

基于低含水量普鲁士蓝正极的准固态钠离子电池
收稿日期: 2024-02-04
修回日期: 2024-04-02
网络出版日期: 2024-04-19
基金资助
国家重点研发计划(2022YFB4101600);国家重点研发计划(2022YFB4101605);国家自然科学基金(52372175);大连市科技创新基金(2023JJ12GX020);中央高校基本科研业务费(DUT22LAB125)
Development of Quasi-solid-state Na-ion Battery Based on Water-minimal Prussian Blue Cathode
Received date: 2024-02-04
Revised date: 2024-04-02
Online published: 2024-04-19
Supported by
National Key R&D Program of China(2022YFB4101600);National Key R&D Program of China(2022YFB4101605);National Natural Science Foundation of China(52372175);Innovation and Technology Fund of Dalian(2023JJ12GX020);Fundamental Research Funds for the Central Universities(DUT22LAB125)
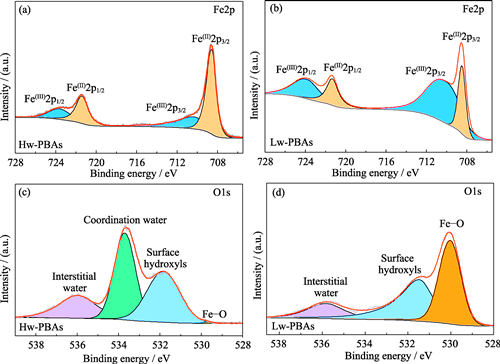
与锂离子电池相比, 钠离子电池具有成本低、低温性能与安全性更佳等优势, 在成本与可靠性敏感的应用领域备受瞩目。高容量、低成本的普鲁士蓝类材料(PBAs)是极具前景的钠离子电池正极材料, 但结构中存在的结晶水导致电池性能快速衰减, 是限制其应用的瓶颈。本研究提出了一种简便易行的热处理策略, 以高效脱除PBAs正极材料中的结晶水, 340次循环后的容量保持率由73%提升到88%。利用原位技术揭示了PBAs正极在充放电过程中, 其晶体结构由三方向立方发生不可逆转变是造成首次库仑效率损失的机制, 并针对性地提出在正极中添加Na2C2O4钠补偿剂可以解决这一问题。在此基础上, 采用高离子电导率、高电化学稳定性的聚乙二醇二丙烯酸酯(PEGDA)准固态电解质, 匹配添加Na2C2O4钠补偿剂的低含水量PBAs正极与硬碳(HC)负极, 构建了高性能准固态钠离子电池。此类电池在20~500 mA·g-1电流密度下的比容量为58~105 mAh·g-1, 并可稳定循环超过200次。研究表明高效脱除结晶水, 可以显著提高PBAs正极的稳定性与比容量。
王琨鹏 , 刘兆林 , 林存生 , 王治宇 . 基于低含水量普鲁士蓝正极的准固态钠离子电池[J]. 无机材料学报, 2024 , 39(9) : 1005 -1012 . DOI: 10.15541/jim20240063
In comparison to Li-ion batteries, Na-ion batteries offer the benefits of low cost, good low-temperature performance, and safety, attracting great attention in the cost- and reliability-sensitive applications. With high capacity and low cost, Prussian blue-like materials (PBAs) stand as promising cathode materials for Na-ion batteries. However, the presence of crystalline water within their structure induces fast performance decay of the battery, serving as a critical bottleneck limiting their application. This work reports a facile thermal treatment strategy to effectively remove crystalline water from PBAs cathode materials, improving capacity retention from 73% to 88% after 340 cycles. The in-situ analysis uncovers that the initial loss of Coulombic efficiency of PBAs cathode is a result of its irreversible transformation from a trigonal form to cubic phase during the charging and discharging process. This issue can be addressed by introducing of Na2C2O4 to compensate the irreversible Na loss in the cathode. On this basis, a high-performance quasi-solid-state Na-ion battery is built by pairing a low-water-content PBAs cathode with Na2C2O4 additive and a hard carbon (HC) anode within a poly(ethylene glycol) diacrylate (PEGDA)-based quasi-solid-state electrolyte with high ionic conductivity and electrochemical stability. This battery exhibits the specific capacities ranging from 58 to 105 mAh·g-1 at current densities from 20 to 500 mA·g-1, capable of sustaining stable cycling for over 200 cycles. This study underscores the significant improvement in stability and capacity of PBAs cathode materials by the efficient removal of crystalline water in them.

| [1] | WANG W L, GANG Y, PENG J, et al. Effect of eliminating water in Prussian blue cathode for sodium-ion batteries. Adv. Funct. Mater., 2022, 32(25): 2111727. |
| [2] | MENG X Y, LIU Y Z, WANG Z Y, et al. A quasi-solid-state rechargeable cell with high energy and superior safety enabled by stable redox chemistry of Li2S in gel electrolyte. Energy Environ. Sci., 2021, 14(4): 2278. |
| [3] | CHE H Y, CHEN S L, XIE Y Y, et al. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ. Sci., 2017, 10(5): 1075. |
| [4] | LI W K, ZHAO N, BI Z J, et al. Na3Zr2Si2PO12 ceramic electrolytes for Na-ion battery: preparation using spray-drying method and its property. J. Inorg. Mater., 2022, 37(2): 189. |
| [5] | LI D, LEI C, LAI H, et al. Recent advancements in interface between cathode and garnet solid electrolyte for all solid state Li-ion batteries. J. Inorg. Mater., 2019, 34(7): 694. |
| [6] | KIM K J, BALAISH M, WADAGUCHI M, et al. Solid-state Li-metal batteries: challenges and horizons of oxide and sulfide solid electrolytes and their interfaces. Adv. Energy Mater., 2021, 11(1): 2002689. |
| [7] | GAO H, GUO B, SONG J, et al. A composite gel-polymer/glass- fiber electrolyte for sodium-ion batteries. Adv. Energy Mater., 2015, 5(9): 1402235. |
| [8] | LIU Y Z, MENG X Y, SHI Y, et al. Long-life quasi-solid-state anode-free batteries enabled by Li compensation coupled interface engineering. Adv. Mater., 2023, 35(42): e2305386. |
| [9] | DU G Y, TAO M L, LI J, et al. Low-operating temperature, high- rate and durable solid-state sodium-ion battery based on polymer electrolyte and Prussian blue cathode. Adv. Energy Mater., 2020, 10(5): 1903351. |
| [10] | PENG J, ZHANG W, LIU Q N, et al. Prussian blue analogues for sodium-ion batteries: past, present, and future. Adv. Mater., 2022, 34(15): 2108384. |
| [11] | LU Y H, WANG L, CHENG J G, et al. Prussian blue: a new framework of electrode materials for sodium batteries. Chem. Commun., 2012, 48(52): 6544. |
| [12] | S?NGELAND C, MOGENSEN R, BRANDELL D, et al. Stable cycling of sodium metal all-solid-state batteries with polycarbonate- based polymer electrolytes. ACS Appl. Poly. Mater., 2019, 1(4): 825. |
| [13] | KIM T, AHN S H, SONG Y Y, et al. Prussian blue-type sodium-ion conducting solid electrolytes for all solid-state batteries. Angew. Chem. Int. Ed., 2023, 62(42): e202309852. |
| [14] | SONG J, WANG L, LU Y H, et al. Removal of interstitial H2O in hexacyanometallates for a superior cathode of a sodium-ion battery. J. Am. Chem. Soc., 2015, 137(7): 2658. |
| [15] | LIU Y, FAN S, GAO Y, et al. Isostructural synthesis of iron-based Prussian blue analogs for sodium-ion batteries. Small, 2023, 19(43): e2302687. |
| [16] | WANG W, GANG Y, HU Z, et al. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat. Commun., 2020, 11: 980. |
| [17] | YOU Y, YU X Q, YIN Y X, et al. Sodium iron hexacyanoferrate with high Na content as a Na-rich cathode material for Na-ion batteries. Nano Res., 2014, 8(1): 117. |
| [18] | REN W H, QIN M S, ZHU Z X, et al. Activation of sodium storage sites in Prussian blue analogues via surface etching. Nano Lett., 2017, 17(8): 4713. |
| [19] | ZHANG H, GAO Y, PENG J, et al. Prussian blue analogues with optimized crystal plane orientation and low crystal defects toward 450 Wh·kg-1 alkali-ion batteries. Angew. Chem. Int. Ed., 2023, 62(27): e202303953. |
| [20] | ZHANG Z H, AVDEEV M, CHEN H C, et al. Lithiated Prussian blue analogues as positive electrode active materials for stable non-aqueous lithium-ion batteries. Nat. Commun., 2022, 13: 7790. |
| [21] | JIANG M, HOU Z, MA H, et al. Resolving deactivation of low-spin Fe sites by redistributing electron density toward high- energy sodium storage. Nano Lett., 2023, 23(22): 10423. |
| [22] | TANG Z, ZHANG R, WANG H Y, et al. Revealing the closed pore formation of waste wood-derived hard carbon for advanced sodium-ion battery. Nat. Commun., 2023, 14: 6024. |
| [23] | NIU Y B, GUO Y J, YIN Y X, et al. High-efficiency cathode sodium compensation for sodium-ion batteries. Adv. Mater., 2020, 32(33): e2001419. |
/
| 〈 |
|
〉 |