无机材料学报 ›› 2020, Vol. 35 ›› Issue (10): 1169-1176.DOI: 10.15541/jim20200005 CSTR: 32189.14.10.15541/jim20200005
所属专题: 生物材料论文精选(2020); 【虚拟专辑】生物检测与成像(2020~2021)
收稿日期:2020-01-05
修回日期:2020-02-03
出版日期:2020-10-20
网络出版日期:2020-10-20
通讯作者:
杨勇, 研究员. E-mail:yangyong@mail.sic.ac.cn作者简介:程琴(1994-), 女, 硕士研究生. E-mail: cq18817206957@163.com
CHENG Qin1,2( ), YANG Yong2(
), YANG Yong2( ), YANG Lili2
), YANG Lili2
Received:2020-01-05
Revised:2020-02-03
Published:2020-10-20
Online:2020-10-20
Contact:
YANG Yong, professor. E-mail: yangyong@mail.sic.ac.cn.About author:CHENG Qin(1994-). female, Master candidate. E-mail: cq18817206957@163.com.
Supported by:摘要:
具有类酶活性的无机纳米材料因其高稳定性和高灵敏度而具有广阔的应用前景, 因而调节其类酶活性对于促进纳米酶的发展具有重要意义。本研究通过简单的液相还原法合成了具有良好均匀性和稳定性的Pt-Au枝状纳米颗粒(Pt-Au DNPs), 研究了动力学参数与纳米颗粒结构之间的关系, 发现Pt-Au DNPs的组成和结构对其类氧化酶活性有很大影响。同时利用其类氧化酶活性催化TMB(3,3′,5,5′-四甲基联苯胺)氧化来比色检测抗坏血酸(AA)。对AA的定量分析结果显示, 在1~15 μmol/L范围存在良好的线性关系, 检出限为78 nmol/L。同时, 发现虽然连续反应会降低Pt-Au DNP的催化性能, 但其仍具有重复使用的潜力, 这在可视化检测AA中并不常见。这项研究不仅提出了合成Pt-Au DNPs的方法, 而且还显示了其在生物样品中进行AA含量分析的潜在应用前景。
中图分类号:
程琴, 杨勇, 杨莉莉. 具有高类氧化酶活性的铂-金枝状纳米粒子用于检测抗坏血酸[J]. 无机材料学报, 2020, 35(10): 1169-1176.
CHENG Qin, YANG Yong, YANG Lili. Pt-Au Dendritic Nanoparticles with High Oxidase-like Activity for Detection of Ascorbic Acid[J]. Journal of Inorganic Materials, 2020, 35(10): 1169-1176.
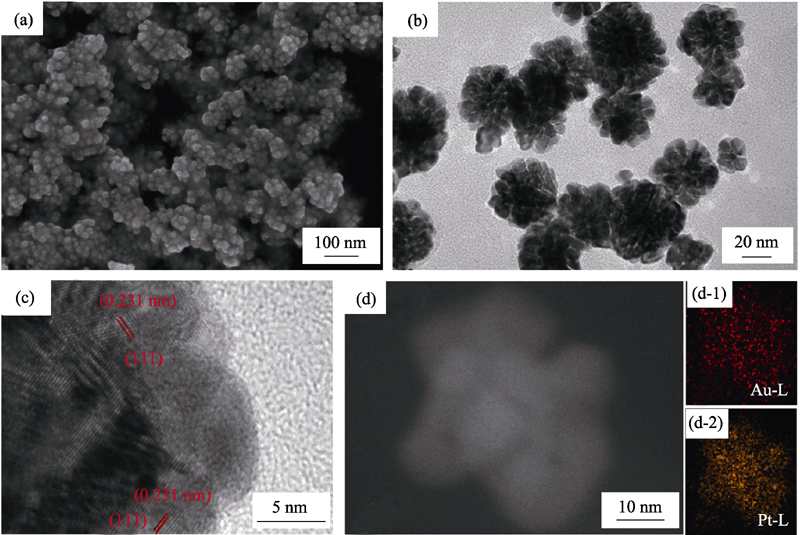
Fig. 1 (a) SEM and (b) TEM images of the as-synthesized Pt-Au DNPs (after 20 min reaction); (c) HRTEM image, (d) STEM image and EDS mapping for Au, Pt elements distribution of one single Pt-Au nanoparticle (d-1: Au and d-2:Pt)
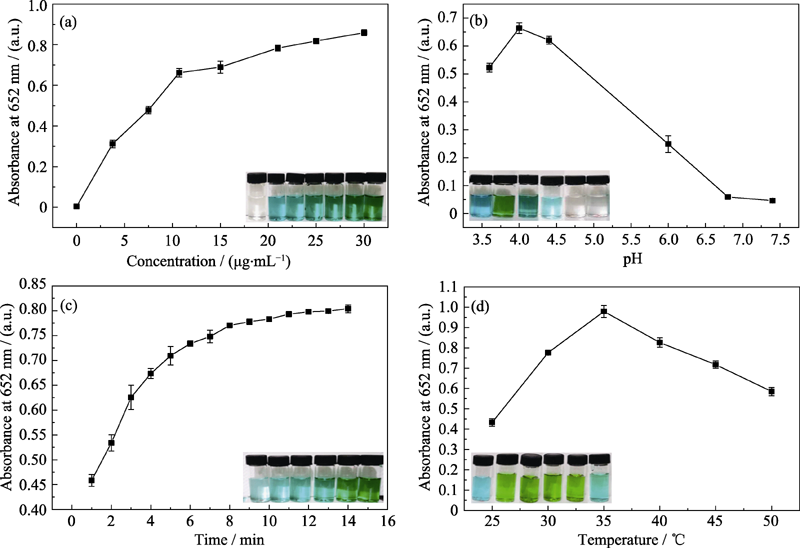
Fig. 6 Effects of different experimental conditions on the oxidase-like activity of Pt-Au DNPs. The Absorbance spectra and visual color changes of TMB in presence of different (a) Pt-Au DNPs concentration, (b) pH, (c) time, (d) temperature, respectively, with insets showing the corresponding photos of the reaction solutions
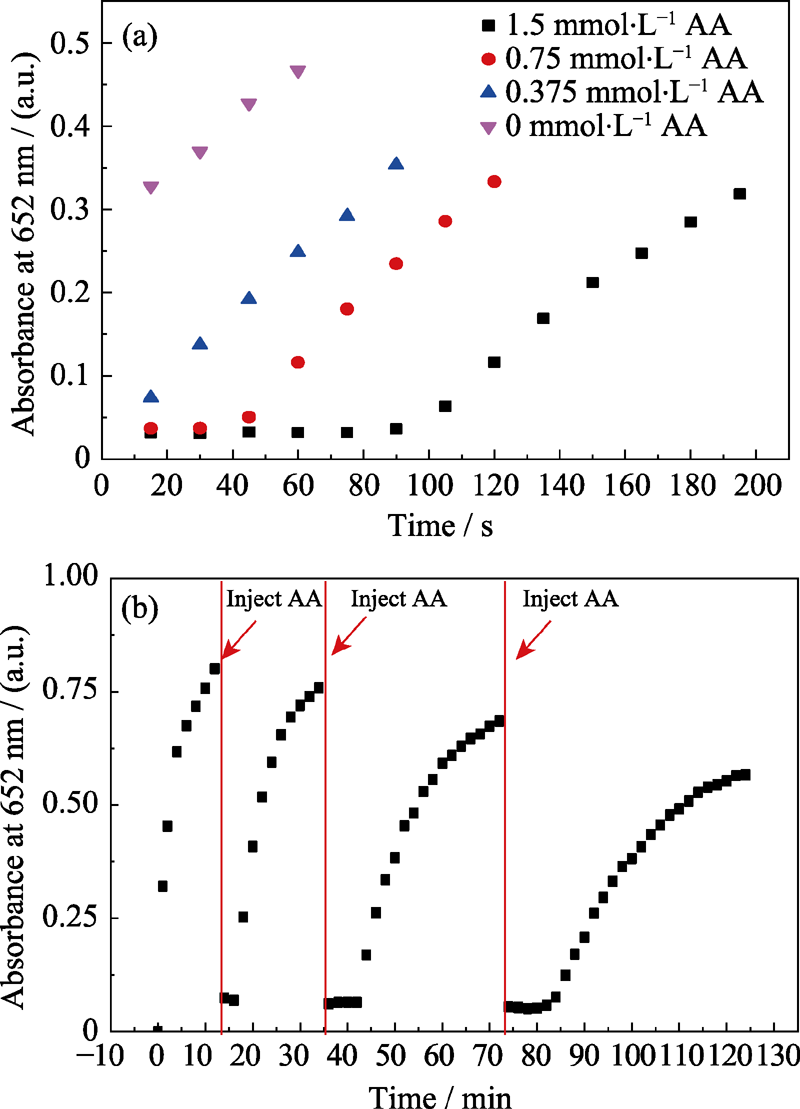
Fig. 8 Time-dependent absorbance for (a) Pt-Au DNPs catalyzed TMB oxidation in the presence of different concentrations of AA and (b) ox-TMB reduced by AA
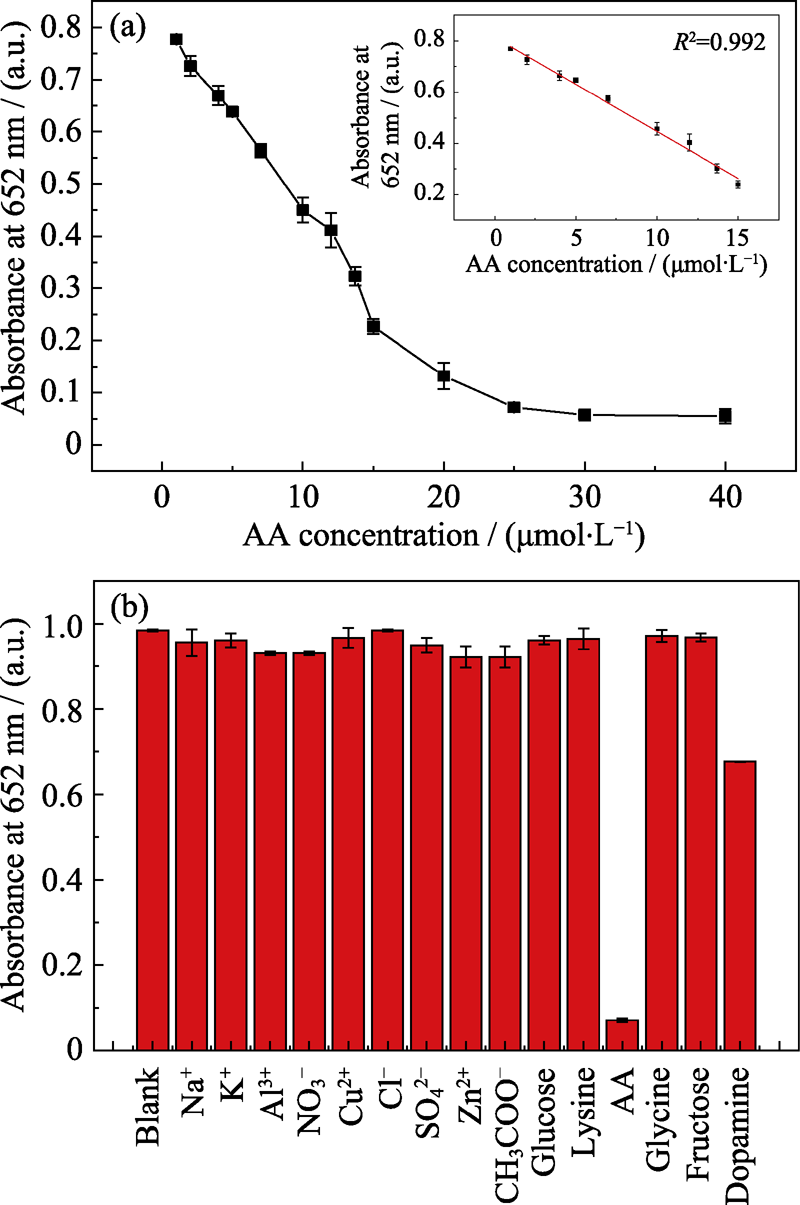
Fig. 9 (a) Dose-response curve for different concentrations of AA standard solutions, with inset showing the linear calibration plot for AA, and (b) interference of different interfering substances in the AA detection, all interfering substances were at a concentration of 25 μmol/L
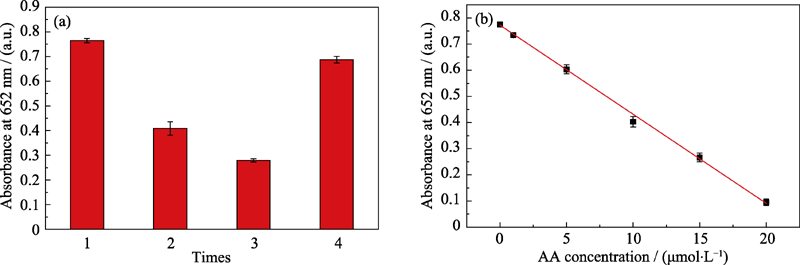
Fig. S8 (a) Pt-Au DNPs continuously catalyzed the oxidation of TMB (each time incubation for 10 min after reducing ox-TMB by AA, with the fourth time showing the result of centrifugal washing of Pt-Au DNPs before incubation), and (b) linear calibration curve for AA after 20 min of TMB second oxidation
| Sample | Added/(μmol·L-1) | Found/(μmol·L-1) | Recovery/% | RSD/(%, n=3) |
|---|---|---|---|---|
| Juice | 0 | 5.53 | - | 4.1 |
| 3.12 | 8.86 | 103.71 | 3.2 | |
| 6.25 | 12.01 | 103.68 | 3.4 |
Table S1 Detection of AA in real juice sample
| Sample | Added/(μmol·L-1) | Found/(μmol·L-1) | Recovery/% | RSD/(%, n=3) |
|---|---|---|---|---|
| Juice | 0 | 5.53 | - | 4.1 |
| 3.12 | 8.86 | 103.71 | 3.2 | |
| 6.25 | 12.01 | 103.68 | 3.4 |
| Catalyst | Substrate | Km/(mmol·L-1) | Ref. |
|---|---|---|---|
| BiW9Cu3 | TMB | 0.29 | [ |
| CeO2 | 0.8-3.8 | [ | |
| Hg2+/Citrate-AgNPs | 0.23 | [ | |
| N-CQDs | 0.515 | [ | |
| Cu-Ag/rGO | 0.634 | [ | |
| Cu NPs | 1.047 | [ | |
| Lysozyme-PtNPs | 0.63 | [ | |
| Fe3O4@C | 0.38 | [ | |
| Pt-Au DNPs | 0.22 | This work |
Table S2 Comparative table of steady-state kinetic parameter for Pt-Au DNPs and other materials
| Catalyst | Substrate | Km/(mmol·L-1) | Ref. |
|---|---|---|---|
| BiW9Cu3 | TMB | 0.29 | [ |
| CeO2 | 0.8-3.8 | [ | |
| Hg2+/Citrate-AgNPs | 0.23 | [ | |
| N-CQDs | 0.515 | [ | |
| Cu-Ag/rGO | 0.634 | [ | |
| Cu NPs | 1.047 | [ | |
| Lysozyme-PtNPs | 0.63 | [ | |
| Fe3O4@C | 0.38 | [ | |
| Pt-Au DNPs | 0.22 | This work |
| Catalyst | Linear range/(μmol·L-1) | LOD/(μmol·L-1) | Ref. |
|---|---|---|---|
| FeCo NPs@PNC | 0.5-28 | 0.38 | [ |
| N-CQDs | 5-40 | 1.773 | [ |
| Cu-Ag/rGO | 1-30 | 3.6 | [ |
| Cu NPs | 1-10 | 0.68 | [ |
| LaF3:Ce,Tb | 8-10 | 2.4 | [ |
| CuO/Pt | 1-600 | 0.796 | [ |
| MIL-88 | 2.57-10.1 | 1.03 | [ |
| Pt-Au DNPs | 1-15 | 0.078 | This work |
Table S3 Comparison of earlier reports for the AA detection.
| Catalyst | Linear range/(μmol·L-1) | LOD/(μmol·L-1) | Ref. |
|---|---|---|---|
| FeCo NPs@PNC | 0.5-28 | 0.38 | [ |
| N-CQDs | 5-40 | 1.773 | [ |
| Cu-Ag/rGO | 1-30 | 3.6 | [ |
| Cu NPs | 1-10 | 0.68 | [ |
| LaF3:Ce,Tb | 8-10 | 2.4 | [ |
| CuO/Pt | 1-600 | 0.796 | [ |
| MIL-88 | 2.57-10.1 | 1.03 | [ |
| Pt-Au DNPs | 1-15 | 0.078 | This work |
| [1] |
REYES A C, PLACHE D C, KOUDELKA A P , et al. Enzyme architecture: breaking down the catalytic cage that activates orotidine 5′-monophosphate decarboxylase for catalysis. Journal of the American Chemical Society, 2018,140:17580-17590.
DOI URL PMID |
| [2] | LIU J, HU X, HOU S , et al. Au@Pt core/shell nanorods with peroxidase- and ascorbate oxidase-like activities for improved detection of glucose. Sensors and Actuators B-Chemical, 2012,166:708-714. |
| [3] |
WU T, MA Z, LI P , et al. Colorimetric detection of ascorbic acid and alkaline phosphatase activity based on the novel oxidase mimetic of Fe-Co bimetallic alloy encapsulated porous carbon nanocages. Talanta, 2019,202:354-361.
DOI URL PMID |
| [4] |
CHEN M, WANG Z, SHU J , et al. Mimicking a natural enzyme system: cytochrome C oxidase-like activity of Cu2O nanoparticles by receiving electrons from cytochrome C. Inorganic Chemistry, 2017,56(16):9400-9403.
DOI URL PMID |
| [5] |
HWANG E T, YTATAVAIRT R, CHUNG J , et al. New functional amorphous calcium phosphate nanocomposites by enzyme-assisted biomineralization. ACS Applied Materials & Interfaces, 2013,5(3):532-537.
DOI URL PMID |
| [6] |
LIU Y, WU H, CHONG Y , et al. Platinum nanoparticles: efficient and stable catechol oxidase mimetics. ACS Applied Materials & Interfaces, 2015,7(35):19709-19717.
DOI URL PMID |
| [7] |
LIU J B, JIANG X M, WANG L M , et al. Ferroxidase-like activity of Au nanorod/Pt nanodot structures and implications for cellular oxidative stress. Nano Research, 2015,8(12):4024-4037.
DOI URL |
| [8] |
ZHANG K, HU X, LIU J , et al. Formation of PdPt alloy nanodots on gold nanorods: tuning oxidase-like activities via composition. Langmuir, 2011,27(6):2796-2803.
DOI URL PMID |
| [9] |
GAO L Z, NIE L, ZHANG J B , et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotechnology, 2007,2(9):577-583.
DOI URL PMID |
| [10] |
QIN W, SU L, YANG C , et al. Colorimetric detection of sulfite in foods by a TMB-O2-Co3O4 nanoparticles detection system. Journal of Agricultural and Food Chemistry, 2014,62(25):5827-5834.
DOI URL |
| [11] | VYADA P K, SINGH V K, CHANDRA S , et al. Green synthesis of fluorescent carbon quantum dots from azadirachta indica leaves and their peroxidase-mimetic activity for the detection of H2O2 and ascorbic acid in common fresh fruits. ACS Biomaterials Science & Engineering, 2019,5:623-632. |
| [12] |
HU Y, GAO X J, ZHU Y , et al. Nitrogen-doped carbon nanomaterials as highly active and specific peroxidase mimics. Chemistry of Materials, 2018,30:6431-6439.
DOI URL |
| [13] |
WANG G L, XU X F, WU X M , et al. Visible-light-stimulated enzymelike activity of graphene oxide and its application for facile glucose sensing. Journal of Physical Chemistry C, 2014,118(48):28109-28117.
DOI URL |
| [14] |
CHEN W, FANG X, LI H , et al. DNA-mediated inhibition of peroxidase-like activities on platinum nanoparticles for simple and rapid colorimetric detection of nucleic acids. Biosensors & Bioelectronics, 2017,94:169-175.
DOI URL PMID |
| [15] | ARAYSCHUKIAT S, KONGTES C, BARTHEL A , et al. Ascorbic acid as a bifunctional hydrogen bond donor for the synthesis of cyclic carbonates from CO2 under ambient conditions. ACS Sustainable Chemistry & Engineering, 2017,5:6392-6397. |
| [16] |
HATAMIE A, RAHMATI R, REZVANI E , et al, Yttrium hexacyanoferrate microflowers on freestanding three-dimensional graphene substrates for ascorbic acid detection. ACS Applied Nano Materials, 2019,2:2212-2221.
DOI URL |
| [17] |
ZHAO L, LIAO K, WONG L , et al. Electro-oxidation of ascorbic acid by cobalt core-shell nanoparticles on a H-terminated Si(100) and by nanostructured cobalt-coated Si nanowire electrodes. ACS Applied Materials & Interfaces , 2013,5(7):2410-2416.
DOI URL PMID |
| [18] |
JANG H I, LEE H G . Stability of chitosan nanoparticles for l-ascorbic acid during heat treatment in aqueous solution. Journal of Agricultural and Food Chemistry, 2008,56(6):1936-1941.
DOI URL PMID |
| [19] |
SINGH V, MONDAL P C, LAKSHMANAN J Y , et al. “Turn on” electron-transfer-based selective detection of ascorbic acid via copper complexes immobilized on glass. Analyst, 2012,137:3216-3219.
DOI URL |
| [20] |
MALASHIKJINA N, PAVLOV V . DNA-decorated nanoparticles as nanosensors for rapid detection of ascorbic acid. Biosensors & Bioelectronics, 2012,33(1):241-246.
DOI URL PMID |
| [21] |
GOKMEN V, KAHRAMAN N, DEMIR N , et al. Enzymatically validated liquid chromatographic method for the determination of ascorbic and dehydroascorbic acids in fruit and vegetables. Journal of Chromatography A, 2000,881(1):309-316.
DOI URL |
| [22] |
DU J, SHAO Q, YIN S , et al. Colorimetric chemodosimeter based on diazonium-gold-nanoparticle complexes for sulfite ion detection in solution. Small, 2012,8:3412-3416.
DOI URL PMID |
| [23] |
ZHANG X, HE S, CHEN Z , et al. CoFe2O nanoparticles as oxidase mimic-mediated chemiluminescence of aqueous luminol for sulfite in white wines. Journal of Agricultural and Food Chemistry, 2013,61(4):840-847.
DOI URL |
| [24] |
WANG X, HAN Q, CAI S , et al. Excellent peroxidase mimicking property of CuO/Pt nanocomposites and their application as an ascorbic acid sensor. Analyst, 2017,142:2500-2506.
DOI URL PMID |
| [25] |
HE W, HAN X, JIA H , et al. AuPt alloy nanostructures with tunable composition and enzyme-like activities for colorimetric detection of bisulfide. Scientific Reports, 2017,7:40103.
DOI URL PMID |
| [26] |
ZHENG Y, ZENG J, RUDITSKIY A , et al. Oxidative etching and its role in manipulating the nucleation and growth of noble-metal nanocrystals. Chemistry of Materials, 2014,26:22-33.
DOI URL |
| [27] |
MA L, WANG C, GONG M , et al. Control over the branched structures of platinum nanocrystals for electrocatalytic applications ACS Nano, 2012,6:9797-9806.
DOI URL PMID |
| [28] |
XIONG Y J, CHEN J Y, WILEY B , et al. Understanding the role of oxidative etching in the polyol synthesis of Pd nanoparticles with uniform shape and size. Journal of the American Chemical Society , 2005,127(20):7332-7333.
DOI URL PMID |
| [29] |
RAM S, FECHT H J . Modulating up-energy transfer and violet- blue light emission in gold nanoparticles with surface adsorption of poly(vinyl pyrrolidone) molecules. Journal of Physical Chemistry C, 2011,115(16):7817-7828.
DOI URL |
| [30] | TSUJI M, JIANG P, HIKINO S , et al. Toward to branched platinum nanoparticles by polyol reduction: a role of poly(vinylpyrrolidone) molecules. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 2008,317:23-31. |
| [31] |
XIONG Y, WASHIO I, CHEN J , et al. Poly(vinyl pyrrolidone): A dual functional reductant and stabilizer for the facile synthesis of noble metal nanoplates in aqueous solutions. Langmuir, 2006,22(20):8563-8570.
DOI URL PMID |
| [32] |
CAO Y, YANG Y, SHAN Y , et al. Large-scale template-free synthesis of ordered mesoporous platinum nanocubes and their electrocatalytic properties. Nanoscale, 2015,7(46):19461-19467.
DOI URL PMID |
| [33] |
ZHANG P, SUN D, CHO A , et al. Modified carbon nitride nanozyme as bifunctional glucose oxidase-peroxidase for metal- free bioinspired cascade photocatalysis. Nature Communications, 2019,10:940.
DOI URL PMID |
| [34] |
LAI W, ZHUANG J , et al. Novel colorimetric immunoassay for ultrasensitive monitoring of brevetoxin b based on enzyme- controlled chemical conversion of sulfite to sulfate. Journal of Agricultural and Food Chemistry, 2015,63(7):1982-1989.
DOI URL PMID |
|
CHAI D H, MA Z, YAN H , et al. Synergistic effect of sandwich polyoxometalates and copper-imidazole complexes for enhancing the peroxidase-like activity. RSC Advances, 2015,5:78771-78779.
DOI URL PMID |
| [1] | 郭大刚,徐可为,岳进,赵晓云,憨勇. 含锶磷灰石骨水泥浆体的pH值及其固化体的体外细胞毒性评价[J]. 无机材料学报, 2005, 20(5): 1159-1166. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||