无机材料学报 ›› 2022, Vol. 37 ›› Issue (10): 1073-1078.DOI: 10.15541/jim20220077 CSTR: 32189.14.10.15541/jim20220077
王烈林( ), 谢华, 谢宇骐, 胡平涛, 尹雯, 任馨玥, 丁芸
), 谢华, 谢宇骐, 胡平涛, 尹雯, 任馨玥, 丁芸
收稿日期:2022-02-16
修回日期:2022-03-23
出版日期:2022-10-20
网络出版日期:2022-04-07
作者简介:王烈林(1982-), 男, 博士, 副研究员. E-mail: wanglielin@swust.edu.cn
基金资助:
WANG Lielin( ), XIE Hua, XIE Yuqi, HU Pingtao, YIN Wen, REN Xinyue, DING Yun
), XIE Hua, XIE Yuqi, HU Pingtao, YIN Wen, REN Xinyue, DING Yun
Received:2022-02-16
Revised:2022-03-23
Published:2022-10-20
Online:2022-04-07
About author:WANG Lielin (1982-), male, PhD, associate professor. E-mail: wanglielin@swust.edu.cn
Supported by:摘要:
A2B2O7烧绿石以其高包容性和物理化学稳定性而成为高放废物固化体候选基材。研究通过喷雾热解-高温烧结制备了Nd2Zr2O7烧绿石A、B位钍掺杂Nd1.8Th0.2Zr2O7和Nd2Zr1.8Th0.2O7固化体, 利用不同检测手段分析样品结构, 并通过MCC-1方法研究了样品化学稳定性。Nd2Zr2O7烧绿石A和B位掺杂Th均能形成纯烧绿石结构, 掺杂将造成烧绿石结构中48f氧位置参数增大, 烧绿石向无序化结构转变。A位掺杂导致烧绿石AO8六面体结构扭曲, B位掺杂导致BO6八面体部分形变。Nd1.8Th0.2 Zr2O7和Nd2Zr1.8Th0.2O7固化体在42 d周期后Th离子浸出率在10-5 g·m-2·d-1量级, 说明锕系核素Th在Nd2Zr2O7的A、B位均能很好地被包容, 固化体表现出优异的物理化学性能。
中图分类号:
王烈林, 谢华, 谢宇骐, 胡平涛, 尹雯, 任馨玥, 丁芸. Nd2Zr2O7烧绿石A、B位晶格固化钍的结构演化及化学稳定性研究[J]. 无机材料学报, 2022, 37(10): 1073-1078.
WANG Lielin, XIE Hua, XIE Yuqi, HU Pingtao, YIN Wen, REN Xinyue, DING Yun. Structural Evolution and Chemical Durability of Thorium-incorporated Nd2Zr2O7 Pyrochlore at A and B Sites[J]. Journal of Inorganic Materials, 2022, 37(10): 1073-1078.
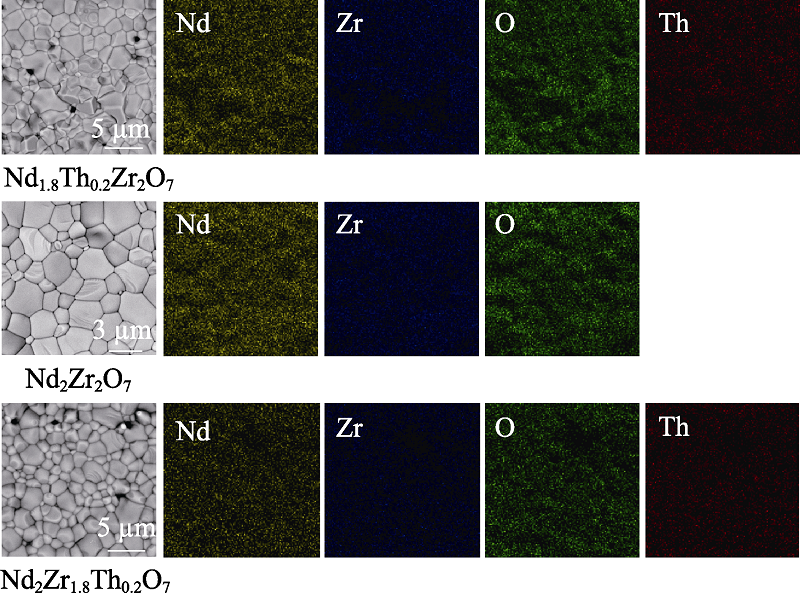
图2 样品Nd1.8Th0.2 Zr2O7、Nd2Zr2O7和Nd2Zr1.8Th0.2O7的SEM照片及EDS元素分布图
Fig. 2 SEM images and EDS elements mappings of samples Nd1.8Th0.2 Zr2O7, Nd2Zr2O7 and Nd2Zr1.8Th0.2O7
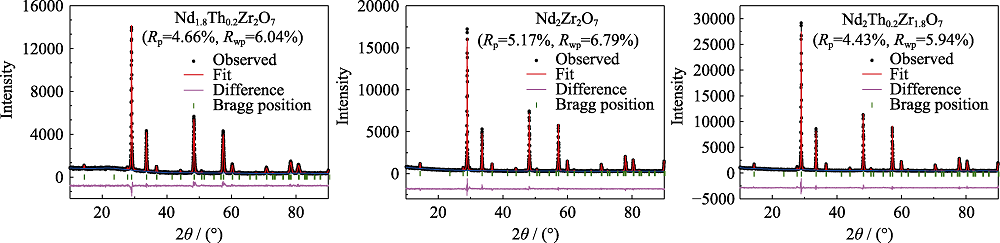
图3 样品Nd1.8Th0.2 Zr2O7、Nd2Zr2O7和Nd2Zr1.8Th0.2O7 的XRD结构精修分析
Fig. 3 Rietveld refinement patterns of samples Nd1.8Th0.2Zr2O7, Nd2Zr2O7 and Nd2Zr1.8Th0.2O7
| Nd1.8Th0.2Zr2O7 | Nd2Zr2O7 | Nd2Zr1.8Th0.2O7 | |
|---|---|---|---|
| Lattice parameter/nm | 1.06370(2) | 1.06744(5) | 1.06781(4) |
| Unit cell volume/nm3 | 1.203 | 1.216 | 1.217 |
| x-parameter of 48f oxygen | 0.3322(6) | 0.3287(6) | 0.3365(5) |
| A-O48f bond distance | 2.5932(5) | 2.6285(4) | 2.5713(4) |
| B-O48f bond distance | 2.0737(3) | 2.0660(2) | 2.1017(2) |
| A-B bond distance | 3.7607(4) | 3.7739(1) | 3.7753(2) |
| A-8a bond distance | 2.3029(2) | 2.3110(1) | 2.3118(3) |
| Rp | 4.66% | 5.17% | 4.43% |
| Rwp | 6.04% | 6.79% | 5.94% |
| χ2 | 2.614 | 1.932 | 2.237 |
表1 样品Nd1.8Th0.2 Zr2O7、Nd2Zr2O7和Nd2Zr1.8Th0.2O7的Rietveld精修数据
Table 1 Rietveld refinement parameters of samples Nd1.8Th0.2 Zr2O7, Nd2Zr2O7 and Nd2Zr1.8Th0.2O7
| Nd1.8Th0.2Zr2O7 | Nd2Zr2O7 | Nd2Zr1.8Th0.2O7 | |
|---|---|---|---|
| Lattice parameter/nm | 1.06370(2) | 1.06744(5) | 1.06781(4) |
| Unit cell volume/nm3 | 1.203 | 1.216 | 1.217 |
| x-parameter of 48f oxygen | 0.3322(6) | 0.3287(6) | 0.3365(5) |
| A-O48f bond distance | 2.5932(5) | 2.6285(4) | 2.5713(4) |
| B-O48f bond distance | 2.0737(3) | 2.0660(2) | 2.1017(2) |
| A-B bond distance | 3.7607(4) | 3.7739(1) | 3.7753(2) |
| A-8a bond distance | 2.3029(2) | 2.3110(1) | 2.3118(3) |
| Rp | 4.66% | 5.17% | 4.43% |
| Rwp | 6.04% | 6.79% | 5.94% |
| χ2 | 2.614 | 1.932 | 2.237 |
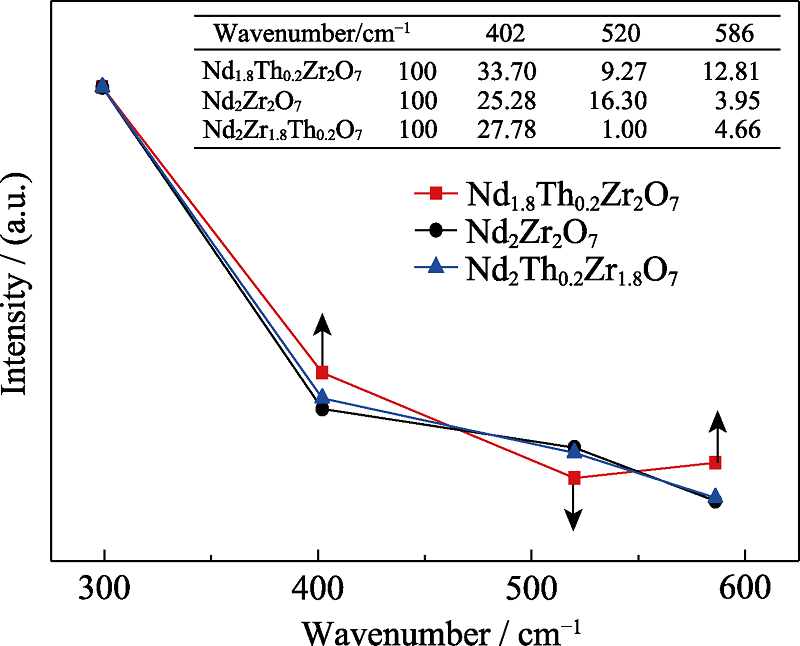
图6 样品Nd1.8Th0.2Zr2O7、Nd2Zr2O7和Nd2Zr1.8Th0.2O7的Raman峰强度变化
Fig. 6 Raman peak intensity variation of samples Nd1.8Th0.2Zr2O7, Nd2Zr2O7 and Nd2Zr1.8Th0.2O7 The peak intensity is normalized to the intensity of 299 cm-1
| [1] |
RINGWOOD A E, KESSON S E, WARE N G, et al. Immobilization of high level nuclear reactor wastes in SYNROC. Nature, 1979, 278: 219-223.
DOI URL |
| [2] |
ORLOVA A I, OJOVAN M I. Ceramic mineral waste-forms for nuclear waste immobilization. Materials, 2019, 12(16): 2638.
DOI URL |
| [3] |
CLARK B M, TUMURUGOTI P, SUNDARAM S K, et al. Preparation and characterization of multiphase ceramic designer waste forms. Scientific Reports, 2021, 11: 4512.
DOI PMID |
| [4] |
WANG S X, BEGG B D, WANG L M, et al. Radiation stability of gadolinium zirconate: a waste form for plutonium disposition. Journal of Materials Research, 1999, 14(12): 4470-4473.
DOI URL |
| [5] |
YANG K, KEITH B, ZHU W, et al. Multicomponent pyrochlore solid solutions with uranium incorporation-a new perspective of materials design for nuclear applications. Journal of the European Ceramic Society, 2021, 41(4): 2870-2882.
DOI URL |
| [6] | LIAN J, WANG L M, HAIRE R G, et al. Ion beam irradiation in La2Zr2O7-Ce2Zr2O7pyrochlore. Nuclear Instruments and Methods in Physics Research Section B, 2004, 218: 236-243. |
| [7] | SHARMA S K, GROVER V, TYAGI A K, et al. Probing the temperature effects in the radiation stability of Nd2Zr2O7 pyrochlore under swift ion irradiation. Materialia, 2019, 6: 2589-1529. |
| [8] |
CHAKOUMAKOS B C. Systematics of the pyrochlore structure type, ideal A2B2X6Y. Journal of Solid State Chemistry, 1984, 53(1): 120-129
DOI URL |
| [9] | SUN J, ZHOU J, HU Z, et al. Controllable sites and high-capacity immobilization of uranium in Nd2Zr2O7 pyrochlore. Journal of Synchrotron Radiation, 2022, 29: 37-44 |
| [10] |
BELIN R C, VALENZA P J, RAISON P E, TILLARD M. Synthesis and Rietveld structure refinement of americium pyrochlore Am2Zr2O7. Journal of Alloys and Compounds, 2008, 448(1/2): 321-324.
DOI URL |
| [11] |
KULKARNI N K, SAMPATH S, VENUGOPAL V. Preparation and characterisation of Pu-pyrochlore: [La1-xPux]2Zr2O7 (x=0-1). Journal of Nuclear Materials, 2000, 281(2/3): 248-250.
DOI URL |
| [12] |
MANDAL B P, GARG N, SHARMA S M, et al. Solubility of ThO2 in Gd2Zr2O7 pyrochlore: XRD, SEM and Raman spectroscopic studies. Journal of Nuclear Materials, 2009, 392(1): 95-99.
DOI URL |
| [13] |
KUTTY K V G, ASUVATHRAMAN R, MADHAVAN R R, et al. Actinide immobilization in crystalline matrix: a study of uranium incorporation in gadolinium zirconate. Journal of Physics and Chemistry of Solids, 2005, 66(2/3/4): 596-601.
DOI URL |
| [14] |
TANG Z, HUANG Z Y, HAN W, et al. Uranium-incorporated pyrochlore La2(UxMgxZr1-2x)2O7 nuclear waste form: structure and phase stability. Inorganic Chemistry, 2020, 59(14): 9919-9926.
DOI URL |
| [15] |
LIAN J, CHEN J, WANG L M, et al. Radiation-induced amorphization of rare-earth titanate pyrochlores. Physical Review B, 2003, 68(1): 134107.
DOI URL |
| [16] | WANG S X, LUMPKIN G R, WANG L M, et al. Ion irradiation- induced amorphization of six zirconolite compositions. Nuclear Instruments and Methods in Physics Research Section B, 2000, 166-167(2): 293-298. |
| [17] |
NÄSTREN C, JARDIN R, SOMERS J, et al. Actinide incorporation in a zirconia based pyrochlore (Nd1.8An0.2)Zr2O7+x (An=Th, U, Np, Pu, Am). Journal of Solid State Chemistry, 2009, 182(1): 1-7.
DOI URL |
| [18] |
THAKUR A, SINGH B, KRISHNANI P D. In-core fuel management for AHWR. Annals of Nuclear Energy, 2013, 57: 47-58.
DOI URL |
| [19] | DAI Z M. Thorium molten salt reactor nuclear energy system (TMSR). Molten Salt Reactors and Thorium Energy, 2017, 17: 531-540. |
| [20] |
WANG L L, LI J B, XIE H, et al. Solubility, structure transition and chemical durability of Th-doped Nd2Zr2O7 pyrochlore. Progress in Nuclear Energy, 2021, 137: 103774.
DOI URL |
| [21] |
TOBY B H, EXPGUI. A graphical user interface for GSAS. Journal of Applied Crystallography, 2001, 34: 210-213.
DOI URL |
| [22] | ASTM. Standard test method for static leaching of monolithic waste forms for disposal of radioactive waste. Annual Book of ASTM Standards. C1220-92. 1992, 12(1): 681-695. |
| [23] |
STRACHAN D M. Results from long-term use of the MCC-1 static leach test method. Nuclear and Chemical Waste Management, 1983, 4(2): 177-188.
DOI URL |
| [24] |
MANDAL B P, KRISHNA P S R, TYAGI A K. Order-disorder transition in the Nd2-yYyZr2O7 system: Probed by X-ray diffraction and Raman spectroscopy. Journal of Solid State Chemistry, 2010, 183(1): 41-45.
DOI URL |
| [25] |
QU Z, WAN C, PAN W. Thermal expansion and defect chemistry of MgO-doped Sm2Zr2O7. Chemistry of Materials, 2007, 19(20): 4913.
DOI URL |
| [26] |
VANDENBORRE M T, HUSSON E, CHATRY J P, et al. Rare- earth titanates and stannates of pyrochlore structure; vibrational spectra and force fields. Journal of Raman Spectroscopy, 1983, 14: 63-71.
DOI URL |
| [27] |
NANDI C, PHATAK R, KESARI S, et al. Phase evolution in [Nd1-xUx]2Zr2O7+δ system in oxidizing and reducing conditions: a nuclear waste form. Journal of Nuclear Materials, 2021, 556(1): 153208.
DOI URL |
| [28] | HAYAKAWA I, KAMIZONO H. Durability of an La2Zr2O7 waste form in water. Journal of Nuclear Materials, 1993, 28: 513-517. |
| [29] |
WEBER W J, NAVROTSKY A, STEFANOVSKY S, et al. Materials science of high-level nuclear waste immobilization. MRS Bulletin, 2009, 34: 46-53.
DOI URL |
| [30] |
GONG B W, YANG K, LIAN J A, et al. Machine learning- enabled prediction of chemical durability of A2B2O7 pyrochlore and fluorite. Computational Materials Science, 2021, 200: 110820.
DOI URL |
| [31] |
FENG Z Q, XIE H, WANG L L, et al. Glass-ceramics with internally crystallized pyrochlore for the immobilization of uranium wastes. Ceramics International, 2019, 45(14): 16999-17005.
DOI URL |
| [1] | 刘建, 王凌坤, 许保亮, 赵倩, 王耀萱, 丁艺, 张胜泰, 段涛. 熔盐法低温合成掺钕ZrSiO4陶瓷的物相演变和化学稳定性[J]. 无机材料学报, 2023, 38(8): 910-916. |
| [2] | 罗世淋, 张胜泰, 许保亮, 王凌坤, 段思逸菡, 丁艺, 赵倩, 段涛. YIG陶瓷对三价锕系模拟核素的固化行为研究[J]. 无机材料学报, 2022, 37(7): 757-763. |
| [3] | 曾建军, 张魁宝, 陈代梦, 郭海燕, 邓婷, 刘奎. 真空烧结制备(La0.2Nd0.2Sm0.2Gd0.2Er0.2)2Zr2O7高熵透明陶瓷[J]. 无机材料学报, 2021, 36(4): 418-424. |
| [4] | 孙亚平, 王洪龙, 褚健, 王绪, 潘社奇, 张铭. 陶瓷固化体的浸出行为及其机理[J]. 无机材料学报, 2019, 34(5): 461-468. |
| [5] | 杨春利, 严 敏, 李 伟. In、Ta共掺对BaCeO3烧结性能及稳定性的影响[J]. 无机材料学报, 2016, 31(9): 955-960. |
| [6] | 王烈林, 谢 华, 陈青云, 王 茜, 龙 勇, 邓 超, 张可心. 锆基烧绿石Nd2Zr2O7固化锕系核素钍[J]. 无机材料学报, 2015, 30(1): 81-86. |
| [7] | 姜金龙, 王 琼, 黄 浩, 张 霞, 王玉宝, 耿庆芬. 紫外光辐照下钛硅共掺杂类金刚石薄膜微结构的演化[J]. 无机材料学报, 2014, 29(9): 941-946. |
| [8] | 丁 娟, 刘睿恒, 顾 辉, 陈立东 . YbyCo4Sb12/Yb2O3热电复合材料的高温稳定性研究[J]. 无机材料学报, 2014, 29(2): 209-214. |
| [9] | 毕 磊,陶泽天,彭冉冉,刘 卫. 质子陶瓷膜燃料电池电解质材料的研究进展[J]. 无机材料学报, 2010, 25(1): 1-7. |
| [10] | 周吉峙,徐 霞,张 怡,钱光人. 铅羟基磷灰石的形成与稳定性[J]. 无机材料学报, 2009, 24(2): 259-263. |
| [11] | 许 峰,胡小方,卢 斌,赵建华,伍小平,袁清习. 碳化硼固相烧结微观结构演化的同步辐射CT观测[J]. 无机材料学报, 2009, 24(1): 175-181. |
| [12] | 钱光人,白红梅,孙福成,周吉峙,孙为民,徐 霞. 含镉羟基磷灰石的形成及其稳定性[J]. 无机材料学报, 2008, 23(5): 1016-1020. |
| [13] | 汪秀全,陈奇,宋鹂,李会平,陆剑英. 多孔SiO2-TiO2块材的结构与性质[J]. 无机材料学报, 2006, 21(1): 181-186. |
| [14] | 黄文旵,周萘,Day Delbert E,Ray Chandra S. Cr2O3对高放核废料磷酸盐玻璃固化体的影响[J]. 无机材料学报, 2005, 20(4): 842-850. |
| [15] | 景晓宁,倪勇,何陵辉,赵建华. 陶瓷烧结过程孔隙演化的二维相场模拟[J]. 无机材料学报, 2002, 17(5): 1078-1082. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||