无机材料学报 ›› 2024, Vol. 39 ›› Issue (11): 1235-1244.DOI: 10.15541/jim20240158 CSTR: 32189.14.10.15541/jim20240158
所属专题: 【材料计算】材料模拟计算(202506); 【结构材料】陶瓷基复合材料(202506)
收稿日期:2024-04-01
修回日期:2024-06-21
出版日期:2024-11-20
网络出版日期:2024-06-24
通讯作者:
关 康, 副教授. E-mail: mskguan@scut.edu.cn;作者简介:马永杰(1999-), 男, 硕士研究生. E-mail: mayongjie@mail.nwpu.edu.cn
基金资助:
MA Yongjie1( ), LIU Yongsheng1, GUAN Kang2(
), LIU Yongsheng1, GUAN Kang2( ), ZENG Qingfeng3(
), ZENG Qingfeng3( )
)
Received:2024-04-01
Revised:2024-06-21
Published:2024-11-20
Online:2024-06-24
Contact:
GUAN Kang, associate professor. E-mail: mskguan@scut.edu.cn;About author:MA Yongjie (1999-), male, Master candidate. E-mail: mayongjie@mail.nwpu.edu.cn
Supported by:摘要:
通过化学气相渗透工艺, 利用以CH4和C2H5OH为前驱体制备碳/碳复合材料, 可以提高沉积速率且易得到高织构的热解炭。探究其反应机制可以更好地用于计算流体力学(CFD)研究。化学反应机制往往包含大量自由基和反应, 而以实验为主手动构建反应机制很容易遗漏重要物质和反应。本研究利用反应机制生成器(RMG)构建了CH4+C2H5OH+Ar体系详细的气相热解动力学机制, 其涵盖31种核心物质和214个核心反应, 预测了主要物质形成和消耗的趋势, 模拟结果与实验结果趋势相吻合。通过详细的动力学机制研究和反应物以及部分重要产物灵敏度分析, 识别了影响关键物质生成和消耗的反应。反应路径分析揭示了详细机制中不同物质之间的关系, 并确定了机制中的核心物质。在温度1373 K、压力10 kPa条件下, 依据灵敏度分析和路径分析的结果对详细机制进行了简化, 得到包含18种物质和44个反应的气相简化动力学机制。该简化机制在保留关键物质的同时显著提高了计算效率, 为进一步CFD研究和应用提供了更为便利的基础。
中图分类号:
马永杰, 刘永胜, 关康, 曾庆丰. CH4+C2H5OH+Ar体系热解的气相动力学研究[J]. 无机材料学报, 2024, 39(11): 1235-1244.
MA Yongjie, LIU Yongsheng, GUAN Kang, ZENG Qingfeng. Gas-phase Kinetic Study of Pyrolysis in the System of CH4+C2H5OH+Ar[J]. Journal of Inorganic Materials, 2024, 39(11): 1235-1244.
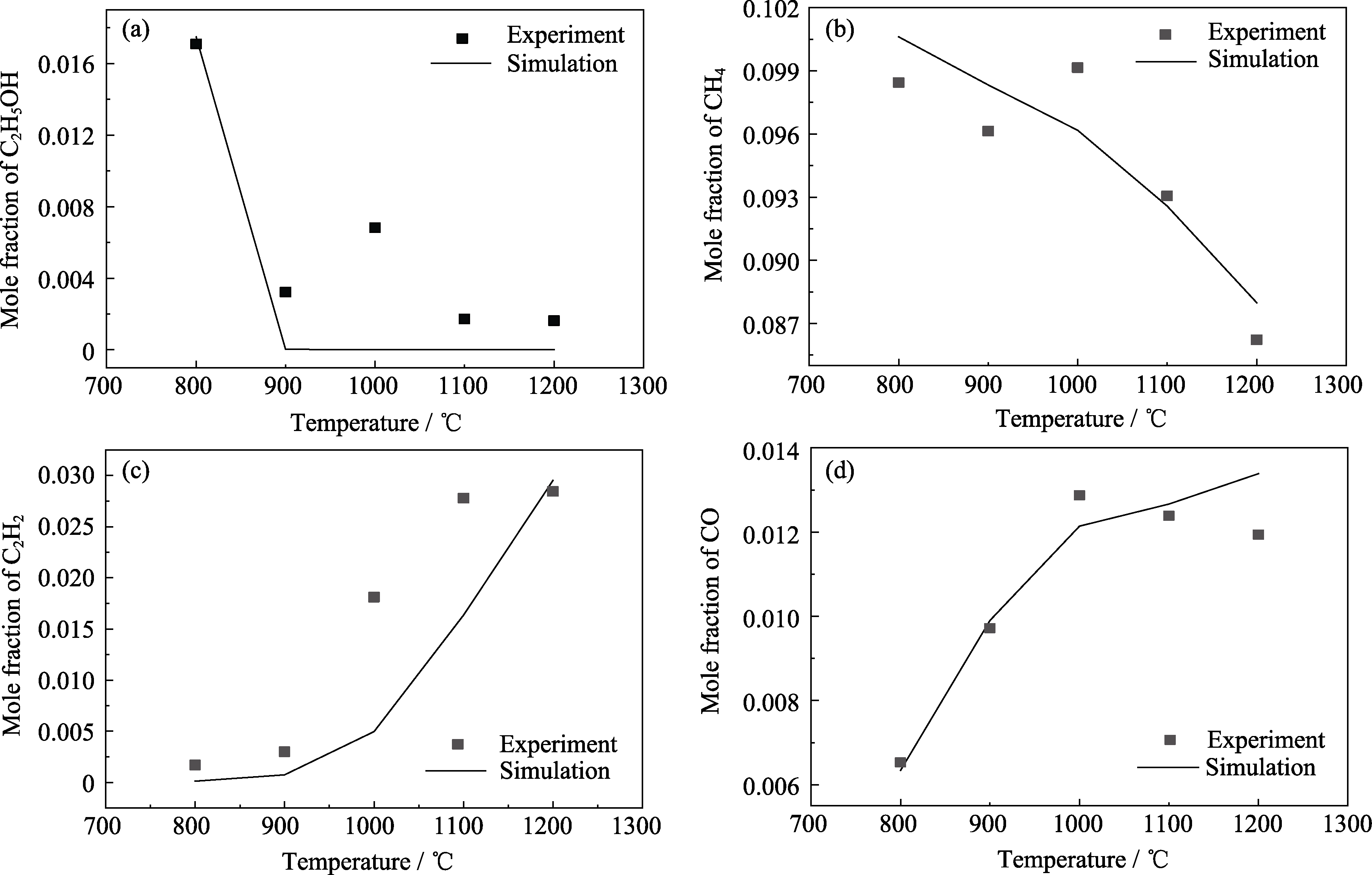
图1 CH4+C2H5OH+Ar体系热解实验与模拟对比
Fig. 1 Comparison of pyrolysis experimental data with simulations of CH4+C2H5OH+Ar system (a) C2H5OH; (b) CH4; (c) C2H2; (d) CO
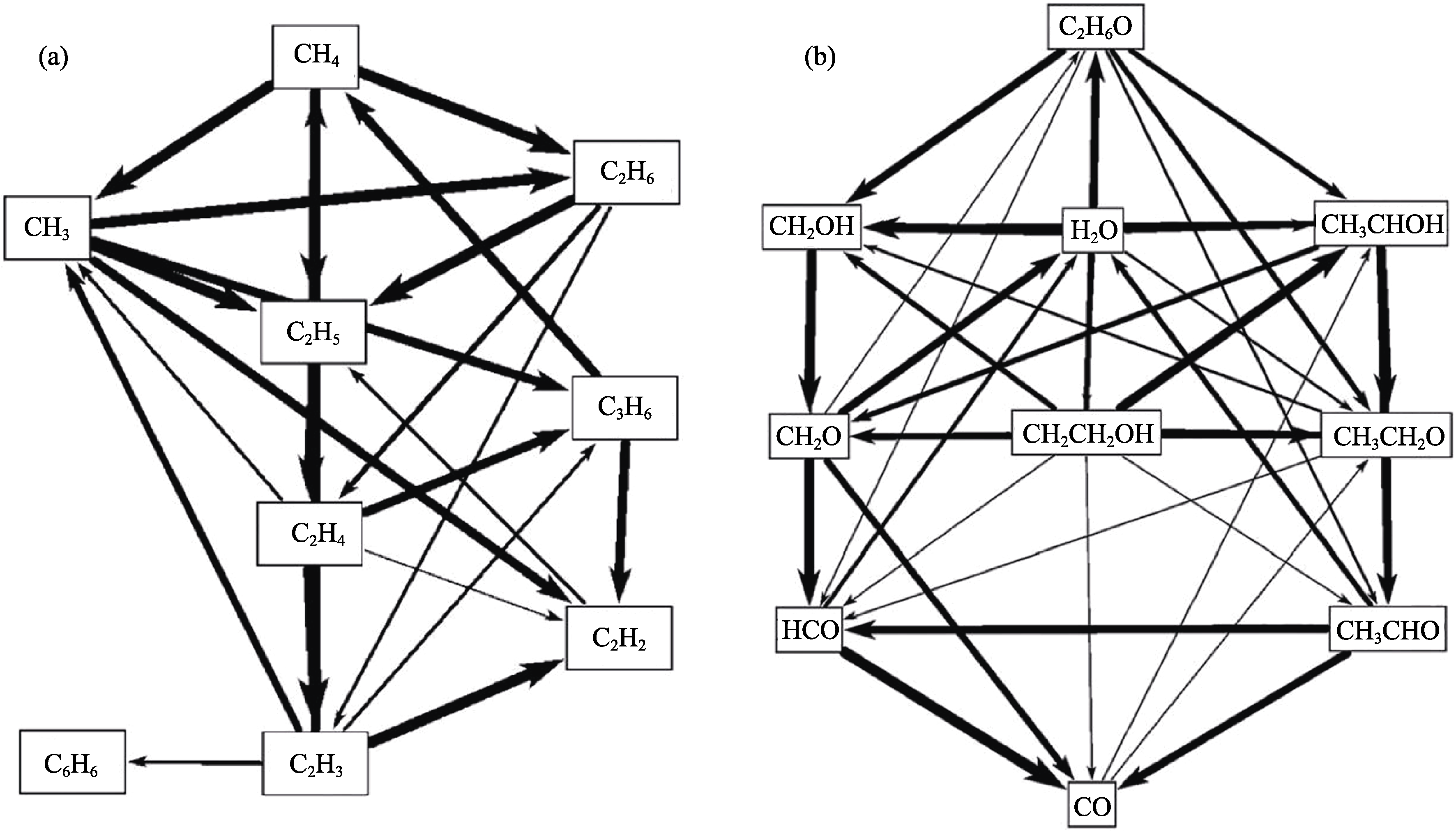
图6 CH4+C2H5OH+Ar 混合物热解路径图
Fig. 6 Thermal decomposition path diagrams of CH4+C2H5OH+Ar mixture (a) CH4 pyrolysis pathway diagram; (b) C2H5OH pyrolysis pathway diagram
| No. | Reaction | *A | n | E/(kJ·mol-1) |
|---|---|---|---|---|
| 1 | C2H4+M<=>H+C2H3+M | 3.98×1017 | 0 | 411.144 |
| 2 | H+C2H4<=>H2+C2H3 | 1.32×107 | 2.5 | 47.168 |
| 3 | H+H+M<=>H2+M | 1.0 | 0 | 0 |
| 4 | CH4+M<=>H+CH3+M | 1.0×1017 | 0 | 359.232 |
| 5 | CH3+C2H4<=>CH4+C2H3 | 5.0×1012 | 0 | 54.428 |
| 6 | H2+CH3<=>H+CH4 | 2.75×104 | 2.5 | 39.435 |
| 7 | 2CH3+M<=>C2H6+M | 3.08×1041 | -7.0 | 11.568 |
| 8 | CH3+CH4<=>H+C2H6 | 8.0×1013 | 2.0 | 167.472 |
| 9 | C2H5<=>H+C2H4 | 2.04×1015 | 0 | 144.713 |
| 10 | C2H6<=>H+C2H5 | 2.08×1038 | -7.1 | 445.928 |
| 11 | 2CH3<=>H+C2H5 | 3.01×1013 | 0 | 56.576 |
| 12 | CH3+CH4<=>H2+C2H5 | 1.0×1013 | 0 | 96.296 |
| 13 | H+C2H5<=>H2+C2H4 | 2.0×1012 | 0 | 0 |
| 14 | 2C2H4<=>C2H3+C2H5 | 1.82×1014 | 0 | 270.183 |
| 15 | C2H3+C2H6<=>C2H4+C2H5 | 1.08×10-3 | 4.5 | 14.653 |
| 16 | H+C2H6<=>H2+C2H5 | 5.4×102 | 3.5 | 21.813 |
| 17 | CH3+C2H6<=>CH4+C2H5 | 5.5×10-1 | 4.0 | 37.757 |
| 18 | H+C3H6<=>CH3+C2H4 | 3.4×1013 | 0 | 14.650 |
| 19 | C3H6<=>CH3+C2H3 | 2.5×1014 | 0 | 417.378 |
| 20 | C2H4+M<=>H2+C2H2+M | 8.0×1012 | 0.4 | 371.641 |
| 21 | H+C2H3<=>H2+C2H2 | 3.0×1013 | 0 | 0 |
| 22 | C3H6<=>CH4+C2H2 | 1.8×1012 | 0 | 293.076 |
| 23 | C2H3+M<=>H+C2H2+M | 7.94×1014 | 0 | 130.088 |
| 24 | 2H+H2<=>2H2 | 9.0×1016 | -0.6 | 0 |
| 25 | CH3+C2H3<=>CH4+C2H2 | 2.0×1013 | 0 | 0 |
| 26 | 2H+H2O<=>H2+H2O | 6.0×1019 | -1.25 | 0 |
| 27 | H2O+C2H4(+M)<=>C2H5OH(+M) | 1.0×100 | 0 | 0 |
| 28 | 2C2H5<=>C2H4+C2H6 | 6.9×1013 | -0.35 | 0 |
| 29 | 2C2H3<=>C2H2+C2H4 | 2.9597×1013 | -0.312 | 0 |
| 30 | CH3+C2H5<=>CH4+C2H4 | 6.57×1014 | -0.68 | 0 |
| 31 | C2H3+C2H5<=>C2H2+C2H6 | 2.1064×1013 | -0.251 | 0 |
| 32 | CH3+CH2OH(+M)<=>C2H5OH(+M) | 1.0 | 0 | 0 |
| 33 | CH3+CH2OH(+M)<=>H2O+C2H4(+M) | 1.0 | 0 | 0 |
| 34 | C2H5OH(+M)<=>H2+CH3CHO(+M) | 7.24×1011 | 0.095 | 381.028 |
| 35 | HCO+CH3(+M) <=>CH3CHO(+M) | 1.0 | 0 | 0 |
| 36 | H+HCO<=>H2+CO | 7.34×1013 | 0 | 0 |
| 37 | HCO+CH3<=>CO+CH4 | 2.648×1013 | 0 | 0 |
| 38 | H2O+HCO<=>H+H2O+CO | 1.5×1018 | -1.0 | 71.176 |
| 39 | H+CH3CHO<=>H2+CO+CH3 | 2.05×109 | 1.16 | 10.069 |
| 40 | CH3+CH3CHO<=>CO+CH3+CH4 | 2.72×106 | 1.77 | 24.786 |
| 41 | HCO+M<=>CO+H+M | 1.87×1017 | -1.0 | 71.176 |
| 42 | CH3CHO(+M)<=>CO+CH4(+M) | 1.0 | 0 | 0 |
| 43 | HCO+C2H3<=>CO+C2H4 | 9.033×1013 | 0 | 0 |
| 44 | HCO+C2H5<=>CO+C2H6 | 4.3×1013 | 0 | 0 |
表S1 CH4+C2H5OH+Ar体系热解的简化反应机制
Table S1 Simplified pyrolysis mechanism of CH4+C2H5OH+Ar system
| No. | Reaction | *A | n | E/(kJ·mol-1) |
|---|---|---|---|---|
| 1 | C2H4+M<=>H+C2H3+M | 3.98×1017 | 0 | 411.144 |
| 2 | H+C2H4<=>H2+C2H3 | 1.32×107 | 2.5 | 47.168 |
| 3 | H+H+M<=>H2+M | 1.0 | 0 | 0 |
| 4 | CH4+M<=>H+CH3+M | 1.0×1017 | 0 | 359.232 |
| 5 | CH3+C2H4<=>CH4+C2H3 | 5.0×1012 | 0 | 54.428 |
| 6 | H2+CH3<=>H+CH4 | 2.75×104 | 2.5 | 39.435 |
| 7 | 2CH3+M<=>C2H6+M | 3.08×1041 | -7.0 | 11.568 |
| 8 | CH3+CH4<=>H+C2H6 | 8.0×1013 | 2.0 | 167.472 |
| 9 | C2H5<=>H+C2H4 | 2.04×1015 | 0 | 144.713 |
| 10 | C2H6<=>H+C2H5 | 2.08×1038 | -7.1 | 445.928 |
| 11 | 2CH3<=>H+C2H5 | 3.01×1013 | 0 | 56.576 |
| 12 | CH3+CH4<=>H2+C2H5 | 1.0×1013 | 0 | 96.296 |
| 13 | H+C2H5<=>H2+C2H4 | 2.0×1012 | 0 | 0 |
| 14 | 2C2H4<=>C2H3+C2H5 | 1.82×1014 | 0 | 270.183 |
| 15 | C2H3+C2H6<=>C2H4+C2H5 | 1.08×10-3 | 4.5 | 14.653 |
| 16 | H+C2H6<=>H2+C2H5 | 5.4×102 | 3.5 | 21.813 |
| 17 | CH3+C2H6<=>CH4+C2H5 | 5.5×10-1 | 4.0 | 37.757 |
| 18 | H+C3H6<=>CH3+C2H4 | 3.4×1013 | 0 | 14.650 |
| 19 | C3H6<=>CH3+C2H3 | 2.5×1014 | 0 | 417.378 |
| 20 | C2H4+M<=>H2+C2H2+M | 8.0×1012 | 0.4 | 371.641 |
| 21 | H+C2H3<=>H2+C2H2 | 3.0×1013 | 0 | 0 |
| 22 | C3H6<=>CH4+C2H2 | 1.8×1012 | 0 | 293.076 |
| 23 | C2H3+M<=>H+C2H2+M | 7.94×1014 | 0 | 130.088 |
| 24 | 2H+H2<=>2H2 | 9.0×1016 | -0.6 | 0 |
| 25 | CH3+C2H3<=>CH4+C2H2 | 2.0×1013 | 0 | 0 |
| 26 | 2H+H2O<=>H2+H2O | 6.0×1019 | -1.25 | 0 |
| 27 | H2O+C2H4(+M)<=>C2H5OH(+M) | 1.0×100 | 0 | 0 |
| 28 | 2C2H5<=>C2H4+C2H6 | 6.9×1013 | -0.35 | 0 |
| 29 | 2C2H3<=>C2H2+C2H4 | 2.9597×1013 | -0.312 | 0 |
| 30 | CH3+C2H5<=>CH4+C2H4 | 6.57×1014 | -0.68 | 0 |
| 31 | C2H3+C2H5<=>C2H2+C2H6 | 2.1064×1013 | -0.251 | 0 |
| 32 | CH3+CH2OH(+M)<=>C2H5OH(+M) | 1.0 | 0 | 0 |
| 33 | CH3+CH2OH(+M)<=>H2O+C2H4(+M) | 1.0 | 0 | 0 |
| 34 | C2H5OH(+M)<=>H2+CH3CHO(+M) | 7.24×1011 | 0.095 | 381.028 |
| 35 | HCO+CH3(+M) <=>CH3CHO(+M) | 1.0 | 0 | 0 |
| 36 | H+HCO<=>H2+CO | 7.34×1013 | 0 | 0 |
| 37 | HCO+CH3<=>CO+CH4 | 2.648×1013 | 0 | 0 |
| 38 | H2O+HCO<=>H+H2O+CO | 1.5×1018 | -1.0 | 71.176 |
| 39 | H+CH3CHO<=>H2+CO+CH3 | 2.05×109 | 1.16 | 10.069 |
| 40 | CH3+CH3CHO<=>CO+CH3+CH4 | 2.72×106 | 1.77 | 24.786 |
| 41 | HCO+M<=>CO+H+M | 1.87×1017 | -1.0 | 71.176 |
| 42 | CH3CHO(+M)<=>CO+CH4(+M) | 1.0 | 0 | 0 |
| 43 | HCO+C2H3<=>CO+C2H4 | 9.033×1013 | 0 | 0 |
| 44 | HCO+C2H5<=>CO+C2H6 | 4.3×1013 | 0 | 0 |

图S2 含氧物质的绝对产率
Fig. S2 Absolute rate of production of oxygen-containing substances (a) C2H5OH; (b) CH2OH; (c) CH2O; (d) H2O; (e) CH3CHO; (f) HCO; (g) CO
| [1] | CHEN S, QIU X, ZHANG B, et al. Advances in antioxidation coating materials for carbon/carbon composites. Journal of Alloys and Compounds, 2021, 886: 161143. |
| [2] | JIN X, FAN X, LU C, et al. Advances in oxidation and ablation resistance of high and ultra-high temperature ceramics modified or coated carbon/carbon composites. Journal of the European Ceramic Society, 2018, 38(1): 1. |
| [3] | FU Q, ZHANG P, ZHUANG L, et al. Micro/nano multiscale reinforcing strategies toward extreme high-temperature applications: take carbon/carbon composites and their coatings as the examples. Journal of Materials Science & Technology, 2022, 96: 31. |
| [4] | BHONG M, KHAN T K H, DEVADE K, et al. Review of composite materials and applications. Materials Today: Proceedings, 2023, DOI: 10.1016/j.matpr.2023.10.026. |
| [5] | MANAWI Y M, IHSANULLAH, SAMARA A, et al. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials, 2018, 11(5): 822. |
| [6] | ZHENG L, WANG Y, QIN J, et al. Scalable manufacturing of carbon nanotubes on continuous carbon fibers surface from chemical vapor deposition. Vacuum, 2018, 152: 84. |
| [7] | SHAH K A, TALI B A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: a review on carbon sources, catalysts and substrates. Materials Science in Semiconductor Processing, 2016, 41: 67. |
| [8] | WEI X, CHENG L, ZHANG L, et al. Numerical simulation of effect of methyltrichlorosilane flux on isothermal chemical vapor infiltration process of C/SiC composites. Journal of the American Ceramic Society, 2006, 89(9): 2762. |
| [9] | LI H, LI A, BAI R, et al. Numerical simulation of chemical vapor infiltration of propylene into C/C composites with reduced multi-step kinetic models. Carbon, 2005, 43(14): 2937 |
| [10] | KIM H G, JI W, KWON H J, et al. Full-scale multi-physics numerical analysis of an isothermal chemical vapor infiltration process for manufacturing C/C composites. Carbon, 2021, 172: 174. |
| [11] | REN B, ZHANG S, HE L, et al. Effect of oxygen and hydrogen on microstructure of pyrolytic carbon deposited from thermal decomposition of methane and ethanol. Journal of Solid State Chemistry, 2018, 261: 86. |
| [12] | REN J, LI K, ZHANG S, et al. Preparation of carbon/carbon composite by pyrolysis of ethanol and methane. Materials & Design, 2015, 65: 174. |
| [13] | LI A, ZHANG S, REZNIK B, et al. Chemistry and kinetics of chemical vapor deposition of pyrolytic carbon from ethanol. Proceedings of the Combustion Institute, 2011, 33(2): 1843. |
| [14] | LI A, ZHANG S, REZNIK B, et al. Synthesis of pyrolytic carbon composites using ethanol as precursor. Industrial & Engineering Chemistry Research, 2010, 49(21): 10421. |
| [15] | MARINOV N M. A detailed chemical kinetic model for high temperature ethanol oxidation. International Journal of Chemical Kinetics, 1999, 31(3): 183. |
| [16] | MINAKOV A V, SIMUNIN M M, RYZHKOV I I. Modelling of ethanol pyrolysis in a commercial CVD reactor for growing carbon layers on alumina substrates. International Journal of Heat and Mass Transfer, 2019, 145: 118764. |
| [17] | HU C, LI H, ZHANG S, et al. A molecular-level analysis of gas-phase reactions in chemical vapor deposition of carbon from methane using a detailed kinetic model. Journal of Materials Science, 2016, 51(8): 38976. |
| [18] | SHINDE V M, PRADEEP P. Detailed gas-phase kinetics and reduced reaction mechanism for methane pyrolysis involved in CVD/CVI processes. Journal of Analytical and Applied Pyrolysis, 2021, 154: 104998. |
| [19] | GAO C W, ALLEN J W, GREEN W H, et al. Reaction mechanism generator: automatic construction of chemical kinetic mechanisms. Computer Physics Communications, 2016, 203: 212. |
| [20] | CURTISS C F, HIRSCHFELDER J O. Integration of stiff equations. Proceedings of the National Academy of Sciences, 1952, 38(3): 235. |
| [21] | BENSON S W, BUSS J H. Additivity rules for the estimation of molecular properties. Thermodynamic properties. Journal of Chemical Physics, 1958, 29(3): 546. |
| [22] | BENSON S W. Thermochemical kinetics:methods for the estimation of thermochemical data and rate parameters. New York: John Wiley and Sons, 1976. |
| [23] | HASHEMI H, CHRISTENSEN J M, GLARBORG P. High- pressure pyrolysis and oxidation of ethanol. Fuel, 2018, 218: 247. |
| [24] | SUSNOW R G, DEAN A M, GREEN W H, et al. Rate-based construction of kinetic models for complex systems. Journal of Physical Chemistry A, 1997, 101(20): 3731. |
| [25] | LIU M, GRINBERG D A, JOHNSON M S, et al. Reaction mechanism generator v3.0: advances in automatic mechanism generation. Journal of Chemical Information and Modeling, 2021, 61(6): 2686. |
| [26] | HUANG Q, CHEN Y, BAO Z, et al. PFR model for high-pressure reaction flow of fuel. Combustion Science and Technology, 2022, 194(11): 2268. |
| [27] | GUPTA A, NIGAM S, SHINDE V M. Gas-phase kinetic of boron carbide chemical vapor deposition using BCl3+ CH4+ H2 mixture. Journal of the American Ceramic Society, 2022, 105(6): 3885. |
| [28] | BRÜGGERT M, HU Z, HÜTTINGER K J. Chemistry and kinetics of chemical vapor deposition of pyrocarbon: VI. influence of temperature using methane as a carbon source. Carbon, 1999, 37(12): 2021. |
| [1] | 刘宇峰, 俸翔, 王金明, 许正辉, 李同起, 焦星剑, 王雅雷, 熊翔. 高性能针刺碳/碳复合材料的制备与性能[J]. 无机材料学报, 2020, 35(10): 1105-1111. |
| [2] | 闫明洋, 杨敏, 李红, 任慕苏, 俞鸣明, 孙晋良. 原位生长的气相生长碳纤维增强C/C复合材料的制备及其弯曲性能[J]. 无机材料学报, 2018, 33(11): 1161-1166. |
| [3] | 郑金煌, 李贺军, 崔红, 王毅, 邓海亮, 殷忠义, 姚冬梅, 苏红. C/C复合材料拉伸强度与针刺成型参数相关性研究[J]. 无机材料学报, 2017, 32(11): 1147-1153. |
| [4] | 张雨雷,李贺军,姚西媛,付前刚,李克智. C/SiC/Si-Mo-Cr复合涂层碳/碳复合材料力学性能研究[J]. 无机材料学报, 2008, 23(4): 725-728. |
| [5] | 张磊磊,李贺军,李克智,李新涛,翟言强,张雨雷. 碳/碳复合材料表面粗糙度对成骨细胞生长行为的影响[J]. 无机材料学报, 2008, 23(2): 341-345. |
| [6] | 孙万昌,李贺军,卢锦花,白瑞成,黄勇. 不同层次界面对C/C复合材料断裂行为的影响[J]. 无机材料学报, 2005, 20(6): 1457-1462. |
| [7] | 李劲,陈振华. 催化型化学液相气化渗透沉积制备碳/碳复合材料工艺研究[J]. 无机材料学报, 2005, 20(6): 1450-1456. |
| [8] | 孙万昌,李贺军,卢锦花,白瑞成,黄勇. 不同层次界面对C/C复合材料断裂行为的影响[J]. 无机材料学报, 2005, 20(6): 1257-1462. |
| [9] | 孙万昌,李贺军,白瑞成,黄勇. 微观组织结构对C/C复合材料力学行为的影响[J]. 无机材料学报, 2005, 20(3): 671-676. |
| [10] | 孙万昌,李贺军,陈三平,张守阳,李克智. CLVI制备C/C复合材料的微观结构及力学性能研究[J]. 无机材料学报, 2003, 18(1): 121-128. |
| [11] | 付涛,憨勇,宋忠孝,李金勇,徐可为. 碳/碳复合材料表面诱导沉积生理磷灰石层[J]. 无机材料学报, 2002, 17(1): 189-192. |
| [12] | 张守阳1,李贺军,李克智,孙军. C/C复合材料层间裂纹扩展研究[J]. 无机材料学报, 2002, 17(1): 91-95. |
| [13] | 孙乐民,李贺军,张守阳. 沥青基碳/碳复合材料的组织特性[J]. 无机材料学报, 2000, 15(6): 1111-1116. |
| [14] | 罗瑞盈,金志浩. 添加剂对快速LARGE CVD抗氧化LARGE C/C复合材料力学性能影响[J]. 无机材料学报, 1997, 12(4): 627-631. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||