4
图4
ZIF-67前驱体和Co-NC(0~48 h)样品的(a)氮气吸脱附等温线、(b)孔径分布曲线以及(c) Co-NC(0~48 h)复合材料随刻蚀时间延长BET比表面积及孔体积的变化曲线
Fig. 4
(a) N2 adsorption-desorption isotherms and (b) pore size distributions of ZIF-67 and Co-NC(0~48 h) samples, (c) curves of variation trend of BET surface area and pore volume of Co-NC composites with increased etching time
2.4 样品成分及硫含量分析
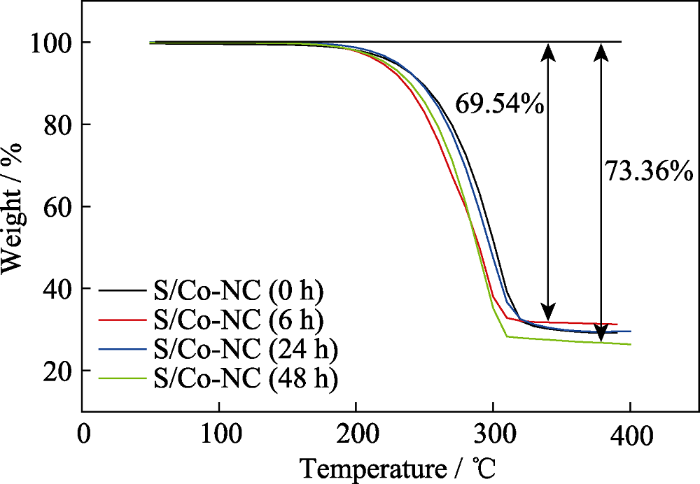
通过在空气中烧结不同刻蚀时间后的Co-NC(0~48 h)样品, 去除样品中的碳, 利用最终剩余的Co3O4计算出原始样品中的Co含量。通过XPS分析复合材料中的N含量。最终所得的各样品的Co、N含量数据列于表2。从表2中可以看出, 未刻蚀样品, 即Co-NC的Co含量为37.55wt%, 随着刻蚀时间的延长, Co含量逐渐下降至23.29wt%(6 h)、16.24wt%(24 h)和15.93wt%(48 h), 且当刻蚀时间超过24 h后很难除去剩余的金属Co。Co-NC (0~48 h)复合材料中的氮元素主要来源于ZIF-67的有机配体2-甲基咪唑的高温裂解, 且经过XPS分析, 四组样品中的氮/碳比基本一致, 约为0.1065。同样, 采用热分析方法对载硫后的样品进行硫含量分析。如图5所示, 四组样品的硫含量介于69.54wt% (Co-NC)和73.36wt% (Co-NC(48 h))之间, 与实验设计的最大载硫量(75%)相差不大, 其差异主要归因于复合材料不同的孔体积和孔结构。
表2 Co-NC复合材料中各元素质量含量及N/C比
Table 2
| Sample | Co-NC(0 h) | Co-NC(6 h) | Co-NC(24 h) | Co-NC(48 h) |
|---|---|---|---|---|
| Co/wt% | 37.55 | 23.29 | 16.24 | 15.93 |
| N/wt% | 6.68 | 8.36 | 8.79 | 8.78 |
| N/C | 0.1070 | 0.1089 | 0.1056 | 0.1045 |
图5
2.5 电化学性能分析
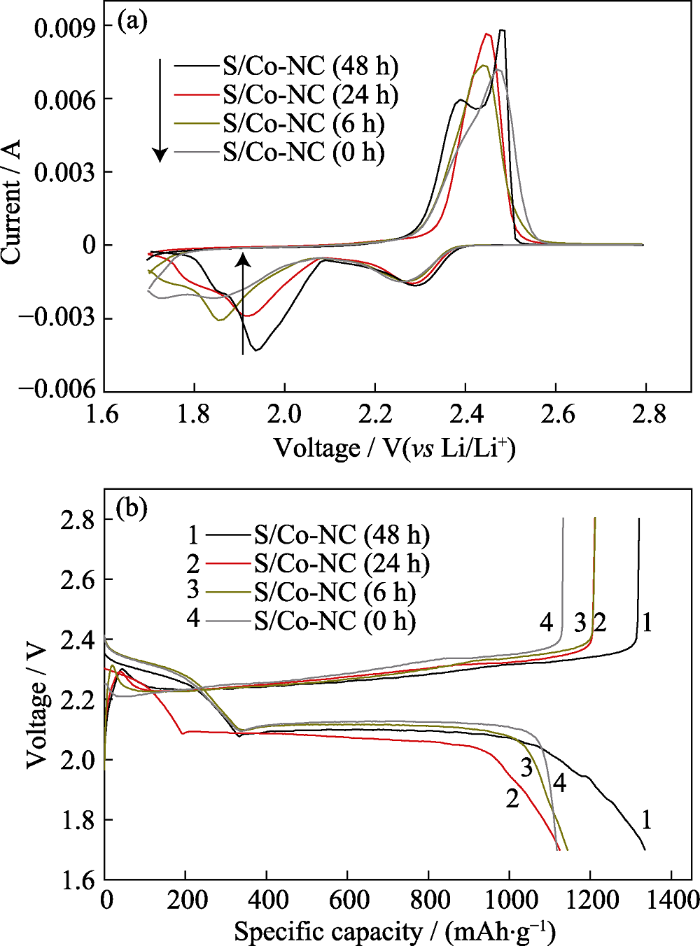
首先对以四种不同Co含量的S/Co-NC(0~48 h)碳硫复合正极材料所组装的扣式电池进行循环伏安(CV)测试, 以研究其在充放电过程中的电化学行为。扫描速率设定为0.1 mV∙s-1, 测试电压范围为1.7~2.8 V (vs Li/Li+)。如图6(a)所示, 四条曲线与典型的单质硫的CV曲线基本一致[7,22]。其中, 位于2.3 V附近的还原峰对应于环状S8分子的开环以及向可溶性长链多硫化锂的转变(Li2Sn, 4≤n≤8); 位于1.9 V附近的较宽的还原峰对应于长链多硫化锂进一步向不可溶的Li2S2和Li2S的还原过程。而对于氧化过程, 四种材料的CV曲线表现出较大差异。以Co-NC(48 h)为载硫基体的锂-硫电池表现出典型的双氧化峰特性, 位于2.35和2.48 V附近的两个氧化峰分别对应于Li2S2和Li2S向长链多硫化锂的固-液转变以及进一步向单质硫的转变。而以其他三种材料为载硫基体的锂-硫电池则只有一个较宽的氧化峰出现, 表明氧化过程进行的不彻底, 活性物质利用率较低[4]。其原因可归结于过量金属Co的存在占据了部分内部孔道结构, 导致单质S并未完全进入到基体结构内部, 随着氧化还原反应的进行, 位于基体材料表面甚至表层的S直接脱离基体进入电解液中, 造成不可逆损失。
图6
图6
不同Co含量的S/Co-NC(0~48 h)电极的(a) CV曲线和(b)恒电流充放电曲线
Fig. 6
(a) CV curves and (b) discharge-charge profiles of S/Co-NC(0~48 h) electrodes with different Co contents
图6(b)所示为以四种不同Co含量的S/Co-NC电极所进行的恒电流充放电测试, 电流密度为0.1C (1C=1675 mA∙g-1), 测试电压范围为1.7~2.8 V (vs Li/Li+)。四条曲线都包含两个放电平台, 2.3 V左右的平台对应于CV曲线中的第一个还原峰, 即S向长链多硫化锂的转变, 相应的, 2.1 V左右的长平台对应于CV曲线的第二个还原峰(开始出峰位置约为2.1 V), 对应可溶性长链多硫化锂进一步向不溶性Li2S2和Li2S的转变。其中, S/Co-NC(48 h)表现出 最高的初始容量, 反映出该电极较高的活性物质利用率。
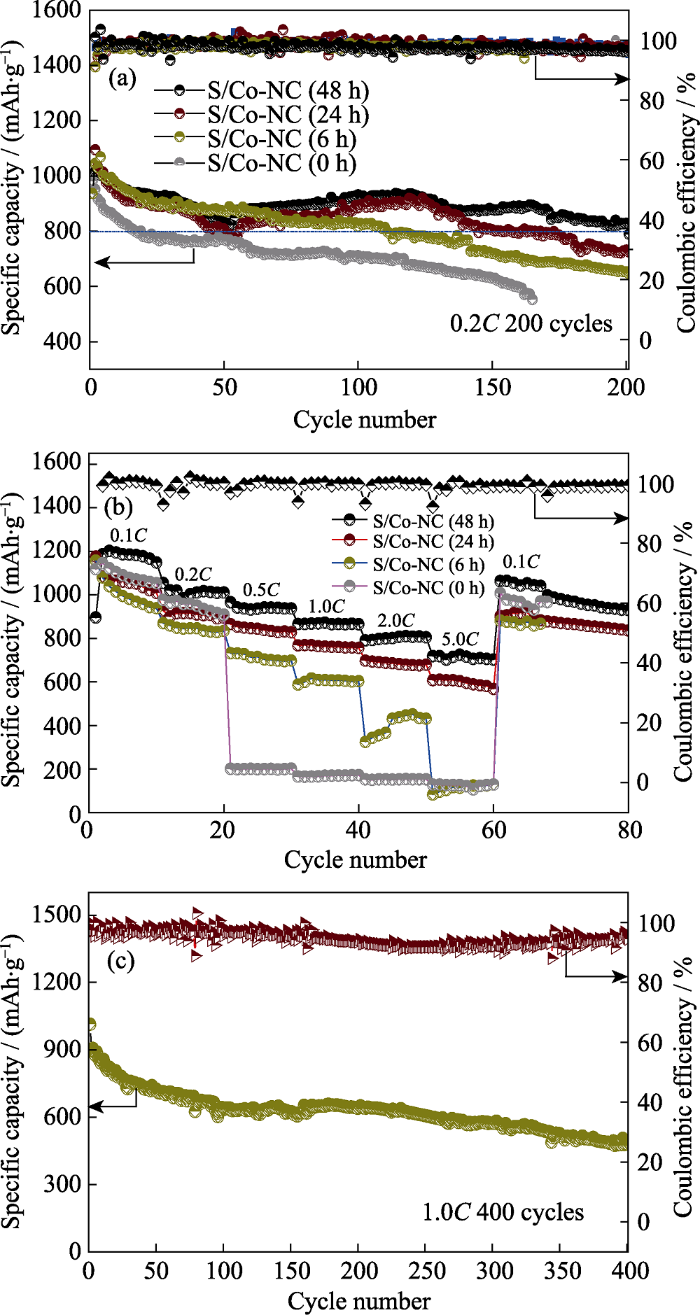
图7(a)所示为四种不同Co含量的S/Co-NC(0~ 48 h)复合材料电极在0.2C电流密度下的循环性能曲线。S/Co-NC(48 h)电极表现出最高的初始放电容量, 达到1095 mAh∙g-1, 接下来的50圈内容量下降较快, 到第50圈时容量为832.1 mAh∙g-1。比容量的下降与多硫化锂在电解液中的溶解和扩散有关。在接下来的循环中比容量趋于稳定, 并在200次循环后仍然保留有789.2 mAh∙g-1的较高可逆容量, 为初始容量的72.07%。从第50圈到200圈循环的容量保持率为94.84%, 库伦效率接近100%, 表明该电极结构起到了较好的束硫效果, 由飞梭效应带来的不可逆硫损失程度较低, 活性物质在充放电过程中具有较高的利用率。相比之下, 其他各组电池则表现出较低的初始容量, 分别为1015.7 (S/Co-NC (24 h))、981.9 (S/Co-NC(6 h))和937 mAh∙g-1(S/Co- NC)。经过200次循环后分别剩余725.2 (S/Co-NC (24 h))、647 (S/Co-NC(6 h))和552.7 mAh∙g-1 (S/Co-NC第165次循环后电池失效)的可逆容量, 相对应的容量保持率分别为71.39%、65.89%和58.98%(前165次循环对应的容量保持率)。综上分析, 随着Co含量的逐渐减少, 对应的正极材料循环性能逐渐改善, 当刻蚀时间为 48 h, 即对应的Co含量为15.93wt%时, 所对应的正极材料具有最佳的循环性能。
图7
图7
不同Co含量的S/Co-NC(0~48 h)电极的(a)循环性能曲线、(b)倍率性能曲线以及(c)S/Co-NC(48 h)电极在1.0C电流密度下的长周期循环性能曲线
Fig. 7
(a) Cycle performances and (b) rate capabilities of S/Co-NC(0~48 h) electrodes with different Co contents; (c) Long-cycle performance of S/Co-NC(48 h) at 1.0C current density
图 7(b)为四种正极材料在不同电流密度下(0.1C~5C)的倍率性能曲线。对于S/Co-NC (48 h)电极, 其在0.1C、0.2C、0.5C、1.0C和2.0C电流密度下的比容量分别为1186.1、1054.7、965.9、865.8和791 mAh∙g-1, 甚至在5.0C高倍率下, 其比容量仍然高达718.8 mAh∙g-1, 当电流密度恢复至0.1C时, 其容量恢复到了1063.2 mAh∙g-1, 表现出优异的倍率性能, 说明该电极结构拥有较好的结构稳定性。其他三组电池的性能相对较差。S/Co-NC(24 h)电极表现出仅次于S/Co-NC(48 h)电极的倍率性能。在5.0C倍率下仍然保留有608.6 mAh∙g-1的较高可逆容量, 说明当刻蚀时间达到24 h时基本上已能达到该材料所能发挥的最佳电化学性能。而对于S/Co-NC (6 h)和S/Co-NC(0 h)电极, 其在较大电流密度下均无法正常循环, 性能极差, 其原因可归结于过量的金属Co使整体材料的质量密度增加, 导致实际可用于储硫的多孔碳含量减少, 活性 S 无法全部融到结构内部, 使得基体材料对 S 的限制作用较差。S/Co-NC (48 h)电极在1.0C电流密度下的长周期循环性能见图7(c)。S/Co-NC (48 h)电极在1.0C电流密度下的初始放电容量为1076.8 mAh∙g-1, 经过400次充放电循环后保留容量为563.9 mAh∙g-1, 对应的容量保持率为每次52.37%, 表现出较好的长周期循环稳定性。
3 结论
以ZIF-67为模板, 通过高温退火以及酸处理刻蚀过程制得具有不同Co含量的氮掺杂多孔碳材料(Co-NC), 并应用于锂-硫电池正极材料中。利用SEM、TEM、XRD、BET等表征手段分析了Co-NC多孔碳材料的微观形貌、金属Co的结晶状态以及孔结构状态。随后系统研究了金属Co含量对电极电化学性能的影响。研究表明, Co-NC(0~48 h)中Co含量为15.93wt%时, 复合电极具有最佳的 循环性能, 在0.2C电流密度放电初始容量为1095 mAh∙g-1, 经200次循环后比容量仍高达789.2 mAh∙g-1, 第50次至200次循环的容量保持率为94.84%, 在5.0C电流密度下可达到718.8 mAh∙g-1的可逆容量, 循环性能与倍率性能均得到显著改善。
参考文献
Research and prospect of lithium-sulfur battery aystem
Lithium/sulfur secondary batteries: a review
Development of lithium/sulfur battery and its facing challenge
A review on electric vehicle battery modelling: from lithium-ion toward lithium-sulphur
Accurate prediction of range of an electric vehicle (EV) is a significant issue and a key market qualifier. EV range forecasting can be made practicable through the application of advanced modelling and estimation techniques. Battery modelling and state-of-charge estimation methods play a vital role in this area. In addition, battery modelling is essential for safe charging/discharging and optimal usage of batteries. Much existing work has been carried out on incumbent Lithium-ion (Li-ion) technologies, but these are reaching their theoretical limits and modern research is also exploring promising next-generation technologies such as Lithium–Sulphur (Li–S). This study reviews and discusses various battery modelling approaches including mathematical models, electrochemical models and electrical equivalent circuit models. After a general survey, the study explores the specific application of battery models in EV battery management systems, where models may have low fidelity to be fast enough to run in real-time applications. Two main categories are considered: reduced-order electrochemical models and equivalent circuit models. The particular challenges associated with Li–S batteries are explored, and it is concluded that the state-of-the-art in battery modelling is not sufficient for this chemistry, and new modelling approaches are needed.
A review of recent developments in rechargeable lithium-sulfur batteries
Abstract The research and development of advanced energy-storage systems must meet a large number of requirements, including high energy density, natural abundance of the raw material, low cost and environmental friendliness, and particularly reasonable safety. As the demands of high-performance batteries are continuously increasing, with large-scale energy storage systems and electric mobility equipment, lithium-sulfur batteries have become an attractive candidate for the new generation of high-performance batteries due to their high theoretical capacity (1675 mA h g -1 ) and energy density (2600 Wh kg -1 ). However, rapid capacity attenuation with poor cycle and rate performances make the batteries far from ideal with respect to real commercial applications. Outstanding breakthroughs and achievements have been made to alleviate these problems in the past ten years. This paper presents an overview of recent advances in lithium-sulfur battery research. We cover the research and development to date on various components of lithium-sulfur batteries, including cathodes, binders, separators, electrolytes, anodes, collectors, and some novel cell configurations. The current trends in materials selection for batteries are reviewed and various choices of cathode, binder, electrolyte, separator, anode, and collector materials are discussed. The current challenges associated with the use of batteries and their materials selection are listed and future perspectives for this class of battery are also discussed.
Shuttle phenomenon-the irreversible oxidation mechanism of sulfur active material in Li-S battery
The commercialization of lithium ulfur batteries is hindered by serious capacity fading that mainly results from the irreversible oxidation of sulfur active material during cycling, but so far the underlying oxidation mechanism still remains unclear. The research results reveal that the most practical solvent DOL and DME are not stable and large amount of degradation products with -OLi edge groups become the oxygen source. The shuttle phenomenon is an important reason leading to the irreversible oxidation of sulfur active material, which results from series chemical reductions of lithium polysulfide, and they keep charge conservation by their selves, but prolong the charge process and lead to the overcharging. In charge process, the low speed reactions of long-chain lithium polysulfide oxidized to LixSOy species exist in the electrolyte, which are indistinctly in the systems without overcharging. As the shuttle phenomenon prolongs the charge process, the irreversible oxidation reactions become distinctly, and the LixSOy species are detectable. The overcharging capacity could be explained as a reversible part and an irreversible part. The reversible capacity is from the cathode active material oxidized to long-chain lithium polysulfide and elemental sulfur, and the irreversible capacity is from the long-chain lithium polysulfide oxidized to LixSOy species.
Sulfur-based composite cathode materials for high-energy rechargeable lithium batteries
Designing high-energy lithium-sulfur batteries
Abstract Due to their high energy density and low material cost, lithium-sulfur batteries represent a promising energy storage system for a multitude of emerging applications, ranging from stationary grid storage to mobile electric vehicles. This review aims to summarize major developments in the field of lithium-sulfur batteries, starting from an overview of their electrochemistry, technical challenges and potential solutions, along with some theoretical calculation results to advance our understanding of the material interactions involved. Next, we examine the most extensively-used design strategy: encapsulation of sulfur cathodes in carbon host materials. Other emerging host materials, such as polymeric and inorganic materials, are discussed as well. This is followed by a survey of novel battery configurations, including the use of lithium sulfide cathodes and lithium polysulfide catholytes, as well as recent burgeoning efforts in the modification of separators and protection of lithium metal anodes. Finally, we conclude with an outlook section to offer some insight on the future directions and prospects of lithium-sulfur batteries.
Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes
Amid burgeoning environmental concerns, electrochemical energy storage has rapidly gained momentum. Among the contenders in the ‘beyond lithium’ energy storage arena, the lithium-sulfur (Li-S) battery has emerged as particularly promising, owing to its potential to reversibly store considerable electrical energy at low cost. Whether or not Li-S energy storage will be able to fulfil this potential depends on simultaneously solving many aspects of its underlying conversion chemistry. Here, we review recent developments in tackling the dissolution of polysulfides — a fundamental problem in Li-S batteries — focusing on both experimental and computational approaches to tailor the chemical interactions between the sulfur host materials and polysulfides. We also discuss smart cathode architectures enabled by recent materials engineering, especially for high areal sulfur loading, as well as innovative electrolyte design to control the solubility of polysulfides. Key factors that allow long-life and high-loading Li-S batteries are summarized.
Strongly coupled interfaces between a heterogeneous carbon host and a sulfur-containing guest for highly stable lithium-sulfur batteries: mechanistic insight into capacity degradation
7: 10601-1-10
Confined sulfur in microporous carbon renders superior cycling stability in Li/S batteries
The use of sulfur in the next generation Li-ion batteries is currently precluded by its poor cycling stability caused by irreversible Li2S formation and the dissolution of soluble polysulfides in organic electrolytes that leads to parasitic cell reactions. Here, a new C/S cathode material comprising short-chain sulfur species (predominately S2) confined in carbonaceous subnanometer and the unique charge mechanism for the subnano-entrapped S2 cathodes are reported. The first charge–discharge cycle of the C/S cathode in the carbonate electrolyte forms a new type of thiocarbonate-like solid electrolyte interphase (SEI). The SEI coated C/S cathode stably delivers ≈600 mAh g611 capacity over 4020 cycles (0.0014% loss cycle611) at ≈100% Coulombic efficiency. Extensive X-ray photoelectron spectroscopy analysis of the discharged cathodes shows a new type of S2 species and a new carbide-like species simultaneously, and both peaks disappear upon charging. These data suggest a new sulfur redox mechanism involving a separated Li+/S261 ion couple that precludes Li2S compound formation and prevents the dissolution of soluble sulfur anions. This new charge/discharge process leads to remarkable cycling stability and reversibility.
Lithium-sulfur battery cable made from ultralight,flexible graphene/carbon nanotube/ sulfur composite fibers
Abstract The emergence of flexible and wearable electronic devices with shape amenability and high mobility has stimulated the development of flexible power sources to bring revolutionary changes to daily lives. The conventional rechargeable batteries with fixed geometries and sizes have limited their functionalities in wearable applications. The first-ever graphene-based fibrous rechargeable batteries are reported in this work. Ultralight composite fibers consisting of reduced graphene oxide/carbon nanotube filled with a large amount of sulfur (rGO/CNT/S) are prepared by a facile, one-pot wet-spinning method. The liquid crystalline behavior of high concentration GO sheets facilitates the alignment of rGO/CNT/S composites, enabling rational assembly into flexible and conductive fibers as lithium–sulfur battery electrodes. The ultralight fiber electrodes with scalable linear densities ranging from 0.028 to 0.13 mg cm611 deliver a high initial capacity of 1255 mAh g611 and an areal capacity of 2.49 mAh cm612 at C/20. A shape-conformable cable battery prototype demonstrates a stable discharge characteristic after 30 bending cycles.
Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion
MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity
Highly porous carbon has played an important role in tackling down the energy and environmental problems due to their attractive features such as high specific surface area (SSA), stability, and mass productivity. Especially, the desirable characteristics of the highly porous carbon such as lightweight, fast adsorption/desorption kinetics, and high SSA have attracted extensive attention in the “hydrogen storage” application which is a main bottleneck for the realization of on-board hydrogen fuel cell vehicles. We herein presented porous carbon with hierarchical pore structure derived from highly crystalline metal organic frameworks (denoted as MOF-derived carbon: MDC) without any carbon source and showed it as a promising hydrogen storage adsorbent. MDCs can be fabricated by a simple heat adjustment of MOFs without complicated process and environmental burden. The MDC displayed hierarchical pore structures with high ultramicroporosity, high SSA, and very high total pore volume. Due to its exceptional poro...
A metal-organic framework-derived bifunctional oxygen electrocatalyst
Confining sulfur in N-doped porous carbon microspheres derived from microalgaes for advanced lithium-sulfur batteries
Abstract Lithium-sulfur (Li-S) battery is one of the most attractive candidates for the next-generation energy storage system. However, the intrinsic insulating nature of sulfur and the notorious polysulfide shuttle are the major obstacles, which hinder the commercial application of Li-S battery. Confining sulfur into conductive porous carbon matrices with designed polarized surfaces is regarded as a promising and effective strategy to overcome above issues. Herein, we propose to use microalgaes (Schizochytrium sp.) as low-cost, renewable carbon/nitrogen precursors and biological templates to synthesize N-doped porous carbon microspheres (NPCMs). These rational designed NPCMs can not only render the sulfur-loaded NPCMs (NPCSMs) composites with high electronic conductivity and sulfur content, but also greatly suppress the diffusion of polysulfides by strongly physical and chemical adsorptions. As a result, NPCSMs cathode demonstrates a superior reversible capacity (1030.7 mA h g-1) and remarkable capacity retention (91%) at 0.1 A g-1 after 100 cycles. Even at an extremely high current density of 5 A g-1, NPCSMs still can deliver a satisfactory discharge capacity of 692.3 mAh g-1. This work reveals a sustainable and effective biosynthetic strategy to fabricate N-doped porous carbon matrices for high performance sulfur cathode in Li-S battery, as well as offers a fascinating possibility to rationally design and synthesize novel carbon-based composites.
A honeycomb-like Co@N-C composite for ultrahigh sulfur loading Li-S batteries
Abstract Because of the high theoretical capacity of 1675 mAh g-1 and high energy density of 2600 Wh kg-1, respectively, lithium-sulfur batteries are attracting intense interest. However, it remains an enormous challenge to realize high utilizations and loadings of sulfur in cathode for the practical applications of Li-S batteries. Herein, we design a quasi-2D Co@N-C composite with honeycomb architecture as multi-functional sulfur host via a simple sacrificial templates method. The cellular flake with large surface area and honeycomb architecture can encapsulate much more sulfur leading to high sulfur content (HSC) composites, and by stacking these HSC flakes, a high sulfur loading (HSL) electrode can be realized due to their high layer bulk density. Compared to our previous work in multi-functional Co-N-C composite, the cellular Co@N-C composite displays a distinct enhancement in the sulfur content, sulfur loading, cycle stability and rate performance. Benefitting from the cellular morphology, a composite with HSC of 93.6 wt% and an electrode with HSL of 7.5 mg cm-2 can be obtained simultaneously, which exhibited excellent rate performance up to 10 C (3.6 mg cm-2) and great cycling stability.
Cobalt oxide nanoparticle embedded N-CNTs: lithium ion battery applications
3D,mutually embedded MOF@carbon nanotube hybrid networks for high-performance lithium-sulfur batteries
A novel synergistic composite with multi-functional effects for high-performance Li-S batteries
The rechargeable lithium–sulfur battery is regarded as a promising option for electrochemical energy storage systems owing to its high energy density, low cost and environmental friendliness. Further development of the Li–S battery, however, is still impeded by capacity decay and kinetic sluggishness caused by the polysulfide shuttle and electrode/electrolyte interface issues. Herein, a new type of metal–organic-framework-derived sulfur host containing cobalt and N-doped graphitic carbon (Co–N-GC) was synthesized and reported, in which the catalyzing for S redox, entrapping of polysulfides and an ideal electronic matrix were successfully achieved synchronously, leading to a significant improvement in the Li–S performance. The large surface area and uniform dispersion of cobalt nanoparticles within the N-doped graphitic carbon matrix contributed to a distinct enhancement in the specific capacity, rate performance and cycle stability for Li–S batteries. As a result of this multi-functional arrangement, cathodes with a high sulfur loading of 70 wt% could operate at 1C for over 500 cycles with nearly 100% coulombic efficiency and exhibited an outstanding high-rate response of up to 5C, suggesting that the S@Co–N-GC electrode was markedly improved by the proposed strategy, demonstrating its great potential for use in low-cost and high-energy Li–S batteries.
The mechanism of Li2S activation in lithium-sulfur batteries: can we avoid the polysulfide formation?
61Mechanism of Li2S activation.61Direct conversion of Li2S to sulfur.61Impact of ionic conductivity on Li2S batteries behavior.61Absence of polysulfides during charging.