无机材料学报 ›› 2017, Vol. 32 ›› Issue (10): 1055-1062.DOI: 10.15541/jim20160677 CSTR: 32189.14.10.15541/jim20160677
常希望1, 陈 宁2, 王丽君3, 李福燊2, 卞刘振1, 周国治1
收稿日期:2016-12-12
修回日期:2017-02-08
出版日期:2017-10-20
网络出版日期:2017-09-21
作者简介:常希望(1986–), 男, 博士研究生. E-mail: changxiwang2006@126.com
基金资助:CHANG Xi-Wang1, CHEN Ning2, WANG Li-Jun3, LI Fu-Shen2, BIAN Liu-Zhen1, CHOU Kuo-Chih1
Received:2016-12-12
Revised:2017-02-08
Published:2017-10-20
Online:2017-09-21
About author:CHANG Xi-Wang. E-mail: changxiwang2006@126.com
摘要:
采用第一性原理, 对元素周期表中3~6周期52种元素作为固体氧化物燃料电池(SOFC) Sr为A位系列钙钛矿结构电极材料B位替换元素的相关结构相的结合能进行了系统计算, 据此分析了各元素对生成立方相和六方相结构稳定性影响的趋势。通过对相关体系的成分比例进行推算, 讨论了这些实验体系在稳定性趋势图中的分布规律, 进一步对上述体系的实验数据进行分析, 得到了以Mo-Fe-Co连线为中心的成分优化区域。根据相关氧离子扩散模型的计算, 结果显示该区域形成的原因与氧空位形成能、迁移能以及禁带宽度均较为适中有关。以上理论结合实验的研究为电极材料的成分优化提供了理论指导。
中图分类号:
常希望, 陈 宁, 王丽君, 李福燊, 卞刘振, 周国治. 固体氧化物燃料电池Sr系钙钛矿电极B位元素成分优化规律[J]. 无机材料学报, 2017, 32(10): 1055-1062.
CHANG Xi-Wang, CHEN Ning, WANG Li-Jun, LI Fu-Shen, BIAN Liu-Zhen, CHOU Kuo-Chih. Optimal Principle on Composition of B Site Elements in Perovskite Electrodes with Sr at A Site for Solid Oxide Fuel Cell[J]. Journal of Inorganic Materials, 2017, 32(10): 1055-1062.
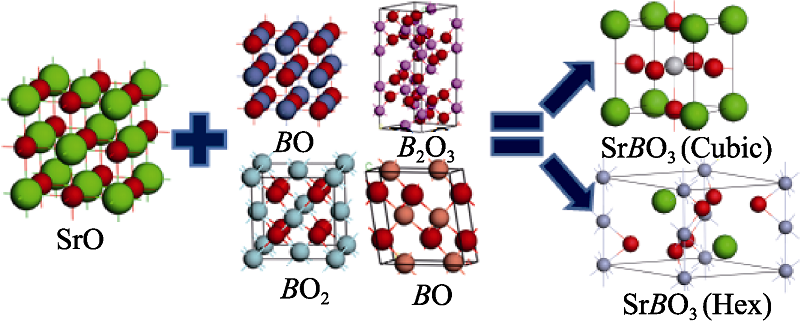
图1 二元氧化物生成立方相和六方相的示意图
Fig. 1 Diagram for the binary oxides reaction to form cubic or hexagonal perovskitesAll of models calculated in this work has been showed above, including SrO, each binary oxide of B site elements, cubic perovskites, and hexagonal perovskites
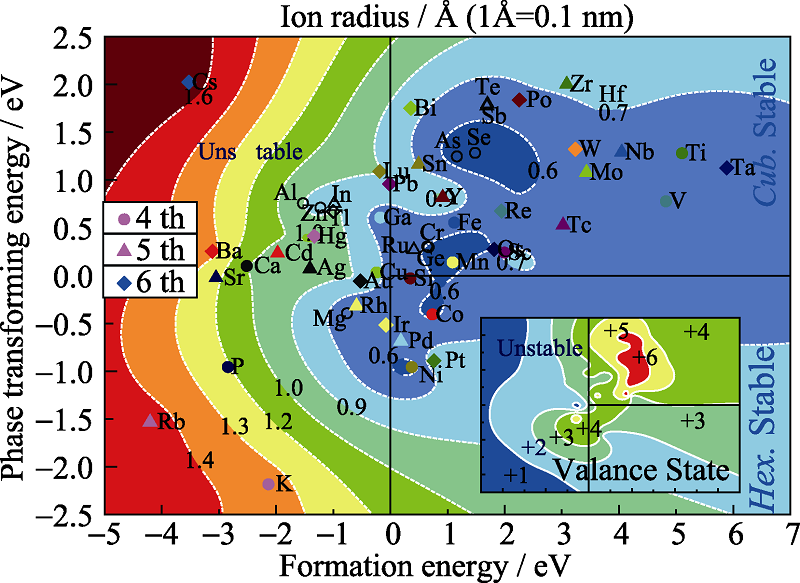
图2 结构稳定性趋势图
Fig. 2 Structure stability tendency diagramThe color in the main graph that changed from red to blue represents that ion radius changed from large to small; the color in the inserted graph that changed from red to blue represents that ion valance states changed from high to low
| Composition | Cathode/ Anode | Electrolyte/ Anode (Cathode) | Cell Supporting Part | Conductivity (S•cm-1)/ Temperature(℃) | Power density (mW·cm-2) /Temperature(℃) |
|---|---|---|---|---|---|
| SrCo0.7Fe0.2Nb0.1O3-δ[ | Cathode | SDC*/NiO-SDC | Electrolyte | 304/350 | 630/800 |
| SrCo0.7Fe0.2Nb0.1O3-δ[ | Cathode | SDC/NiO-SDC | Anode | 1587/600 | |
| SrCo0.8Sc0.2O3-δ[ | Cathode | SDC/NiO-SDC | Anode | 902/600 | |
| SrCo0.9Nb0.1O3-δ[ | Cathode | LSGM*/NiO-SDC | Electrolyte | 462.7/300 | 678/800 |
| SrCo0.9Nb0.1O3-δ[ | Cathode | LSGM/NiO-SDC | Electrolyte | 50/850 | 600/850 |
| SrCo0.95Ti0.05O3-δ[ | Cathode | LSGM/SMF* | Electrolyte | 398/350 | 824/850 |
| SrCo0.97V0.03O3-δ[ | Cathode | LSGM/SMF | Electrolyte | 8/850 | 550/850 |
| SrCo0.95Sn0.05O3-δ[ | Cathode | SDC/NiO-SDC | Anode | 545/550 | 847/700 |
| SrCo0.7Fe0.2Ta0.1O3-δ[ | Cathode | LSGM/NiO-SDC | Electrolyte | 249.4/350 | 652.9/800 |
| SrCo0.95Sb0.05O3-δ | Cathode | LSGM/SMM* | Electrolyte | 500/400[ | 618/850[ |
| SrCo0.9Ta0.1O3-δ[ | Cathode | - | 471/325 | ||
| SrFe0.95Ti0.05O3-δ[ | Cathode | LSGM/NiO-SDC | Electrolyte | 72/650 | 605/800 |
| SrFe0.75Cr0.25O3-δ[ | Symmetry Electrode | - | 22/600 | ||
| SrFe0.7Cu0.3O3-δ[ | Cathode | - | 54/800 | ||
| SrFe0.9Nb0.1O3-δ[ | Cathode | SDC/NiO-SDC | Electrolyte | 104.4/450 | 407/800 |
| SrFe0.75Zr0.25O3-δ[ | Symmetry Electrode | LSGM | Electrolyte | 11.2/650 | 425/800 |
| SrFe0.75Mo0.25O3-δ | Symmetry Electrode | LSGM | Electrolyte | 23.8/650[ | 970/800[ |
| SrMo0.9Fe0.1O3-δ[ | Anode | LSGM/SCF* | Electrolyte | 305/50 | 874/850 |
| SrMo0.9Co0.1O3-δ[ | Anode | LSGM/SCF | Electrolyte | 386/50 | 793/850 |
| SrMo0.9Cr0.1O3-δ[ | Anode | LSGM/SCF | Electrolyte | 365/50 | 755/850 |
| SrTi0.8Nb0.2O3-δ[ | Anode | LSGM/LSCF* | Electrolyte | 794/850 | |
| SrFe0.8Ta0.2O3-δ[ | Cathode | - | 25.9/700 | ||
| SrFe0.9W0.1O3-δ[ | Anode | - | 60.4/700 |
表1 常见SrBO3成分及相关性能数据
Table 1 Compositions of SrBO3 perovskite and their related performance data
| Composition | Cathode/ Anode | Electrolyte/ Anode (Cathode) | Cell Supporting Part | Conductivity (S•cm-1)/ Temperature(℃) | Power density (mW·cm-2) /Temperature(℃) |
|---|---|---|---|---|---|
| SrCo0.7Fe0.2Nb0.1O3-δ[ | Cathode | SDC*/NiO-SDC | Electrolyte | 304/350 | 630/800 |
| SrCo0.7Fe0.2Nb0.1O3-δ[ | Cathode | SDC/NiO-SDC | Anode | 1587/600 | |
| SrCo0.8Sc0.2O3-δ[ | Cathode | SDC/NiO-SDC | Anode | 902/600 | |
| SrCo0.9Nb0.1O3-δ[ | Cathode | LSGM*/NiO-SDC | Electrolyte | 462.7/300 | 678/800 |
| SrCo0.9Nb0.1O3-δ[ | Cathode | LSGM/NiO-SDC | Electrolyte | 50/850 | 600/850 |
| SrCo0.95Ti0.05O3-δ[ | Cathode | LSGM/SMF* | Electrolyte | 398/350 | 824/850 |
| SrCo0.97V0.03O3-δ[ | Cathode | LSGM/SMF | Electrolyte | 8/850 | 550/850 |
| SrCo0.95Sn0.05O3-δ[ | Cathode | SDC/NiO-SDC | Anode | 545/550 | 847/700 |
| SrCo0.7Fe0.2Ta0.1O3-δ[ | Cathode | LSGM/NiO-SDC | Electrolyte | 249.4/350 | 652.9/800 |
| SrCo0.95Sb0.05O3-δ | Cathode | LSGM/SMM* | Electrolyte | 500/400[ | 618/850[ |
| SrCo0.9Ta0.1O3-δ[ | Cathode | - | 471/325 | ||
| SrFe0.95Ti0.05O3-δ[ | Cathode | LSGM/NiO-SDC | Electrolyte | 72/650 | 605/800 |
| SrFe0.75Cr0.25O3-δ[ | Symmetry Electrode | - | 22/600 | ||
| SrFe0.7Cu0.3O3-δ[ | Cathode | - | 54/800 | ||
| SrFe0.9Nb0.1O3-δ[ | Cathode | SDC/NiO-SDC | Electrolyte | 104.4/450 | 407/800 |
| SrFe0.75Zr0.25O3-δ[ | Symmetry Electrode | LSGM | Electrolyte | 11.2/650 | 425/800 |
| SrFe0.75Mo0.25O3-δ | Symmetry Electrode | LSGM | Electrolyte | 23.8/650[ | 970/800[ |
| SrMo0.9Fe0.1O3-δ[ | Anode | LSGM/SCF* | Electrolyte | 305/50 | 874/850 |
| SrMo0.9Co0.1O3-δ[ | Anode | LSGM/SCF | Electrolyte | 386/50 | 793/850 |
| SrMo0.9Cr0.1O3-δ[ | Anode | LSGM/SCF | Electrolyte | 365/50 | 755/850 |
| SrTi0.8Nb0.2O3-δ[ | Anode | LSGM/LSCF* | Electrolyte | 794/850 | |
| SrFe0.8Ta0.2O3-δ[ | Cathode | - | 25.9/700 | ||
| SrFe0.9W0.1O3-δ[ | Anode | - | 60.4/700 |
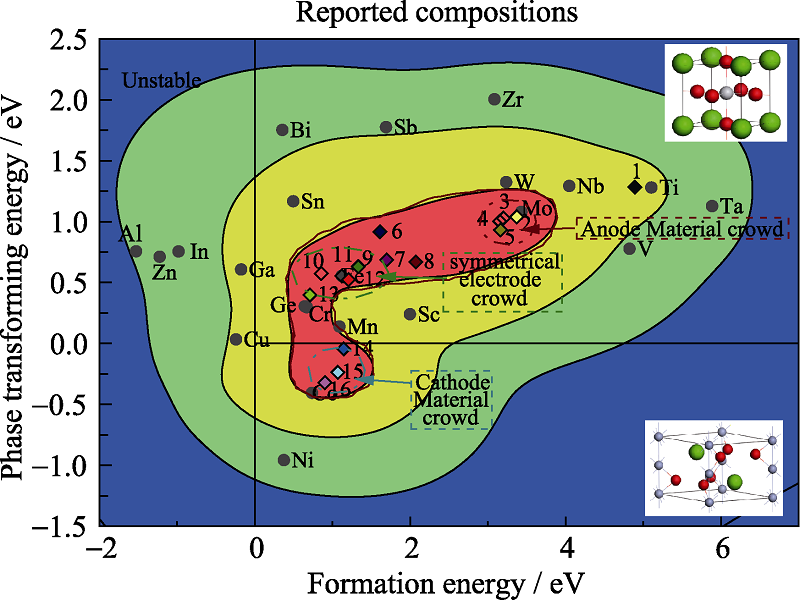
图3 稳定性与实验数据的关系规律
Fig. 3 Relationship between stability and experience data1: SrTi0.8Nb0.2O3-δ; 2: SrMo0.98Ni0.02O3-δ; 3: SrMo0.9Fe0.1O3-δ; 4: SrMo0.9Cr0.1O3-δ; 5: SrMo0.9Co0.1O3-δ; 6: SrFe0.75Zr0.25O3-δ; 7: SrFe0.75 Mo0.25O3-δ; 8: SrFe0.8Ta0.2O3-δ; 9: SrFe0.9W0.1O3-δ; 10: SrFe0.9Al0.1O3-δ; 11: SrFeO2.5+δ; 12: SrFe0.9Sc0.1O3-δ; 13: SrFe0.7Cu0.3O3-δ; 14: SrCo0.7Fe0.2 Nb0.1O3-δ; 15: Sr0.9Ce0.1Co0.9Nb0.1O3-δ; 16: SrCo0.95Nb0.05O3-δ; Color distribution for the amount of compositions reported in references
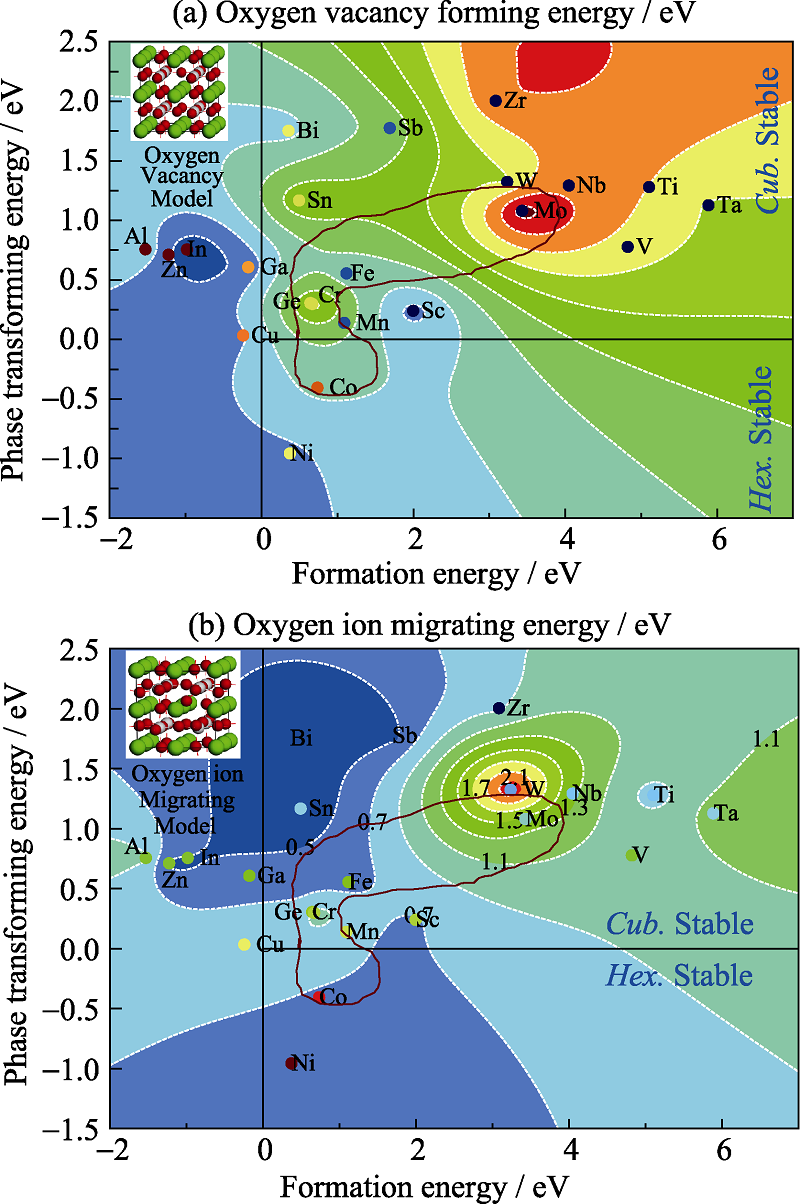
图4 氧空位形成能、迁移能随稳定性的影响规律
Fig. 4 Oxygen vacancy forming and ion migrating energy changing tendency based on structure stability(a) Vacancy forming energy; (b) Ion migrating energy
| [1] | SHANNON R D, PREWITT C T.Effective Ionic radii in oxides and fluorides.Acta Crystallogr. Sect. B: Struct. Sci., 1969, 25(5): 925-946. |
| [2] | MUÑOZ-GARCÍA A B, RITZMANN A M, PAVONE M, et al. Oxygen transport in perovskite-type solid oxide fuel cell materials: insights from quantum mechanics.Acc. Chem. Res., 2014, 47(11): 3340-3348. |
| [3] | CHEN Y, ZHOU W, DING D, et al.Advances in cathode materials for solid oxide fuel cells: complex oxides without alkaline earth metal elements.Adv. Energy Mater., 2015, 5(18): 1500537. |
| [4] | MASTRIKOV Y A, MERKLE R, KOTOMIN E A, et al.Formation and migration of oxygen vacancies in La1-xSrxCo1-yFeyO3-δ perovskites: insight from ab initio calculations and comparison with Ba1-xSrxCo1-yFeyO3-δ.Phys. Chem. Chem. Phys., 2013, 15(3): 911-918. |
| [5] | KOTOMIN E A, MERKLE R, MASTRIKOV Y A, et al.First principles modeling of oxygen mobility in perovskite SOFC cathode and oxygen permeation membrane materials.Electrochem. Soc. Trans., 2011, 35(1): 823-830. |
| [6] | DENG Z, YANG W, LIU W, et al.Relationship between transport properties and phase transformations in mixed-conducting oxides.J. Solid State Chem., 2006, 179(2): 362-369. |
| [7] | NAGAI T, ITO W, SAKON T.Relationship between cation substitution and stability of perovskite structure in SrCoO3-δ-based mixed conductors.Solid State Ionics, 2007, 177(39/40): 3433-3444. |
| [8] | FERNÁNDEZ-ROPERO A J, PORRAS-VÁZQUEZ J M, CABEZA A, et al. High valence transition metal doped strontium ferrites for electrode materials in symmetrical SOFCs.J. Power Sources, 2014, 249: 405-413. |
| [9] | BLENNOW P, HANSEN K K, WALLENBERG L R, et al.Electrochemical characterization and redox behavior of Nb-doped SrTiO3.Solid State Ionics, 2009, 180(1): 63-70. |
| [10] | LI M, ZHOU W, ZHU Z.Recent development on perovskite-type cathode materials based on SrCoO3-δ parent oxide for intermediatetemperature solid oxide fuel cells.Asia-Pac. J. Chem. Eng., 2016, 11(3): 370-381. |
| [11] | XIAO G, LIU Q, WANG S, et al.Synthesis and characterization of Mo-doped SrFeO3-δ as cathode materials for solid oxide fuel cells.J. Power Sources, 2012, 202: 63-69. |
| [12] | NAGAI I, SHIRAKAWA N, IKEDA S, et al.Highest conductivity oxide SrMoO3 grown by a floating-zone method under ultralow oxygen partial pressure.Appl. Phys. Lett., 2005, 87(2): 024105. |
| [13] | LI NA, CHEN NING, LI FUSHEN, et al.Theoretical research on optimization ingredient regulation of BaBO3 series hypoxic materials.Scientia Sinica Phys, Mech & Astron, 2011, 41(9): 1075-1079. |
| [14] | REN YUMEI, CHEN NING, ZHAO HAILEI, et al.Theoretical research on optimization dopant regulation of La2BO4 series mixed conductor materials.J. Inorg. Mater., 2013, 28(8): 841-846. |
| [15] | CHANG XIWANG, CHEN NING, WANG LIJUN, et al.Optimization rule of anode materials for solid oxide fuel cells.J. Inorg. Mater., 2015, 30(10): 1043-1048. |
| [16] | ZHAO JICHENG, A perspective on the materials genome initiative.Chin. J. Nat., 2014, 36(2): 89-104. |
| [17] | PERDEW J P, BURKE K, ERNZERHOF M.Generalized gradient approximation made simple.Phys. Rev. Lett., 1996, 77(18): 3865-3868. |
| [18] | VANDERBILT D.Soft self-consistent pseudopotentials in a generalized eigenvalue formalism.Physical. Review. B, 1990, 41(11): 7892-7895. |
| [19] | YOON J S, YI E J, CHOI B H, et al.Methane oxidation behavior over La0.08Sr0.92Fe0.20Ti0.80O3-δ perovskite oxide for SOFC anode.Ceram. Int., 2014, 40(1): 1525-1529. |
| [20] | AGUADERO A, PEREZ-COLL D, ALONSO J A, et al.A new family of Mo-doped SrCoO3-δ perovskites for application in reversible solid state electrochemical cells.Chem. Mater., 2012, 24(14): 2655-2663. |
| [21] | ZHANG J, XIE K, WEI H, et al.In situ formation of oxygen vacancy in perovskite Sr0.95Ti0.8Nb0.1M0.1O3 (M=Mn, Cr) toward efficient carbon dioxide electrolysis.Sci. Rep., 2014, 4: 7082. |
| [22] | CASCOS V, MARTÍNEZ-CORONADO R, ALONSO J A. New Nb-doped SrCo1-xNbxO3-δ perovskites performing as cathodes in solid-oxide fuel cells.Int. J. Hydrogen Energy, 2014, 39(26): 14349-14354. |
| [23] | MARKOV A A, SHALAEVA E V, TYUTYUNNIK A P, et al.Structural features and enhanced high-temperature oxygen ion transport in SrFe1-xTaxO3-δ.J. Solid State Chem., 2013, 197: 191-197. |
| [24] | ZHANG H, WANG T, DONG X, et al.Preparation and oxygen permeation properties of SrFe(Cu)O3-δ dense ceramic membranes.J. Nat. Gas Chem., 2009, 18(1): 45-49. |
| [25] | LEONIDOVA I A, PATRAKEEVA M V, BAHTEEVAA J A, et al.Oxygen-ion and electron conductivity in Sr2(Fe1-xGax)2O5.J. Solid State Chem., 2006, 179(10): 3045-3051. |
| [26] | LONG Y, KANEKO Y, ISHIWATA S, et al.Synthesis of cubic SrCoO3 single crystal and its anisotropic magnetic and transport properties.J. Phys.: Condens. Matter, 2011, 23(24): 245601. |
| [27] | SØNDENÅ R, RAVINDRAN P, STØLEN S, et al. Electronic structure and magnetic properties of cubic and hexagonal SrMnO3.Phys. Rev. B, 2006, 74(14): 144102. |
| [28] | HODGES J P, SHORT S, JORGENSEN J D, et al.Evolution of oxygen-vacancy ordered crystal structures in the perovskite series SrnFenO3n-1(n=2, 4, 8, and ∞), and the Relationship to electronic and magnetic properties.J. Solid State Chem., 2000, 151(2): 190-209. |
| [29] | MARKOV A A, LEONIDOV I A, PATRAKEEV M V, et al.Structural stability and electrical transport in SrFe1-xMoxO3-δ.Solid State Ionics, 2008, 179(21-26): 1050-1053. |
| [30] | ARÉVALO-LÓPEZ A M, RODGERS J A, SENN M S, et al. “hard-soft” synthesis of SrCrO3-δ superstructure phases.Angew. Chem. Int. Ed., 2012, 51(43): 10791-10794. |
| [31] | LÜ S, YU B, MENG X, et al.Characterization of SrCo0.7Fe0.2Nb0.1O3-δ cathode materials for intermediate-temperature solid oxide fuel cells.J. Power Sources, 2015, 273: 244-254. |
| [32] | ZHU Y, SUNARSO J, ZHOU W, et al.High-performance SrNb0.1Co0.9-xFexO3-δ perovskite cathodes for low-temperature solid oxide fuel cells.J. Mater. Chem. A, 2014, 2: 15454-15462. |
| [33] | ZHOU W., SHAO Z., RAN R., et al.Novel SrSc0.2Co0.8O3-δ as a cathode material for low temperature solid-oxide fuel cell.Electrochem. Commun., 2008, 10(10): 1647-1651. |
| [34] | WANG F, ZHOU Q, HE T, et al.Novel SrCo1-yNbyO3-δ cathode for intermediate-temperature solid oxide fuel cells.J. Power Sources, 2010, 195(12): 3772-3778. |
| [35] | CASCOS V, TRONCOSO L, ALONSO J A.New families of Mn+-doped SrCo1-xMxO3-δ perovskites performing as cathodes in solid-oxide fuel cells.Int. J. Hydrogen Energy, 2015, 40(34): 11333-11341. |
| [36] | WANG S, HSU Y F, YEH C T, et al.Characteristic of SrCo1-xSnxO3-δ cathode materials for use in solid oxide fuel cells.Solid State Ionics, 2012, 227: 10-16. |
| [37] | QU B, LONG W, JIN F, et al.SrCo0.7Fe0.2Ta0.1O3-δ perovskite as a cathode material for intermediate-temperature solid oxide fuel cells.Int. J. Hydrogen Energy, 2014, 39(23): 12074-12082. |
| [38] | AGUADERO A, PÉREZ-COLL D, CALLE C D L, et al. SrCo1-xSbxO3-δ perovskite oxides as cathode materials in solid oxide fuel cells.J. Power Sources, 2009, 192(1): 132-137. |
| [39] | AGUADERO A, ALONSO J A, PÉREZ-COLL D, et al. SrCo0.95Sb0.05O3-δ as cathode material for high power density solid oxide fuel cells.Chem. Mater., 2010, 22(3): 789-798. |
| [40] | ZHOU Q, WEI T, SHI Y, et al.Evaluation and optimization of SrCo0.9Ta0.1O3-δ perovskite as cathodes for solid oxide fuel cell.Curr. Appl. Phys., 2012, 12(4): 1092-1095. |
| [41] | YU X, LONG W, JIN F, et al.Cobalt-free perovskite cathode materials SrFe1-xTixO3-δ and performance optimization for intermediate-temperature solid oxide fuel cells.Electrochim. Acta, 2014, 123: 426-434. |
| [42] | LI Q, XIA T, SUN L, et al.Electrochemical performance of novel cobalt-free perovskite SrFe0.7Cu0.3O3-δ cathode for intermediate temperature solid oxide fuel cells.Electrochim. Acta, 2014, 150: 151-156. |
| [43] | ZHOU Q, ZHANG L, HE T.Cobalt-free cathode material SrFe0.9Nb0.1O3-δ for intermediate-temperature solid oxide fuel cells.Electrochem. Commun., 2010, 12(2): 285-287. |
| [44] | SANTOS-GÓMEZ L D, COMPANA J M, BRUQUE S, et al. Symmetric electrodes for solid oxide fuel cells based on Zr-doped SrFeO3-δ.J. Power Sources, 2015, 279: 419-427. |
| [45] | MENG X, LIU X, HAN D, et al.Symmetrical solid oxide fuel cells with impregnated SrFe0.75Mo0.25O3-δ electrodes.J. Power Sources, 2014, 252: 58-63. |
| [46] | MARTÍNERZ-CORONADO R, ALONSO J A, AGUADERO A, et al. Optimized energy conversion efficiency in solid-oxide fuel cells implementing SrMo1-xFexO3-δ perovskites as anodes.J. Power Sources, 2012, 208: 153-158. |
| [47] | MARTÍNERZ-CORONADO R, ALONSO J A, FERNÁNDEZ-DÍAZ M T. SrMo0.9Co0.1O3-δ: A potential anode for intermediate-temperature solid-oxide fuel cells (IT-SOFC).J. Power Sources, 2014, 258: 76-82. |
| [48] | MARTÍNERZ-CORONADO R, ALONSO J A, AGUADERO A, et al. New SrMo1-xCrxO3-δ perovskites as anodes in solid-oxide fuel cells.Int. J. Hydrogen Energy, 2014, 39(8): 4067-4073. |
| [49] | XIAO G, WANG S, LIN Y, et al.Releasing metal catalysts via phase transition: (NiO)0.05-(SrTi0.8Nb0.2O3)0.95 as a redox stable anode material for solid oxide fuel cells.Appl. Mater. Interfaces, 2014, 6(22): 19990-19996. |
| [50] | SHALAEVA E V, PATRAKEEV M V, MARKOV A A, et al.Ion transport in dual-phase SrFe1-xТаxO3-δ (x=0.03-0.10): effects of redox cycling.J. Solid State Electrochem., 2015, 19(3): 841-849. |
| [51] | MARKOV A A, PATRAKEEV M V, SAVINSKAYA O A, et al.Oxygen nonstoichiometry and high-temperature transport in SrFe1-xWxO3-δ.Solid State Ionics, 2008, 179(1-6): 99-103. |
| [52] | LI X, ZHAO H, SHEN W, et al.Synthesis and properties of Y-doped SrTiO3 as an anode material for SOFCs.J. Power Sources, 2007, 166(1): 47-52. |
| [53] | LEE K J, IGUCHI E.Electronic properties of SrMnO3-x.J. Solid State Chem., 1995, 114(1): 242-248. |
| [54] | MACCHESNEY J B, SHERWOOD R C, POTTER J F.Electric and magnetic properties of the strontium ferrates.J. Chem. Phys., 1965, 43(6): 1907-1913. |
| [55] | HOFFMANN M, BORISOV V S, OSTANIN S, et al.Magnetic properties of defect-free and oxygen-deficient cubic SrCoO3-δ.Phys. Rev. B, 2015, 92(9): 094427. |
| [1] | 张琨, 王宇, 朱腾龙, 孙凯华, 韩敏芳, 钟秦. LaNi0.6Fe0.4O3阴极接触材料导电特性调控及其对SOFC电化学性能的影响[J]. 无机材料学报, 2024, 39(4): 367-373. |
| [2] | 周云凯, 刁亚琪, 王明磊, 张宴会, 王利民. 聚苯胺改性Ti3C2(OH)2抗氧化性的第一性原理计算研究[J]. 无机材料学报, 2024, 39(10): 1151-1158. |
| [3] | 吴晓维, 张涵, 曾彪, 明辰, 孙宜阳. 杂化泛函HSE和PBE0计算CsPbI3缺陷性质的比较研究[J]. 无机材料学报, 2023, 38(9): 1110-1116. |
| [4] | 杨颖康, 邵怡晴, 李柏良, 吕志伟, 王路路, 王亮君, 曹逊, 吴宇宁, 黄荣, 杨长. Cl掺杂对CuI薄膜发光性能增强研究[J]. 无机材料学报, 2023, 38(6): 687-692. |
| [5] | 肖美霞, 李苗苗, 宋二红, 宋海洋, 李钊, 毕佳颖. 表面端基卤化Ti3C2 MXene应用于锂离子电池高容量电极材料的研究[J]. 无机材料学报, 2022, 37(6): 660-668. |
| [6] | 赵宇鹏,贺勇,张敏,史俊杰. 新型二维Zr2CO2/InS异质结可见光催化产氢性能的第一性原理研究[J]. 无机材料学报, 2020, 35(9): 993-998. |
| [7] | 陈雷雷, 邓子旋, 李勉, 李朋, 常可可, 黄峰, 都时禹, 黄庆. 新型MAX相的相图热力学研究[J]. 无机材料学报, 2020, 35(1): 35-40. |
| [8] | 罗凌虹, 胡佳幸, 程亮, 徐序, 吴也凡, 林囿辰. Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Ce0.9Gd0.1O2-δ中低温固体氧化物燃料电池复相阴极的研究[J]. 无机材料学报, 2018, 33(4): 441-446. |
| [9] | 张建峰, 曹惠杨, 王红兵. 新型二维材料MXene的研究进展[J]. 无机材料学报, 2017, 32(6): 561-570. |
| [10] | 王晓媛, 嶋田隆広, 北村隆行. 超薄钛酸铅纳米管铁电性和力电耦合特性的第一性原理研究[J]. 无机材料学报, 2014, 29(3): 309-314. |
| [11] | 陈 弦,杨 杰,蒲 健,李 箭. 平板式SOFC结构热应力的有限元分析[J]. 无机材料学报, 2007, 22(2): 339-343. |
| [12] | 忻隽,郑燕青,施尔畏. 材料压电性能的第一性原理计算回顾与展望[J]. 无机材料学报, 2007, 22(2): 193-200. |
| [13] | 杨乃涛,孟秀霞,谭小耀,李正民. 中温固体氧化物燃料电池阳极的研究[J]. 无机材料学报, 2006, 21(2): 409-414. |
| [14] | 杨勇杰,杨建华,屠恒勇,吕之奕,温廷琏. 铬酸锶镧材料的制备和性能研究[J]. 无机材料学报, 1999, 14(5): 739-744. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||