

CuO/ZnO复合电催化剂的制备及其还原CO2制合成气
收稿日期: 2021-02-13
修回日期: 2021-03-30
网络出版日期: 2021-03-15
基金资助
国家自然科学基金(51961165107);上海国际合作项目(19520761000);上海市自然科学基金(19ZR1464500)
CuO/ZnO Composite Electrocatalyst: Preparation and Reduction of CO2 to Syngas
Received date: 2021-02-13
Revised date: 2021-03-30
Online published: 2021-03-15
Supported by
National Natural Science Foundation of China(51961165107);Shanghai International Cooperation Project(19520761000);Shanghai Natural Science Foundation(19ZR1464500)
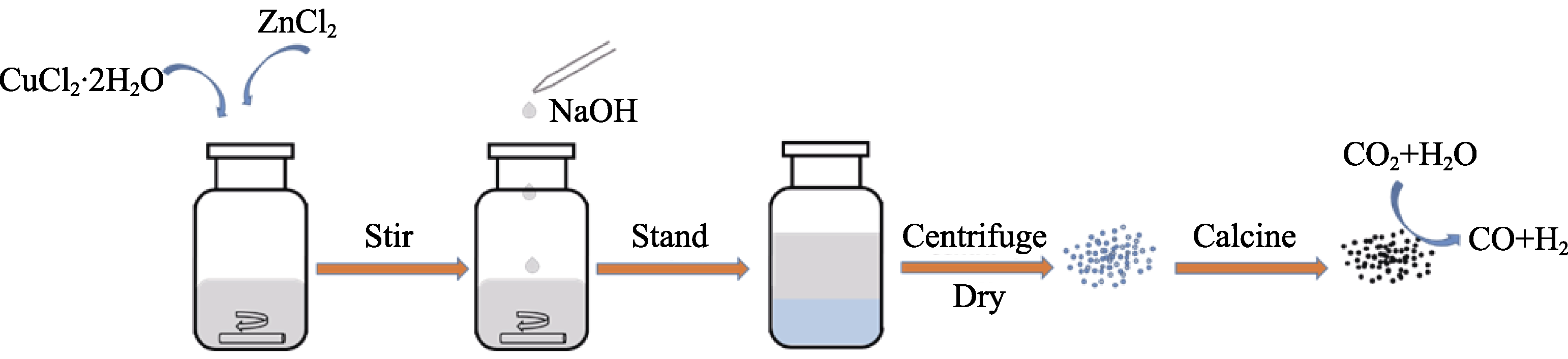
二氧化碳(CO2)还原制备合成气(CO和H2混合气), 不仅可以实现碳循环降低温室效应, 而且能缓解能源危机。而实现CO2资源化利用的关键在于催化剂设计。本研究采用金属离子共沉淀法制备了CuO及CuO/ZnO复合氧化物纳米材料, 通过调节催化剂组分, 探究其在不同电势下电化学CO2还原制备合成气的性能。结果表明: 引入锌(Zn)物种可以减弱中间物CO2•-在催化剂上的吸附强度, 导致CO的法拉第效率(FE)降低, 氢气FE增加, 从而实现不同电势下合成气CO/H2在1/1~1/4范围内的可控调节。尤其是, 当前驱液中铜和锌配比为1 : 2时, 在-0.9 V (vs. RHE)的电势下, CO和H2总FE高达84%。
张清明 , 朱敏 , 周晓霞 . CuO/ZnO复合电催化剂的制备及其还原CO2制合成气[J]. 无机材料学报, 2021 , 36(11) : 1145 -1153 . DOI: 10.15541/jim20210092
The reduction of carbon dioxide (CO2) to syngas (a mixture of CO and H2) can not only realize the carbon cycle and decrease the greenhouse effect but also alleviate the energy crisis. Design of catalyst is the key to realize CO2 resource utilization. Herein, CuO and CuO/ZnO composite were prepared by metal ion co-precipitation method, and their performance of electrochemical CO2 reduction to syngas under different potentials was investigated by adjusting the catalyst components. The results show that the introduction of zinc (Zn) species can decrease the adsorption intensity of intermediate CO2•- on the catalyst, which leads to the decrease of Faraday efficiency (FE) of CO and the increase of FE of H2, thus achieving controllable regulation of CO/H2 in the range of 1/1-1/4 under different applied electrochemical potential. In particular, the total FE of syngas CO/H2 is up to 84% when the ratio of Cu to Zn in the precursor solution is 1 : 2.

Key words: CO2 reduction; syngas; CuO/ZnO; intermediate CO2•-
| [1] | CHANG X X, WANG T, YANG P P, et al. The development of cocatalysts for photoelectrochemical CO2 reduction. Adv. Mater., 2019, 31(31):1804710. |
| [2] | WU J H, HUANG Y, YE W, et al. CO2 reduction: from the electrochemical to photochemical approach. Adv. Sci., 2017, 4(11):1700194. |
| [3] | LI X, YU J G, JARONIEC K, et al. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem. Rev., 2019, 119(6):3962-4179. |
| [4] | REN Y J, ZENG D Q, ONG W. Interfacial engineering of graphitic carbon nitride (g-C3N4)-based metal sulfide heterojunction photocatalysts for energy conversion: a review. Chinese Journal of Catalysis, 2019, 40(3):289-319. |
| [5] | MA S C, LIU J F, SASAKI K, et al. Carbon foam decorated with silver nanoparticles for electrochemical CO2 conversion. Energy Technology, 2017, 5(6):861-863. |
| [6] | WU M F, ZHU C, WANG K, et al. Promotion of CO2 electrochemical reduction via Cu nanodendrites. ACS Appl. Mater. Interfaces, 2020, 12(10):11562-11569. |
| [7] | CHU S, FAN S Z, WANG Y J, et al. Tunable syngas production from CO2 and H2O in an aqueous photoelectrochemical cell. Angew. Chem. Int. Ed., 2016, 55(46):14262-14266. |
| [8] | CHU S, OU P F, RASHID R T, et al. Decoupling strategy for enhanced syngas generation from photoelectrochemical CO2 reduction. iScience, 2020, 23(8):101390. |
| [9] | CHU S, OU P F, GHAMARI P, et al. Photoelectrochemical CO2 reduction into syngas with the metal/oxide interface. J. Am. Chem. Soc., 2018, 140(25):7869-7877. |
| [10] | WOLDU A R. From low to high-index facets of noble metal nanocrystals: a way forward to enhance the performance of electrochemical CO2 reduction. Nanoscale, 2020, 12(16):8626-8635. |
| [11] | TANG J, CHEN D, YAO Q F, et al. Recent advances in noble metal- based nanocomposites for electrochemical reactions. Materials Today Energy, 2017, 6:115-127. |
| [12] | LI C Q, BAEK J B. Recent advances in noble metal (Pt, Ru, and Ir)-based electrocatalysts for efficient hydrogen evolution reaction. ACS Omega, 2020, 5(1):31-40. |
| [13] | YANG Z N, OROPEZA F E, ZHANG K H L. P-block metal-based (Sn, In, Bi, Pb) electrocatalysts for selective reduction of CO2 to formate. APL Materials, 2020, 8(6):060901. |
| [14] | PÉREZ-RODRíGUEZ S, PASTOR E, LÁZARO M J. Noble metal- free catalysts supported on carbon for CO2 electrochemical reduction. Journal of CO2 Utilization, 2017, 18:41-52. |
| [15] | BELL T E, TORRENTE-MURCIANO L. H2 production via ammonia decomposition using non-noble metal catalysts: a review. Topics in Catalysis, 2016, 59(15):1438-1457. |
| [16] | WHITE L J, BARUCH M, PANDER J, et al. Light-driven heterogeneous reduction of carbon dioxide: photocatalysts and photoelectrodes. Chem. Rev., 2015, 115(23):12888-12935 |
| [17] | REN D, FONG J H, YEO B S. The effects of currents and potentials on the selectivities of copper toward carbon dioxide electroreduction. Nat. Commun., 2018, 9(1):925. |
| [18] | DAIYAN R, CHEN R, KUMAR P, et al. Tunable syngas production through CO2 electroreduction on cobalt-carbon composite electrocatalyst. ACS Appl. Mater. Interfaces, 2020, 12(8):9307-9315. |
| [19] | SASTRE F, MUÑOZ-BATISTA M J, KUBACKA A, et al. Efficient electrochemical production of syngas from CO2 and H2O by using a nanostructured Ag/g-C3N4 catalyst. ChemElectroChem, 2016, 3(9):1497-1502. |
| [20] | MA W C, XIE M C, XIE S J, et al. Nickel and indium core-shell co-catalysts loaded silicon nanowire arrays for efficient photoelectrocatalytic reduction of CO2 to formate. Journal of Energy Chemistry, 2021, 54:422-428. |
| [21] | WANG Y H, LIU J L, WANG Y F, et al. Efficient solar-driven electrocatalytic CO2 reduction in a redox-medium-assisted system. Nat. Commun., 2018, 9(1):5003. |
| [22] | ZHAO S K, SHEN Y B, HAO F L, et al. p-n junctions based on CuO-decorated ZnO nanowires for ethanol sensing application. Applied Surface Science, 2021, 538:148140. |
| [23] | XIE Y, XING R Q, LI Q Q, et al. Three-dimensional ordered ZnO- CuO inverse opals toward low concentration acetone detection for exhaled breath sensing. Sensors and Actuators B: Chemical, 2015, 211:255-262. |
| [24] | YE H C, NA W, GAO W G, et al. Carbon-modified CuO/ZnO catalyst with high oxygen vacancy for CO2 hydrogenation to methanol. Energy Technology, 2020, 8(6):2000194. |
| [25] | ZHU L Y, LI H, LIU Z R, et al. Synthesis of the 0D/3D CuO/ZnO heterojunction with enhanced photocatalytic activity. The Journal of Physical Chemistry C, 2018, 122(17):9531-9539. |
| [26] | WANG J J, WANG G J, ZHANG J F, et al. Inversely tuning the CO2 electroreduction and hydrogen evolution activity on metal oxide via heteroatom doping. Angew. Chem. Int. Ed., 2021, 60(14):7602-7606. |
| [27] | CHEN Z, FAN T T, ZHANG Y Q, et al. Wavy SnO2 catalyzed simultaneous reinforcement of carbon dioxide adsorption and activation towards electrochemical conversion of CO2 to HCOOH. Applied Catalysis B: Environmental, 2020, 261:118243. |
| [28] | MOHAMMSDI A R, HABIBI Y A, BAYRAMI CHEN Z, et al. Enhanced anti-bacterial activities of ZnO nanoparticles and ZnO/ CuO nanocomposites synthesized using vaccinium arctostaphylos L. fruit extract. Artif. Cells Nanomed. Biotechnol., 2018, 46:1200-1209. |
| [29] | CHEN F, ZHANG P P, XIAO L W, et al. Structure-performance correlations over Cu/ZnO interface for low-temperature methanol synthesis from syngas containing CO2. ACS Applied Mater Interfaces, 2021, 13(7):8191-8205. |
/
| 〈 |
|
〉 |