无机材料学报 ›› 2023, Vol. 38 ›› Issue (7): 793-799.DOI: 10.15541/jim20220658 CSTR: 32189.14.10.15541/jim20220658
孟波1( ), 肖刚2, 王秀丽1, 涂江平1, 谷长栋1(
), 肖刚2, 王秀丽1, 涂江平1, 谷长栋1( )
)
收稿日期:2022-11-07
修回日期:2022-12-04
出版日期:2022-12-16
网络出版日期:2022-12-27
通讯作者:
谷长栋, 副教授. E-mail: cdgu@zju.edu.cn作者简介:孟 波(1998-), 女, 硕士研究生. E-mail: 22060231@zju.edu.cn
基金资助:
MENG Bo1( ), XIAO Gang2, WANG Xiuli1, TU Jiangping1, GU Changdong1(
), XIAO Gang2, WANG Xiuli1, TU Jiangping1, GU Changdong1( )
)
Received:2022-11-07
Revised:2022-12-04
Published:2022-12-16
Online:2022-12-27
Contact:
GU Changdong, associate professor. E-mail: cdgu@zju.edu.cnAbout author:MENG Bo (1998-), female, Master candidate. E-mail: 22060231@zju.edu.cn
Supported by:摘要:
光热电站需要配备大规模高温储热模块, 金属氧化物可以通过可逆氧化还原反应实现热量的存储与释放。其中锰基氧化物无毒、廉价, 极具潜力, 但可逆性较差。为此, 本研究采用深共溶溶剂离子热合成了锰基氧化物, 探索了合成参数和铁掺杂对其储热性能的影响。离子热合成的MnCO3前驱体在高温下分解释放CO2, 使锰基氧化物具有丰富的孔隙结构, 为氧气的传输与扩散提供通道, 有利于氧化还原反应。离子热合成的Mn2O3比商业Mn2O3反应性能好, 但其氧化反应速度较慢; 合成温度150 ℃、掺杂20% Fe的锰铁氧化物的氧化速率快, 储热密度高达300.66 J/g, 反应可逆性最佳, 可实现长期稳定循环。离子热合成策略可以增加锰氧化物中晶格氧占比, 促进氧空位的迁移, 从而提高可逆性和循环稳定性。
中图分类号:
孟波, 肖刚, 王秀丽, 涂江平, 谷长栋. 离子热合成锰基氧化物及其可逆储热性能[J]. 无机材料学报, 2023, 38(7): 793-799.
MENG Bo, XIAO Gang, WANG Xiuli, TU Jiangping, GU Changdong. Ionic Thermal Synthesis and Reversible Heat Storage Performance of Manganese-based Oxides[J]. Journal of Inorganic Materials, 2023, 38(7): 793-799.
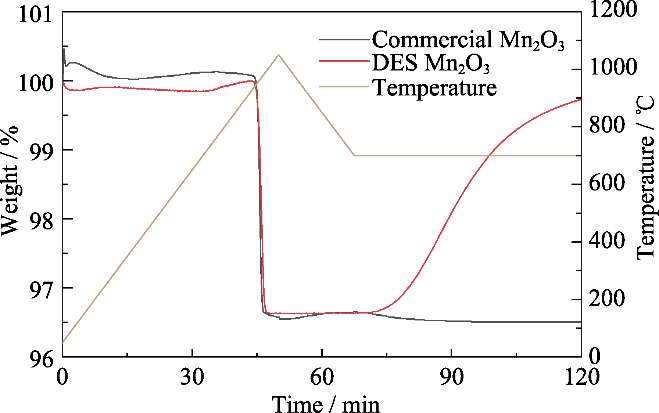
图1 离子热合成Mn2O3与商业Mn2O3氧化还原反应特性
Fig. 1 Redox reaction characteristics of Mn2O3 by ionic thermal synthesis and commercial Mn2O3 Colorful figure is available on website
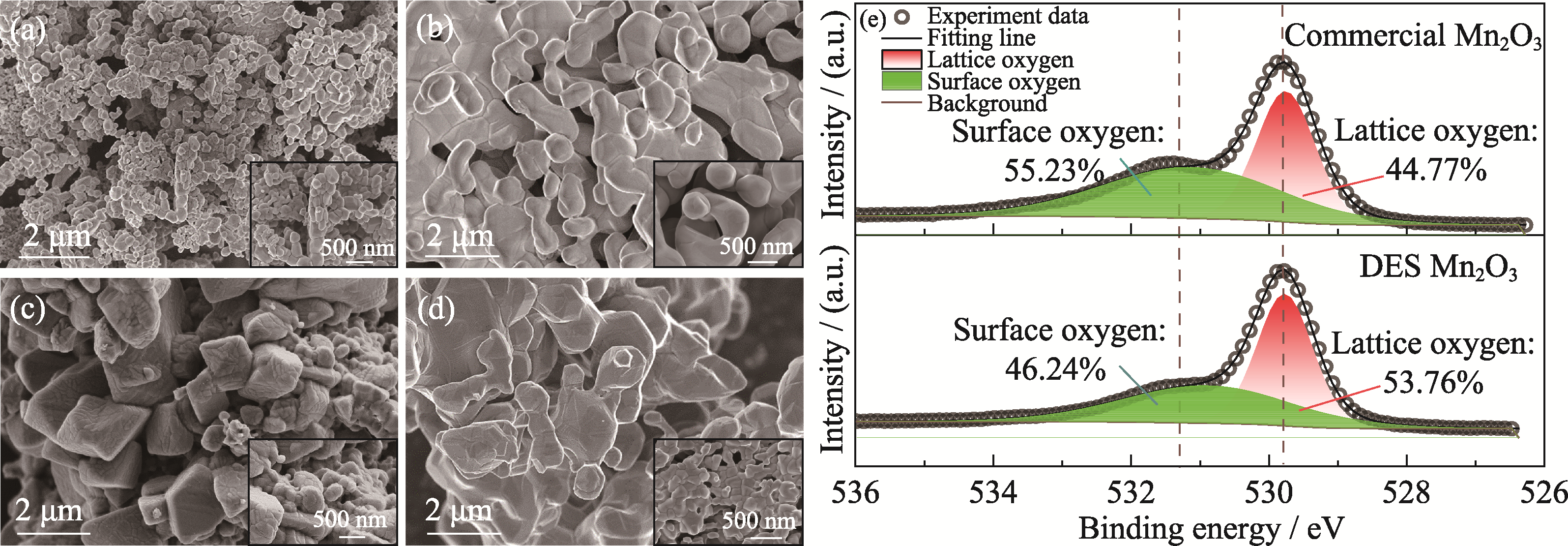
图2 (a~d)Mn2O3循环前后的SEM照片和(e)循环前O1s的XPS图谱
Fig. 2 (a-d) SEM images of samples before and after cycling, and (e) O1s XPS spectra of Mn2O3 before cycling (a) Commercial Mn2O3 before cycling; (b) Commercial Mn2O3 after cycling; (c) DES Mn2O3 before cycling; (d) DES Mn2O3 after cycling
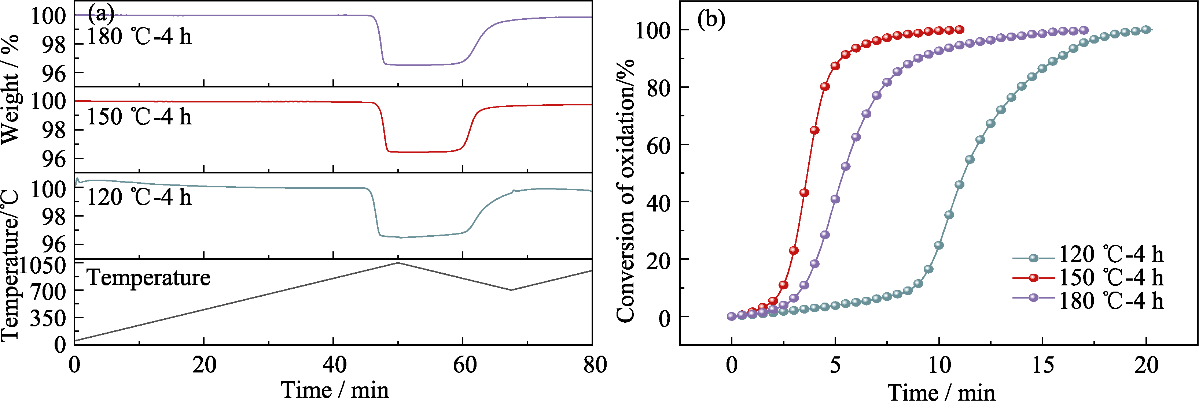
图4 不同离子热合成温度制备样品的(a)TG曲线和(b)氧化反应转化率-时间曲线
Fig. 4 (a) TG curves and (b) oxidation reaction conversion rate-time curves of samples prepared at different ionic thermal synthesis temperatures
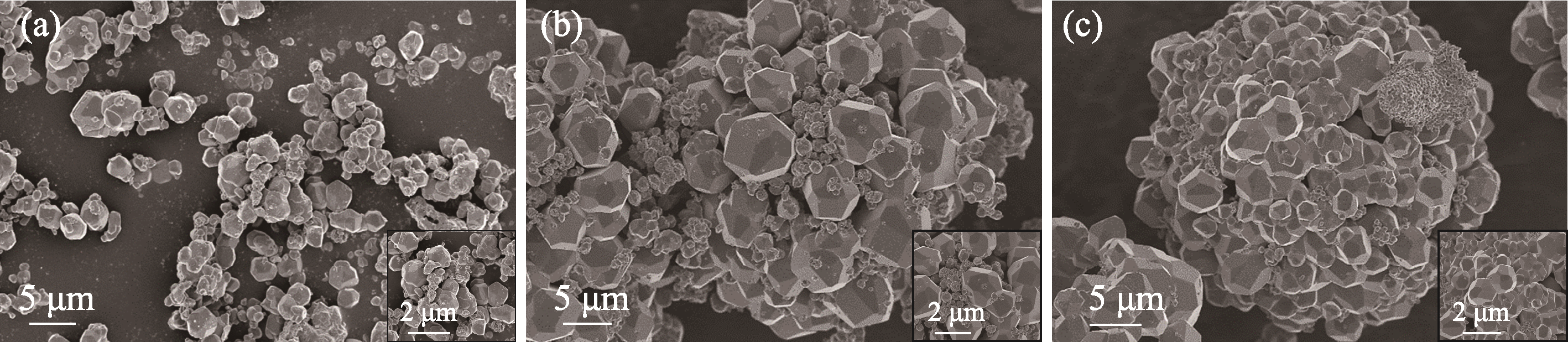
图5 不同离子热合成温度制备样品循环前的SEM照片
Fig. 5 SEM images of samples prepared by ionic thermal synthesis at different temperatures before cycling (a) 120 ℃-4 h; (b) 150 ℃-4 h; (c) 180 ℃-4 h
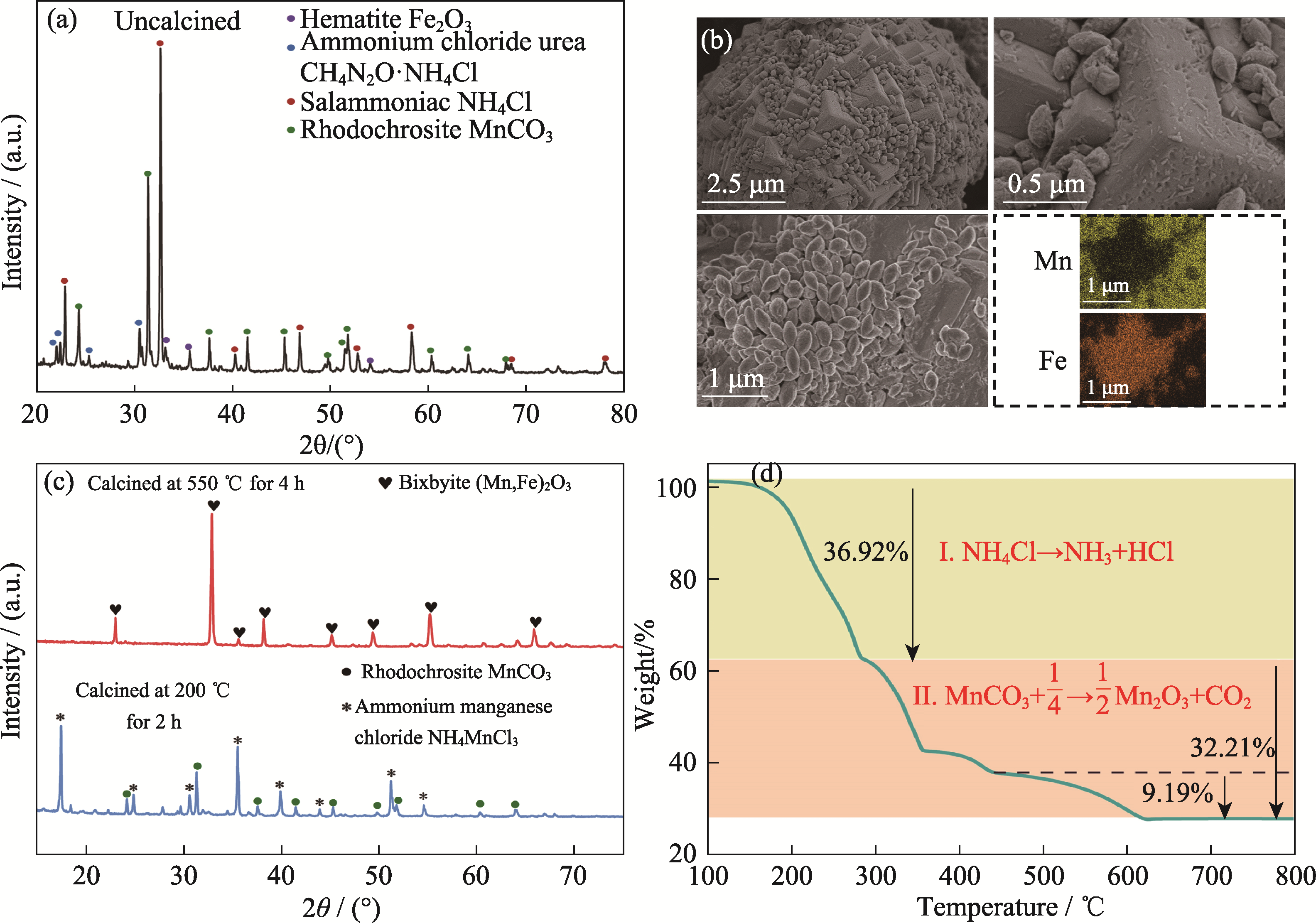
图8 DES合成锰基氧化物前驱物表征
Fig. 8 Characterization of precursors of manganese-based oxide synthesized by DES (a) XRD pattern; (b) SEM-EDS images; (c) XRD patterns under different heat-treatments; (d) TG curve
| [1] |
PFENNINGER S, GAUCHÉ P, LILLIESTAM J, et al. Potential for concentrating solar power to provide baseload and dispatchable power. Nature Climate Change, 2014, 4(8): 689.
DOI |
| [2] |
PRASAD J S, MUTHUKUMAR P, DESAI F, et al. A critical review of high-temperature reversible thermochemical energy storage systems. Applied Energy, 2019, 254: 113733.
DOI URL |
| [3] |
LANCHI M, TURCHETTI L, SAU S, et al. A discussion of possible approaches to the integration of thermochemical storage systems in concentrating solar power plants. Energies, 2020, 13(18): 4940.
DOI URL |
| [4] |
ANDRÉ L, ABANADES S, FLAMANT G, et al. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renewable and Sustainable Energy Reviews, 2016, 64: 703.
DOI URL |
| [5] |
BLOCK T, SCHMUCKER M. Metal oxides for thermochemical energy storage: a comparison of several metal oxide systems. Solar Energy, 2016, 126: 195.
DOI URL |
| [6] |
CARRILLO A J, GONZALEZ-AGUILAR J, ROMERO M, et al. Solar energy on demand: a review on high temperature thermochemical heat storage systems and materials. Chemical Reviews. 2019, 119(7): 4777.
DOI PMID |
| [7] |
WU S, ZHOU C, DOROODCHI E, et al. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energy Conversion and Management, 2018, 168: 421.
DOI URL |
| [8] | BIELSA D, ZAKI A, FAIK A, et al. Efficiency improvement of Mn2O3/Mn3O4 redox reaction by means of different operation strategies. AIP Conference Proceedings, 2019, 2126: 210001. |
| [9] |
CARRILLO A J, SERRANO D P, PIZARRO P, et al. Thermochemical heat storage based on the Mn2O3/Mn3O4 redox couple: influence of the initial particle size on the morphological evolution and cyclability. Journal of Materials Chemistry A, 2014, 2(45): 19435.
DOI URL |
| [10] | CARRILLO A J, SERRANO D P, PIZARRO P, et al. Design of efficient Mn-based redox materials for thermochemical heat storage at high temperatures. AIP Conference Proceedings, 2016, 1734: 050009. |
| [11] |
BIELSA D, ZAKI A, ARIAS P L, et al. Improving the redox performance of Mn2O3/Mn3O4 pair by Si doping to be used as thermochemical energy storage for concentrated solar power plants. Solar Energy, 2020, 204: 144.
DOI URL |
| [12] |
CHEN X, KUBOTA M, YAMASHITA S, et al. Exploring Cu-based spinel/delafossite couples for thermochemical energy storage at medium-high temperature. ACS Applied Energy Materials, 2021, 4(7): 7242.
DOI URL |
| [13] |
ANDRÉ L, ABANADES S, CASSAYRE L, et al. Experimental investigation of Co-Cu, Mn-Co, and Mn-Cu redox materials applied to solar thermochemical energy storage. ACS Applied Energy Materials, 2018, 1(7): 3385.
DOI URL |
| [14] |
HLONGWA N W, SASTRE D, IWUOHA E, et al. Exploring the thermochemical heat storage capacity of AMn2O4(A=Li or Cu) spinels. Solid State Ionics, 2018, 320: 316.
DOI URL |
| [15] |
ABAD A, MENDIARA T, IZQUIERDO M T, et al. Evaluation of the redox capability of manganese-titanium mixed oxides for thermochemical energy storage and chemical looping processes. Fuel Processing Technology, 2021, 211: 106579.
DOI URL |
| [16] |
RANDHIR K, KING K, RHODES N, et al. Oxidation kinetics of magnesium-manganese oxides for high-temperature thermochemical energy storage. Energy Technology, 2020, 8(10): 2000063.
DOI URL |
| [17] |
CARRILLO A J, PIZARRO P, CORONADO J M, et al. Assessing Cr incorporation in Mn2O3/Mn3O4 redox materials for thermochemical heat storage applications. Journal of Energy Storage, 2020, 33: 102028.
DOI URL |
| [18] |
WOKON M, KOHZER A, LINDER M. Investigations on thermochemical energy storage based on technical grade manganese-iron oxide in a lab-scale packed bed reactor. Solar Energy, 2017, 153: 200.
DOI URL |
| [19] |
AL-SHANKITI I A, EHRHART B D, WARD B J, et al. Particle design and oxidation kinetics of iron manganese oxide redox materials for thermochemical energy storage. Solar Energy, 2019, 183: 17.
DOI URL |
| [20] |
WANG B, LI L, SCHAFER F, et al. Thermal reduction of iron-manganese oxide particles in a high-temperature packed-bed solar thermochemical reactor. Chemical Engineering Journal, 2021, 412: 128255.
DOI URL |
| [21] |
GE X, GU C D, WANG X L, et al. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: challenges, opportunities, and future vision. Journal of Materials Chemistry A, 2017, 5(18): 8209.
DOI URL |
| [22] |
LIU Y, CHI X, HANQ, et al. α-MnO2 nanofibers/carbon nanotubes hierarchically assembled microspheres: approaching practical applications of high-performance aqueous Zn-ion batteries. Journal of Power Sources, 2019, 443: 227244.
DOI URL |
| [23] |
ZHANG Z, YU J, ZHANG J, et al. Tailored metastable Ce-Zr oxides with highly distorted lattice oxygen for accelerating redox cycles. Chemical Science, 2018, 9(13): 3386.
DOI PMID |
| [24] |
XIANG D, GU C D, XU H R, et al. Self-assembled structure evolution of Mn-Fe oxides for high temperature thermochemical energy storage. Small, 2021, 17(29): 2101524.
DOI URL |
| [25] | 张杰, 唐定国, 刘浩文, 等. 碳酸锰高温分解制备三氧化二锰研究. 山东化工, 2013, 42(4): 1. |
| [26] |
NOUR E M, TELEB S M, AL-KHSOSY N A, et al. A novel method for the synthesis of metal carbonates. I.synthesis and infrared spectrum of manganese carbonate, MnCO3·H2O, formed by the reaction of urea with manganese(II) salts. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 1997, 27(4): 505.
DOI URL |
| [27] |
XIONG Q Q, TU J P, GE X, et al. One-step synthesis of hematite nanospindles from choline chloride/urea deep eutectic solvent with highly powerful storage versus lithium. Journal of Power Sources, 2015, 274: 1.
DOI URL |
| [1] | 李孟夏, 陆越, 王利斌, 胡先罗. Mn3O4@ZnO核壳结构纳米片阵列的可控合成及其在水系锌离子电池中的应用[J]. 无机材料学报, 2020, 35(1): 86-92. |
| [2] | 徐晓虹, 田江洲, 吴建锋, 张乾坤, 金昊, 杜怿鑫. Fe2O3对原位制备SiCw/SiC太阳能储热陶瓷的结构与性能的影响[J]. 无机材料学报, 2019, 34(10): 1103-1108. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||