无机材料学报 ›› 2024, Vol. 39 ›› Issue (8): 945-954.DOI: 10.15541/jim20240016 CSTR: 32189.14.10.15541/jim20240016
所属专题: 【能源环境】化工催化(202506)
王旭昌1( ), 焦楚钰1, 季卓1, 焦其瑞1, 秦波2, 杜艳泽2, 郑家军1(
), 焦楚钰1, 季卓1, 焦其瑞1, 秦波2, 杜艳泽2, 郑家军1( ), 李瑞丰1
), 李瑞丰1
收稿日期:2024-01-10
修回日期:2024-04-12
出版日期:2024-08-20
网络出版日期:2024-04-19
通讯作者:
郑家军, 教授. E-mail: zhengjiajun@tyut.edu.cn作者简介:王旭昌(1997-), 男, 硕士研究生. E-mail: wangxuchangxc@163.com
WANG Xuchang1( ), JIAO Chuyu1, JI Zhuo1, JIAO Qirui1, QIN Bo2, DU Yanze2, ZHENG Jiajun1(
), JIAO Chuyu1, JI Zhuo1, JIAO Qirui1, QIN Bo2, DU Yanze2, ZHENG Jiajun1( ), LI Ruifeng1
), LI Ruifeng1
Received:2024-01-10
Revised:2024-04-12
Published:2024-08-20
Online:2024-04-19
Contact:
ZHENG Jiajun, professor. E-mail: zhengjiajun@tyut.edu.cnAbout author:WANG Xuchang (1997-), male, Master candidate. E-mail: wangxuchangxc@163.com
Supported by:摘要:
合成ZSM-5沸石通常以小分子多元胺或季铵盐作为有机结构导向剂(OSDA)。无OSDA的水热合成体系避免了使用有机模板和随后的煅烧步骤, 这不仅可以降低合成成本, 而且避免因有机模板燃烧造成环境污染, 是一种环境友善的合成方法。然而, 已有的研究显示, 某些有机物如乙醇, 会或多或少地参与所谓的无模板合成系统。为确保合成体系中不带入有机模板, 本研究以煅烧的商用ZSM-5沸石为晶种, 铝酸钠为铝源, 硅溶胶为硅源, 在无OSDA的体系中成功制备了一种由棒状纳米晶组成的多晶ZSM-5聚集体。详细研究了ZSM-5晶种的硅/铝比、加入量和晶化条件(如晶化温度以及晶化时间)等对ZSM-5沸石合成的影响。研究结果表明:以含质量分数5.6% MFI拓扑结构晶种的凝胶前驱体为原料, 经水热处理48 h, 可制得由尺寸小于100 nm的初级纳米晶粒组成的高结晶度ZSM-5多晶聚集体; ZSM-5晶种的硅/铝比不影响样品的拓扑结构、孔结构, 但低硅/铝比的晶种更有利于目标沸石快速结晶, 并更有利于获得高酸量尤其是较强酸量的ZSM-5。采用甲醇脱水考察合成的多晶ZSM-5的催化性能, 并与商用参比ZSM-5r催化剂进行比较。结果表明, 与参比催化剂相比, 合成的样品因疏松聚集的纳米初级晶粒堆积形成多级孔而具有较长的催化寿命(16 h vs 8 h), 且合成的多晶聚集体催化剂具有较高的芳烃选择性(28.1%~29.8% vs 26.5%)。
中图分类号:
王旭昌, 焦楚钰, 季卓, 焦其瑞, 秦波, 杜艳泽, 郑家军, 李瑞丰. 晶种诱导合成ZSM-5多晶聚集体及其在甲醇制碳氢化合物中的催化性能[J]. 无机材料学报, 2024, 39(8): 945-954.
WANG Xuchang, JIAO Chuyu, JI Zhuo, JIAO Qirui, QIN Bo, DU Yanze, ZHENG Jiajun, LI Ruifeng. Polycrystalline ZSM-5 Aggregates Induced by Seed and Catalytic Performance in Methanol to Hydrocarbon[J]. Journal of Inorganic Materials, 2024, 39(8): 945-954.
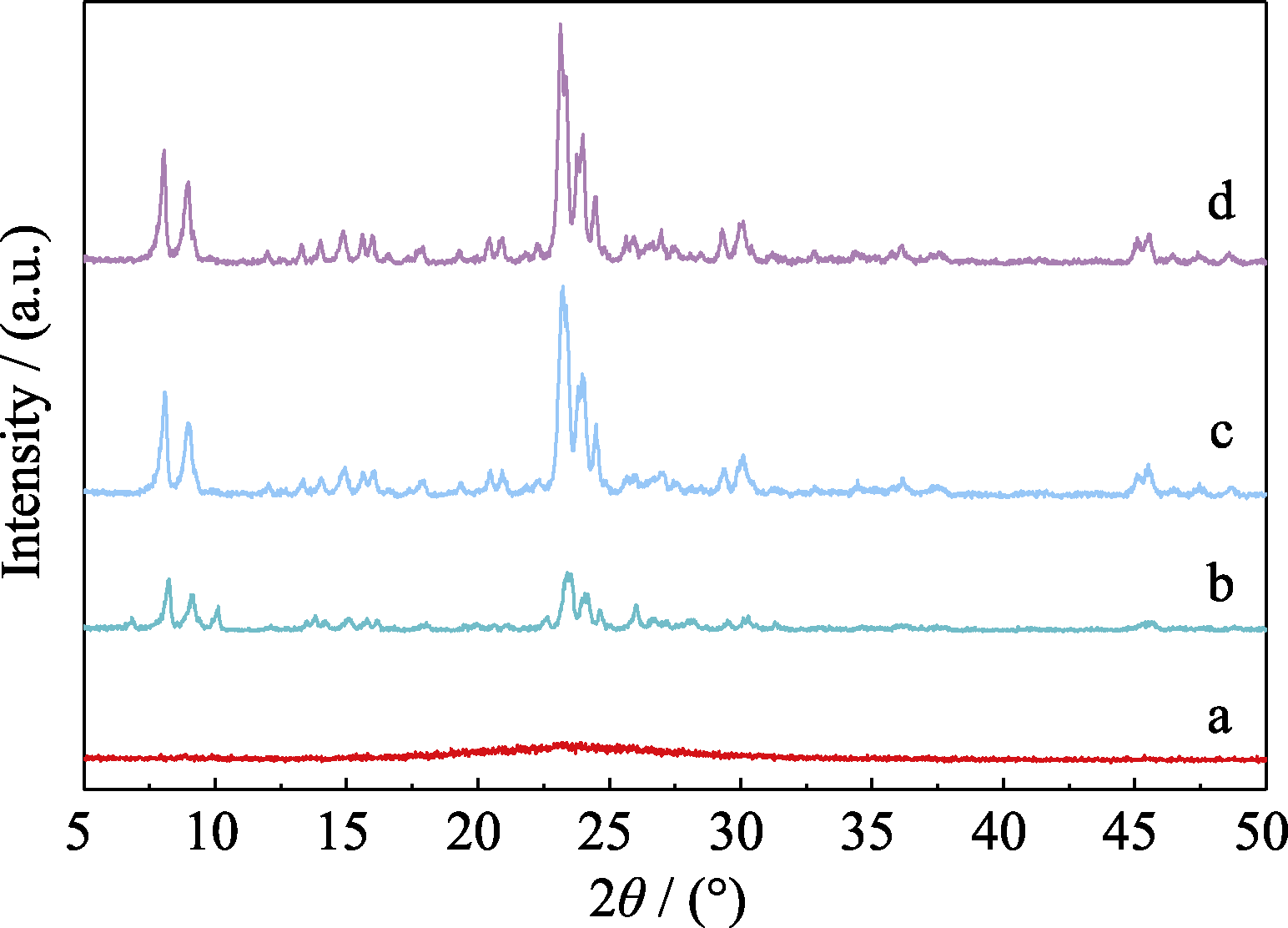
Fig. 1 XRD patterns of the as-synthesized samples added with different amounts of the seeds (a) Z518-0-48; (b) Z518-2.8-48; (c) Z518-5.6-48; (d) Z518-11.2-48.
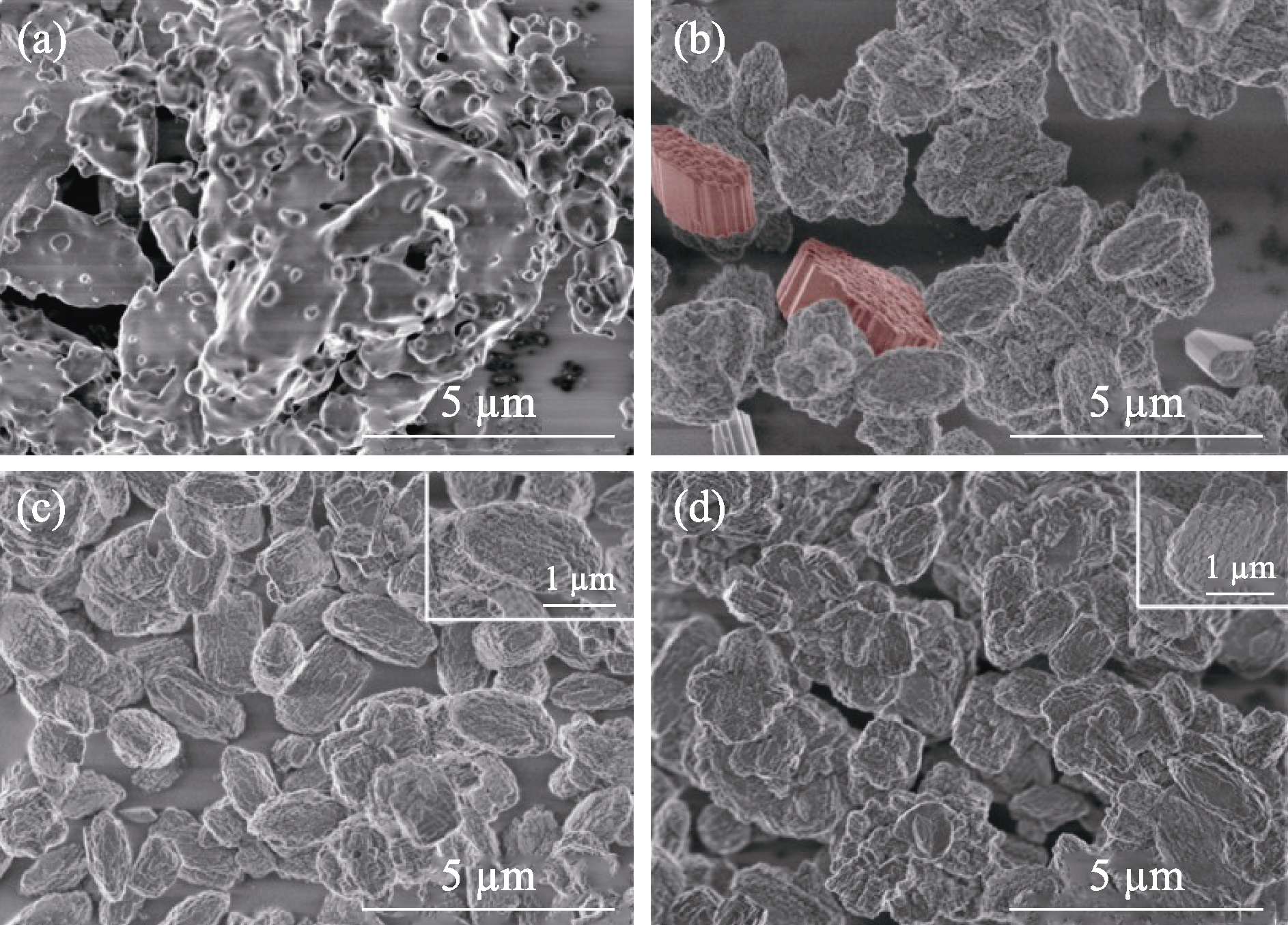
Fig. 2 SEM images of the samples prepared with different amounts of seeds (a) Z518-0-48; (b) Z518-2.8-48; (c) Z518-5.6-48; (d) Z518-11.2-48 The part rendered in red may be MOR zeolite; Colorful figures are available on website
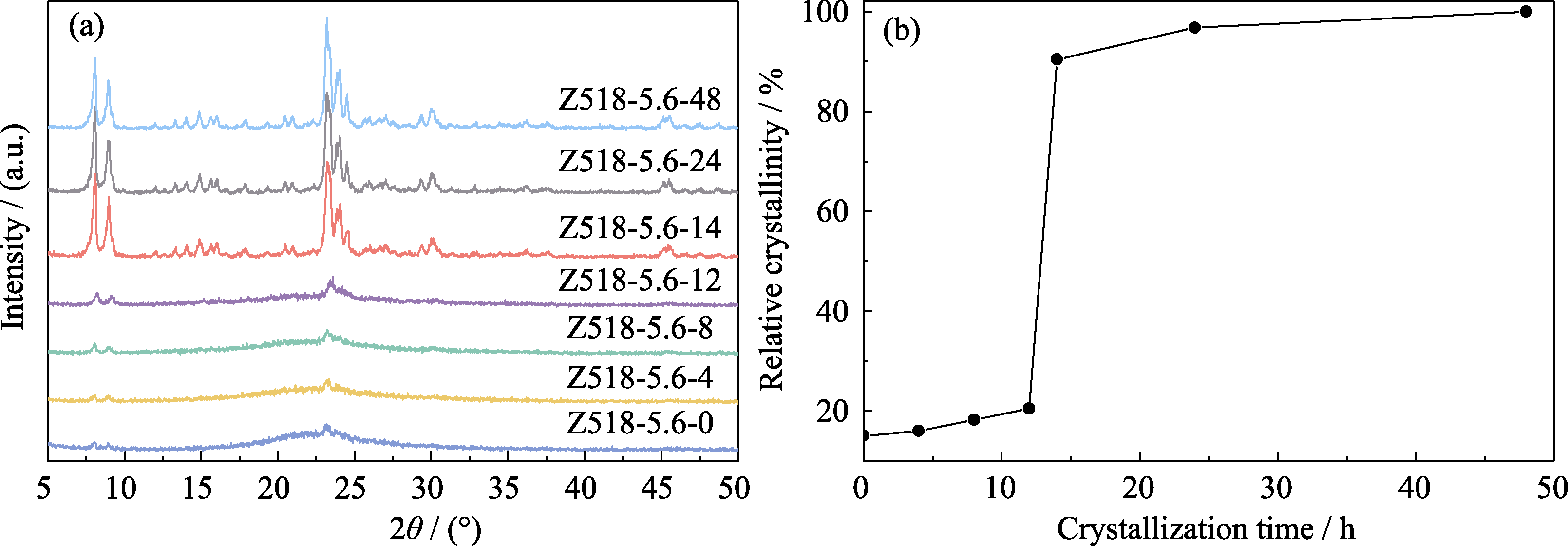
Fig. 3 XRD patterns of the samples with different crystallization time (a) and corresponding crystallization kinetic curve (b) Relative crystallinity (RC) was calculated by comparing the peak areas of XRD patterns in the range of 2θ=22.5°-25° of the samples with those of Z518-5.6-48
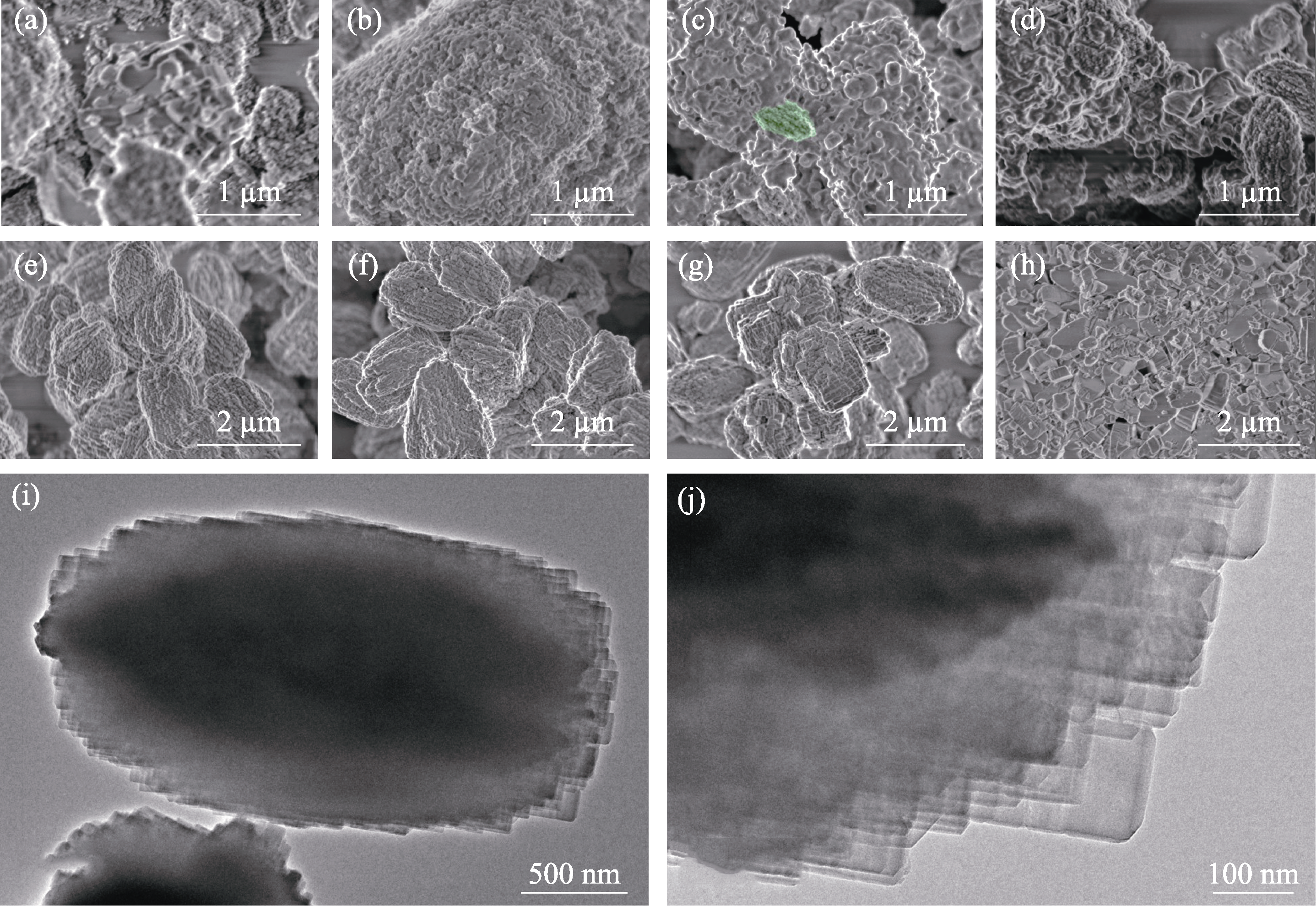
Fig. 5 SEM and TEM images of the as-synthesized samples with different crystallization time SEM images of (a) Z518-5.6-0, (b) Z518-5.6-4, (c) Z518-5.6-8, (d) Z518-5.6-12, (e) Z518-5.6-14, (f) Z518-5.6-24, (g) Z518-5.6-48 and (h) NK-18; (i, j) TEM images of Z518-5.6-48
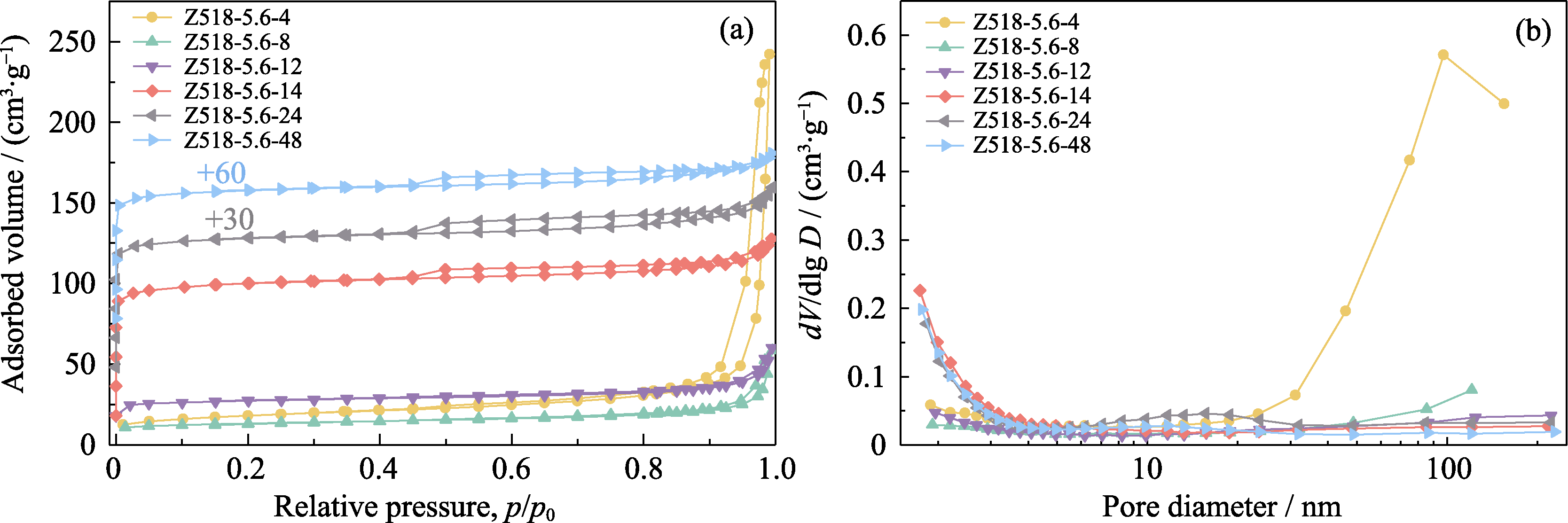
Fig. 6 N2 adsorption-desorption isotherms (a) and corresponding BJH pore size distribution curves (b) of the samples prepared from the same gel precursor treated with different crystallization time
| Sample | SBET/(cm2·g-1) | Smic/(cm2·g-1) | Sext/(cm2·g-1) | Vtotal/(cm3·g-1) | Vmic/(cm3·g-1) | Vext/(cm3·g-1) | *RC/% |
|---|---|---|---|---|---|---|---|
| Z518-5.6-4 | 66 | 13 | 51 | 0.375 | 0.002 | 0.373 | 16.0 |
| Z518-5.6-8 | 48 | 26 | 22 | 0.089 | 0.009 | 0.080 | 18.2 |
| Z518-5.6-12 | 105 | 84 | 21 | 0.093 | 0.031 | 0.062 | 20.5 |
| Z518-5.6-14 | 396 | 355 | 41 | 0.198 | 0.134 | 0.064 | 90.4 |
| Z518-5.6-24 | 390 | 351 | 40 | 0.200 | 0.131 | 0.069 | 96.7 |
| Z518-5.6-48 | 392 | 351 | 41 | 0.213 | 0.132 | 0.081 | 100 |
Table 1 Textural properties of the as-synthesized samples with different crystallization time
| Sample | SBET/(cm2·g-1) | Smic/(cm2·g-1) | Sext/(cm2·g-1) | Vtotal/(cm3·g-1) | Vmic/(cm3·g-1) | Vext/(cm3·g-1) | *RC/% |
|---|---|---|---|---|---|---|---|
| Z518-5.6-4 | 66 | 13 | 51 | 0.375 | 0.002 | 0.373 | 16.0 |
| Z518-5.6-8 | 48 | 26 | 22 | 0.089 | 0.009 | 0.080 | 18.2 |
| Z518-5.6-12 | 105 | 84 | 21 | 0.093 | 0.031 | 0.062 | 20.5 |
| Z518-5.6-14 | 396 | 355 | 41 | 0.198 | 0.134 | 0.064 | 90.4 |
| Z518-5.6-24 | 390 | 351 | 40 | 0.200 | 0.131 | 0.069 | 96.7 |
| Z518-5.6-48 | 392 | 351 | 41 | 0.213 | 0.132 | 0.081 | 100 |
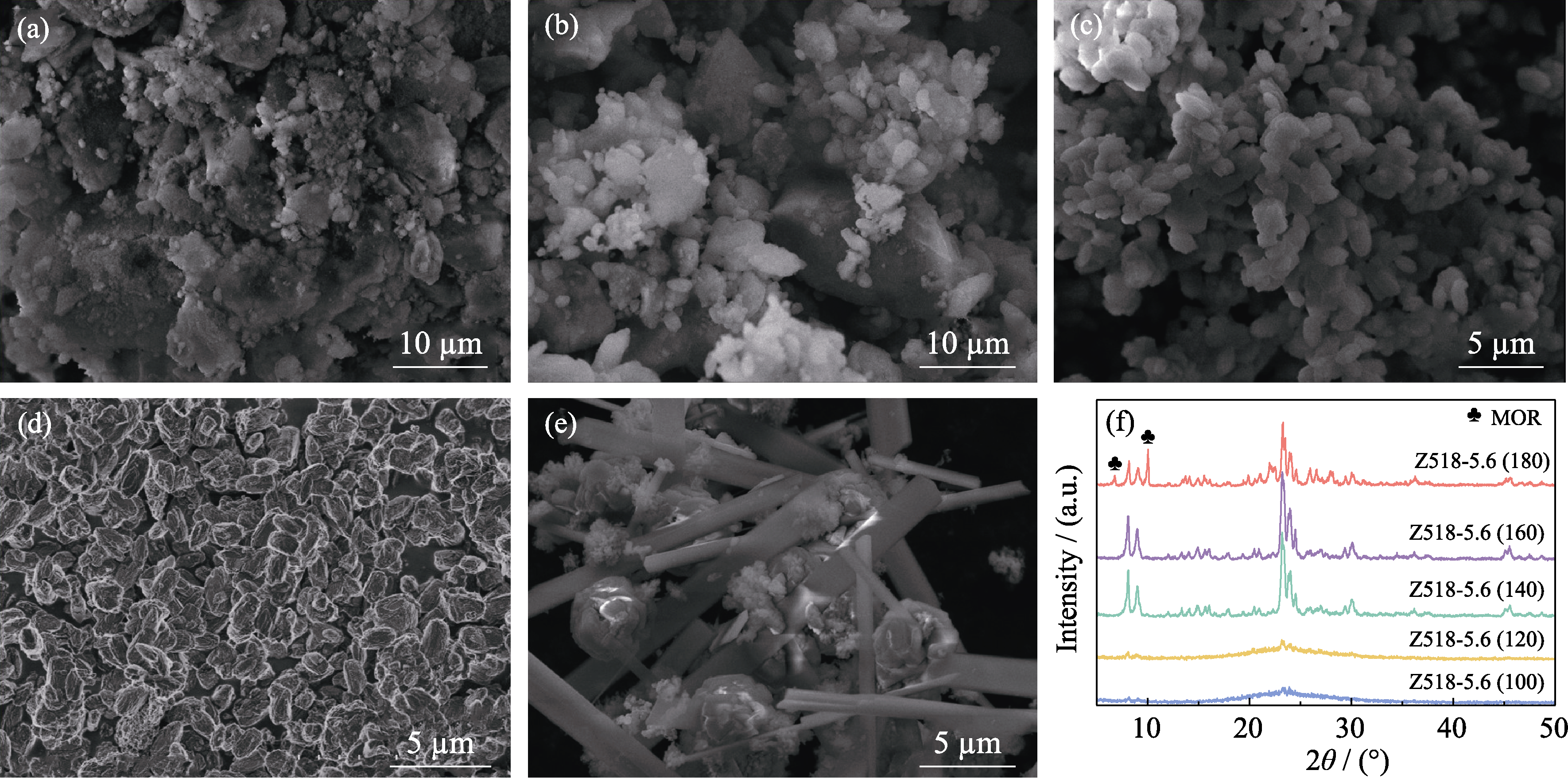
Fig. 7 SEM images and corresponding XRD patterns of the samples synthesized at different crystallization temperatures SEM images of (a) Z518-5.6(100), (b) Z518-5.6(120), (c) Z518-5.6(140), (d) Z518-5.6(160) and (e) Z518-5.6(180); (f) Corresponding XRD patterns of the samples synthesized at different crystallization temperatures All samples were obtained from the same gel precursor as the one yielded the sample Z518-5.6
| Sample | SBET/ (cm2·g-1) | Smic/ (cm2·g-1) | Vtotal/ (cm3·g-1) | Vmic/ (cm3·g-1) | Vmeso/ (cm3·g-1) | *RC/% | **Si/Al | Acid density/(μmol·g-1) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Total | ||||||||
| Z518-5.6-48 | 392 | 352 | 0.19 | 0.13 | 0.06 | 100 | 11.3 | 218 | 65 | 293 | 576 |
| Z527-5.6-48 | 376 | 333 | 0.19 | 0.12 | 0.07 | 99 | 11.3 | 212 | 83 | 224 | 519 |
| Z5200-5.6-48 | 356 | 314 | 0.19 | 0.12 | 0.07 | 99 | 11.6 | 205 | 79 | 221 | 505 |
| ZSM-5r | 377 | 343 | 0.14 | 0.14 | 0.03 | 100 | 17.4 | 168 | 53 | 236 | 457 |
Table 2 Physical and chemical properties of the samples yielded from the similar gel precursor induced by a crystal seed with different Si/Al ratios
| Sample | SBET/ (cm2·g-1) | Smic/ (cm2·g-1) | Vtotal/ (cm3·g-1) | Vmic/ (cm3·g-1) | Vmeso/ (cm3·g-1) | *RC/% | **Si/Al | Acid density/(μmol·g-1) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Total | ||||||||
| Z518-5.6-48 | 392 | 352 | 0.19 | 0.13 | 0.06 | 100 | 11.3 | 218 | 65 | 293 | 576 |
| Z527-5.6-48 | 376 | 333 | 0.19 | 0.12 | 0.07 | 99 | 11.3 | 212 | 83 | 224 | 519 |
| Z5200-5.6-48 | 356 | 314 | 0.19 | 0.12 | 0.07 | 99 | 11.6 | 205 | 79 | 221 | 505 |
| ZSM-5r | 377 | 343 | 0.14 | 0.14 | 0.03 | 100 | 17.4 | 168 | 53 | 236 | 457 |
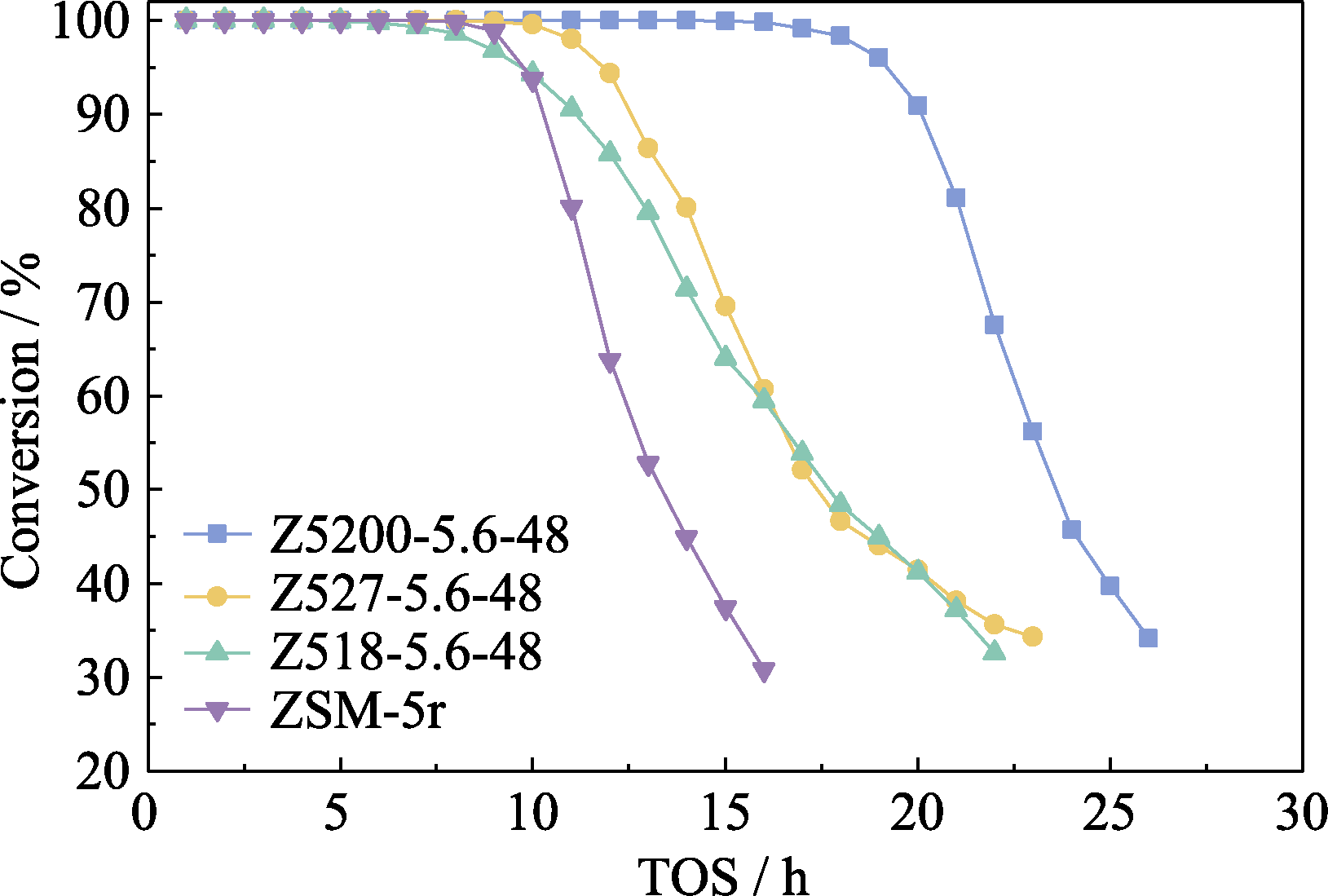
Fig. 8 MTH reaction performance of the samples using crystal seeds with different Si/Al ratios Reaction conditions: T=430 ℃, ptotal=1 atm, WHSV=2.4 h-1, catalysts weight=200 mg
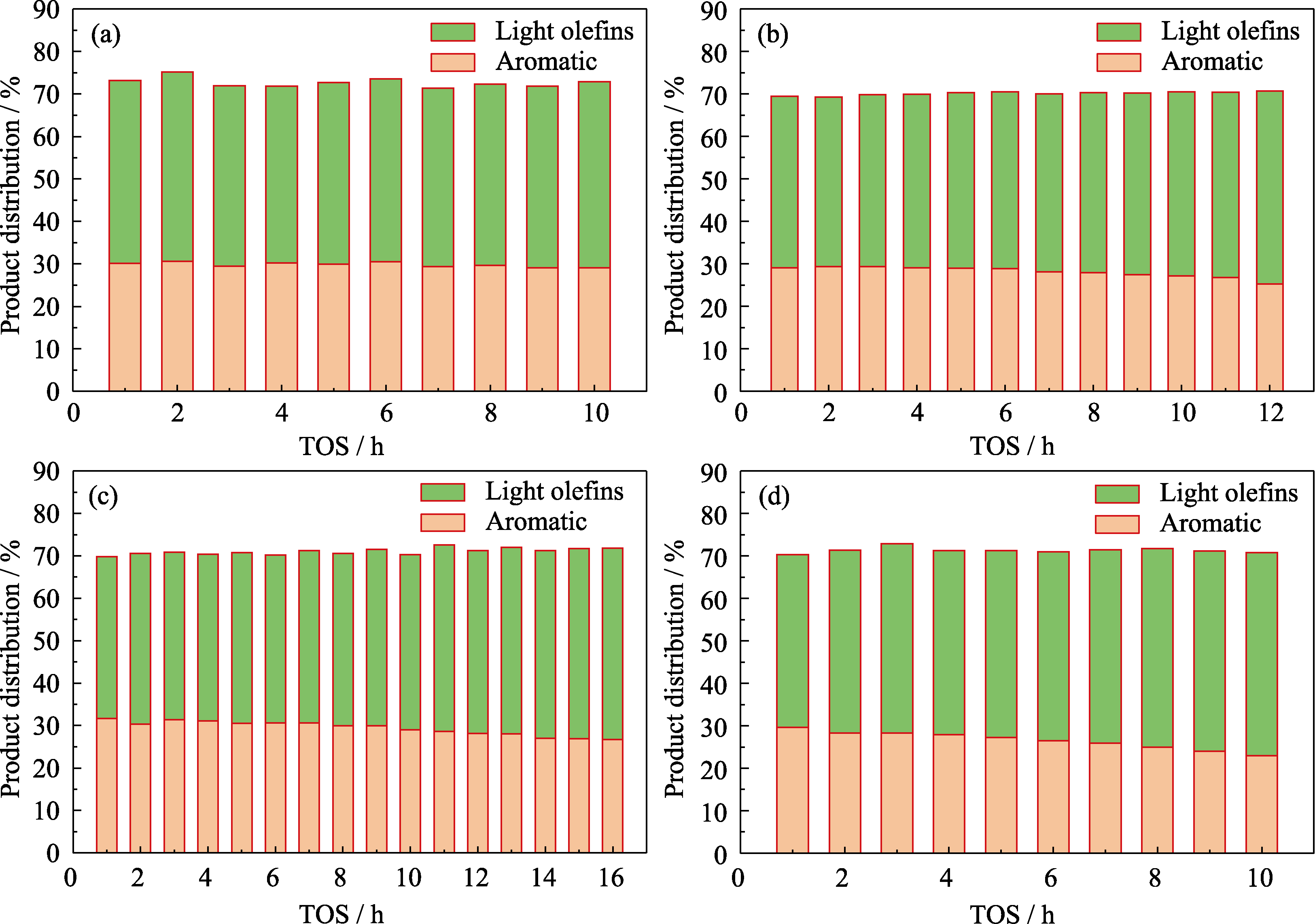
Fig. 9 Selectivity of light olefins over the catalysts with time on stream (a) Z518-5.6-48; (b) Z527-5.6-48; (c) Z5200-5.6-48; (d) ZSM-5r Reaction conditions: T=430 ℃, ptotal=1 atm, WHSV=2.4 h-1, catalysts weight=200 mg
| Sample | SBET/(m2·g-1) | Smic/(m2·g-1) | Sext/(m2·g-1) | Vtotal/(cm3·g-1) | Vmic/(cm3·g-1) | RC/% |
|---|---|---|---|---|---|---|
| Z518-5.6-48 | 392 | 351 | 41 | 0.213 | 0.132 | 100 |
| Z518-11.2-48 | 388 | 346 | 42 | 0.199 | 0.128 | 99 |
Table S1 Pore structure parameter and relative crystallinity of the as-synthesized samples
| Sample | SBET/(m2·g-1) | Smic/(m2·g-1) | Sext/(m2·g-1) | Vtotal/(cm3·g-1) | Vmic/(cm3·g-1) | RC/% |
|---|---|---|---|---|---|---|
| Z518-5.6-48 | 392 | 351 | 41 | 0.213 | 0.132 | 100 |
| Z518-11.2-48 | 388 | 346 | 42 | 0.199 | 0.128 | 99 |
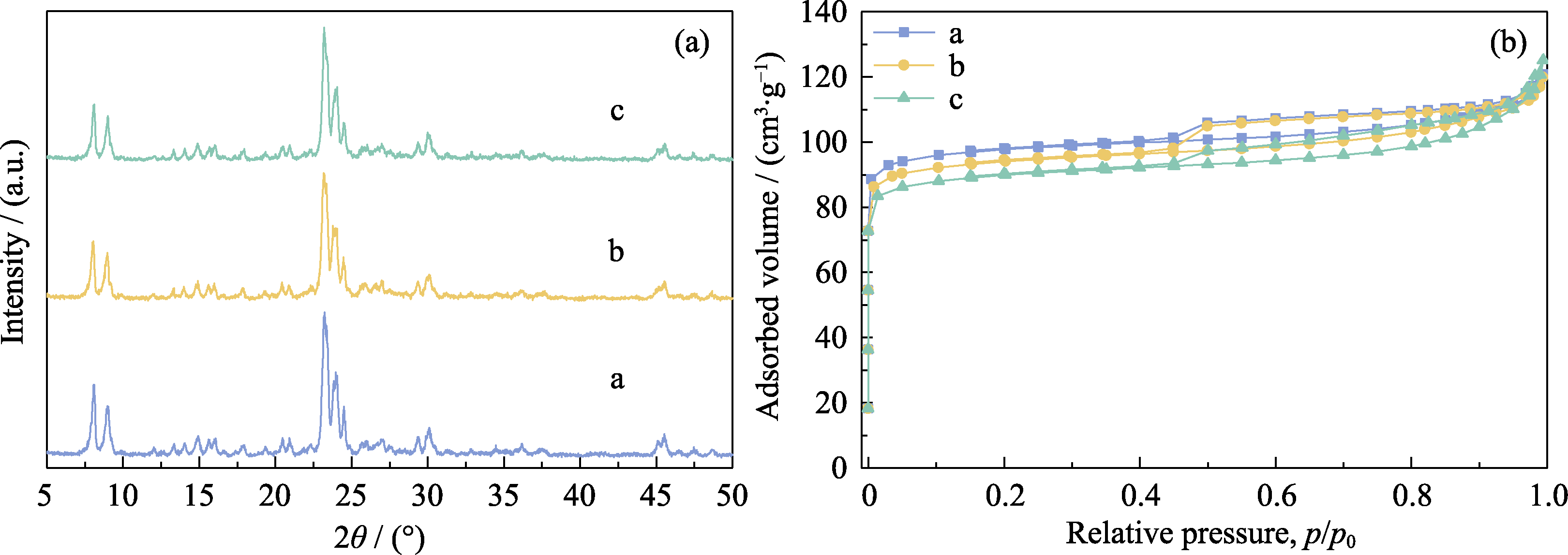
Fig. S4 XRD patterns (a) and N2 adsorption-desorption isotherms (b) of the samples prepared in an OSDA-free system induced by seeds with different Si/Al ratios (a) Z518-5.6-48; (b) Z527-5.6-48; (c) Z5200-5.6-48
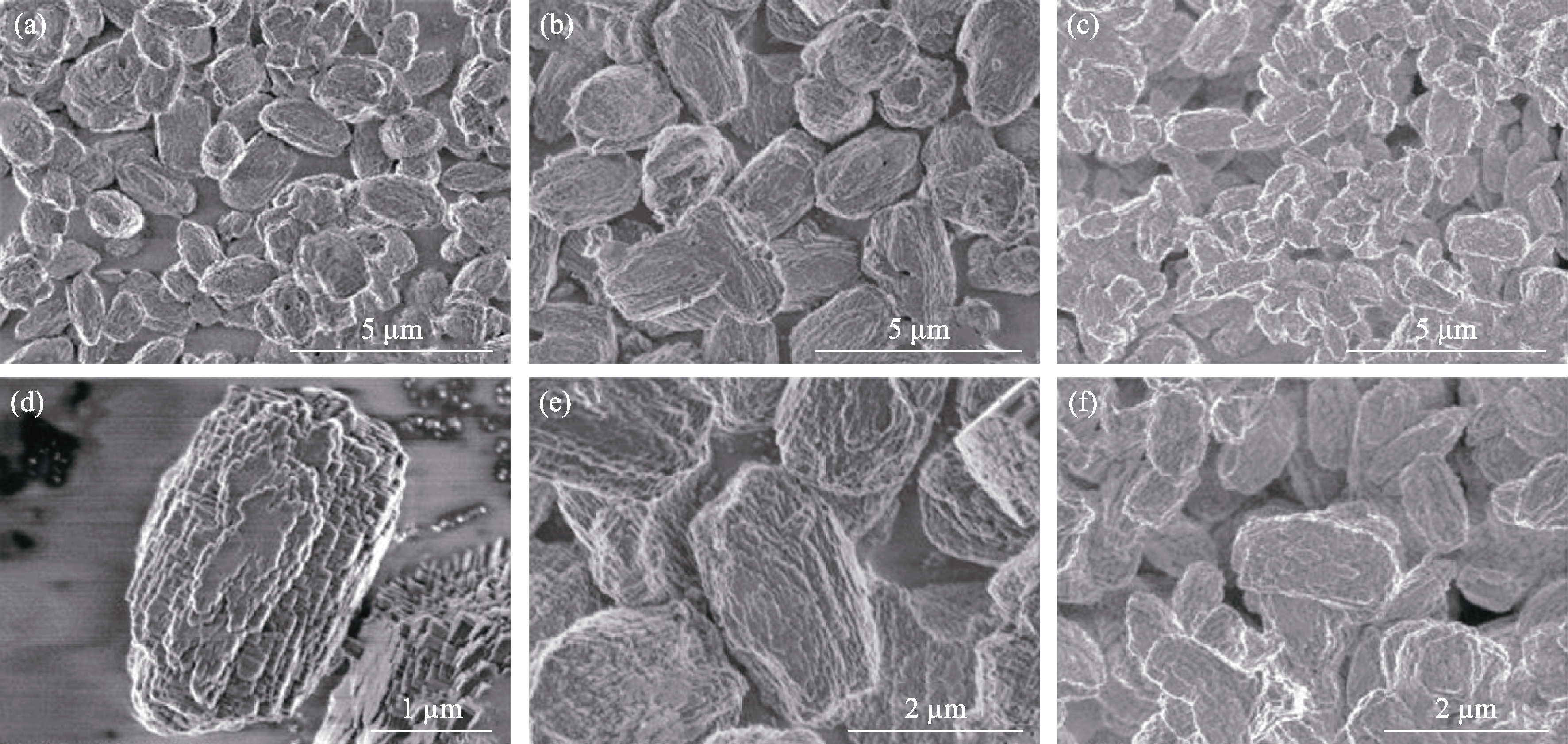
Fig. S5 SEM images of the samples yielded from a similar gel precursor induced by seeds with different Si/Al ratios (a, d) Z518-5.6-48; (b, e) Z527-5.6-48; (c, f) Z5200-5.6-48
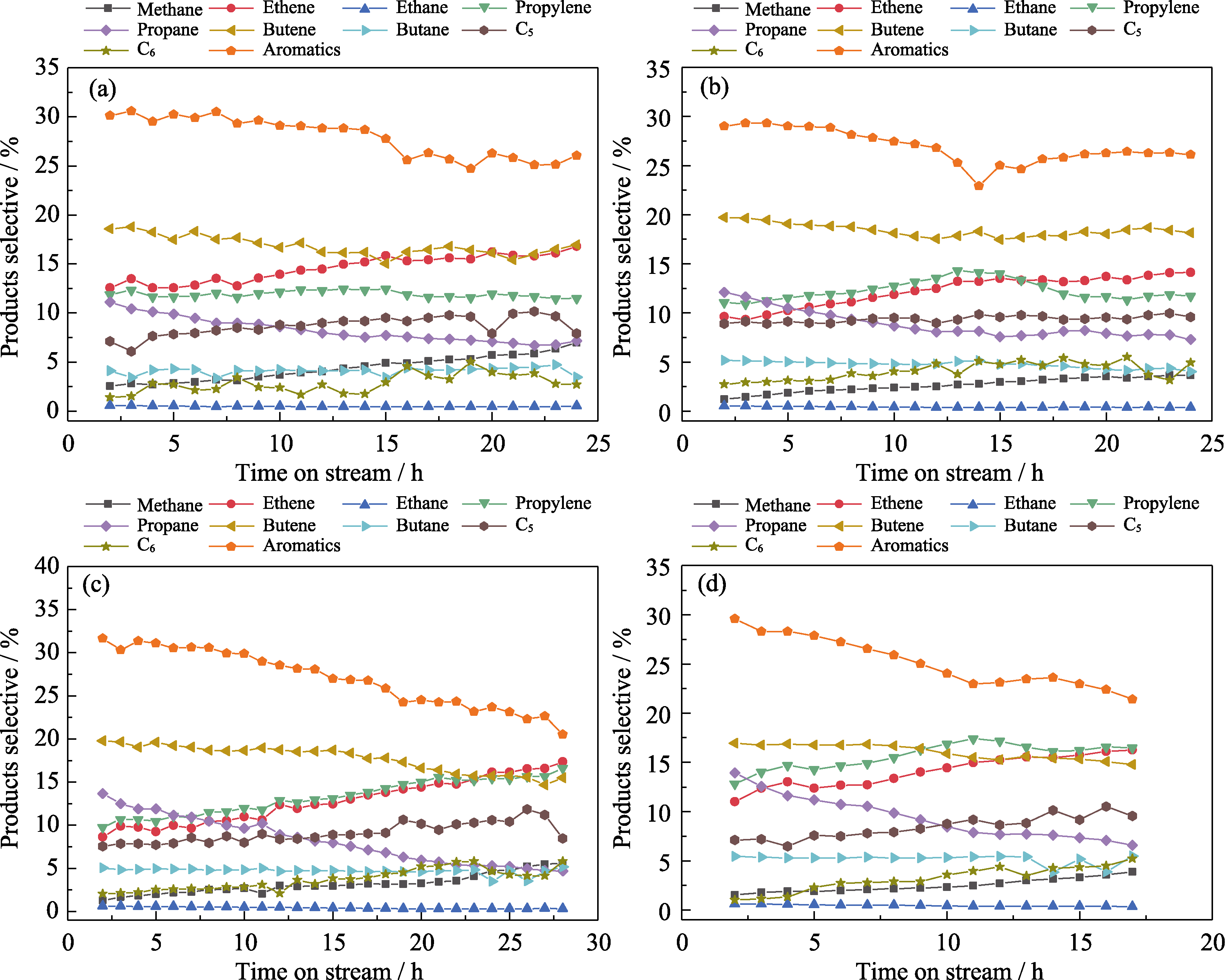
Fig. S8 Selectivity of final products over ZSM-5-x catalysts with time on stream (a) Z518-5.6-48; (b) Z527-5.6-48; (c) Z5200-5.6-48; (d) ZSM-5r. Reaction conditions: T=430 ℃, ptotal=1 atm, WHSV=2.4 h-1, catalysts weight=200 mg
| [1] | PAN M, ZHENG J J, LIU Y J, et al. Construction and practical application of a novel zeolite catalyst for hierarchically cracking of heavy oil. Journal of Catalysis, 2019, 369: 72. |
| [2] | WANG J, ZHANG R Z, HAN L N, et al. Seed-assisted synthesis and characterization of nano and micron ZSM-5 molecular sieves in template-free system. Journal of Solid State Chemistry, 2020, 290: 121536. |
| [3] | LAI R, GAVALAS G R. ZSM-5 membrane synthesis with organic-free mixtures. Microporous and Mesoporous Materials, 2000, 38(2/3): 239. |
| [4] | WANG Y Q, WANG X, WU Q M, et al. Seed-directed and organotemplate-free synthesis of TON zeolite. Catalysis Today, 2014, 226: 103. |
| [5] | YUE Y Y, GU L L, ZHOU Y N, et al. Template-free synthesis and catalytic applications of microporous and hierarchical ZSM-5 zeolites from natural aluminosilicate minerals. Industrial & Engineering Chemistry Research, 2017, 56(36): 10069. |
| [6] | SHESTAKOVA D O, BABINA K A, SLADKOVSKIY D A, et al. Seed-assisted synthesis of hierarchical zeolite ZSM-5 in the absence of organic templates. Materials Chemistry and Physics, 2022, 288: 126432. |
| [7] | ZHANG H Y, WANG L, ZHANG D L, et al. Mesoporous and Al-rich MFI crystals assembled with aligned nanorods in the absence of organic templates. Microporous and Mesoporous Materials, 2016, 233: 133. |
| [8] | GAO Y, WU G, MA F W, et al. Modified seeding method for preparing hierarchical nanocrystalline ZSM-5 catalysts for methanol aromatization. Microporous and Mesoporous Materials, 2016, 226: 251. |
| [9] | NADA M H, LARSEN S C. Insight into seed-assisted template free synthesis of ZSM-5 zeolites. Microporous and Mesoporous Materials, 2017, 239: 444. |
| [10] | HAMIDZADEH M, SAEIDI M, KOMEILI S. Modified seeding method to produce hierarchical nanocrystalline ZSM-5 zeolite. Materials Today Communications, 2020, 25: 101308. |
| [11] | LI Q, CONG W W, XU C Y, et al. New insight into the inductive effect of various seeds on the template-free synthesis of ZSM-5 zeolite. CrystEngComm, 2021, 23: 8641. |
| [12] | FENG F X, DOU T, XIAO Y Z, et al. Effect of solvent on zeolite synthesis. Journal of Natural Gas Chemistry, 1996, 5(4): 351. |
| [13] | ZHANG D S, WANG R J, YANG X X. Application of fractional factorial design to ZSM-5 synthesis using ethanol as template. Microporous and Mesoporous Materials, 2009, 126(1/2): 8. |
| [14] | MA T, ZHANG L M, SONG Y, et al. A comparative synthesis of ZSM-5 with ethanol or TPABr template: distinction of Brønsted/Lewis acidity ratio and its impact on n-hexane cracking. Catalysis Science & Technology, 2018, 8(7): 1923. |
| [15] | UGUINA M A, LUCAS A D, RUIZ F, et al. Synthesis of ZSM-5 from ethanol-containing systems. Influence of the gel composition. Industrial & Engineering Chemistry Research, 1995, 34(2): 451. |
| [16] | FALAMAKI C, EDRISSI M, SOHRABI M. Studies on the crystallization kinetics of zeolite ZSM-5 with 1,6-hexanediol as a structure-directing agent. Zeolites, 1997, 19: 2. |
| [17] | YANG X N, MA X S, WANG X C, et al. Caterpillar-shaped hierarchical ZSM-5 resulted from the self-assembly of regularly primary nano-sized zeolite crystals. Journal of Porous Materials, 2023, 30: 1543. |
| [18] | GROEN J C, ZHU W D, BROUWER S, et al. Direct demonstration of enhanced diffusion in mesoporous ZSM-5 zeolite obtained via controlled desilication. Journal of the American Chemical Society, 2007, 129(2): 355. |
| [19] | ZHANG D Z, JIN C Z, ZOU M M, et al. Mesopore engineering for well-defined mesoporosity in Al-rich aluminosilicate zeolites. Chemistry - A European Journal, 2019, 25(11): 2675. |
| [20] | CHEN L H, SUN M H, WANG Z, et al. Hierarchically structured zeolites: from design to application. Chemical Reviews, 2020, 120(20): 11194. |
| [21] | WANG Y, XIAO F S. Understanding mechanism and designing strategies for sustainable synthesis of zeolites: a personal story. The Chemical Record, 2016, 16(3): 1054. |
| [22] | RAVISHANKAR R, KIRSCHHOCK C, SCHOEMAN B J, et al. Physicochemical characterization of silicalite-1 nanophase material. Journal of Physical Chemistry B, 1998, 102: 2633. |
| [23] | NING W W, YANG X N, ZHENG J J, et al. An environmentally friendly route to prepare hierarchical ZSM-12 using waste liquor as partial nutrients. Materials Chemistry and Physics, 2019, 223: 299. |
| [24] | WANG H Q, SHEN B Y, CHEN X, et al. Modulating inherent lewis acidity at the intergrowth interface of mortise-tenon zeolite catalyst. Nature Communications, 2022, 13: 2924. |
| [25] | SUN C, WANG Y Q, ZHAO A J, et al. Synthesis of nano-sized SAPO-34 with morpholine-treated micrometer-seeds and their catalytic performance in methanol-to-olefin reactions. Applied Catalysis A-General, 2020, 589: 117314. |
| [26] | AKHGAR S, TOWFIGHI J, HAMIDZADEH M. MTO performance over seed-assisted SAPO-34 zeolites synthesized by reducing template consumption. Journal of Materials Research and Technology, 2020, 9: 12126. |
| [27] | TIAN P, WEI Y X, MAO Y, et al. Methanol to olefins (MTO): from fundamentals to commercialization. ACS Catalysis, 2015, 5: 1922. |
| [1] | 魏建文, 张丽娟, 耿琳琳, 李誉, 廖雷, 王敦球. 以ZSM-5/MCM-48为载体制备新型高容量CO2吸附剂的性能及机理研究[J]. 无机材料学报, 2025, 40(7): 833-839. |
| [2] | 张栋强, 路惠惠, 苏娜, 李贵贤, 季东, 赵新红. 活化晶种调节SAPO-34的性质及其对甲醇制烯烃反应催化寿命的增强[J]. 无机材料学报, 2021, 36(1): 101-106. |
| [3] | 徐国皓, 余金鹏, 徐华胜, 李春成, 黄金花, 王鹏飞. CH3COONa处理HZSM-5分子筛及其催化性能[J]. 无机材料学报, 2019, 34(5): 546-552. |
| [4] | 杜艳泽,杨晓娜,宁伟巍,孔庆岚,秦波,郑家军,李文林,李瑞丰. “蒸汽相转化”法制备球形多级Y沸石[J]. 无机材料学报, 2019, 34(2): 225-232. |
| [5] | 冯守爱, 周俊, 杨玄宇, 刘鸿, 黄江锋, 白家峰, 程晓维, 邓勇辉. 晶种导向蒸汽辅助晶化法全硅BETA沸石的合成研究[J]. 无机材料学报, 2018, 33(9): 963-968. |
| [6] | 王有和, 王晓东, 徐经纬, 孙洪满, 吴成成, 阎子峰, 季生福. 酸碱复合处理制备多级孔ZSM-5分子筛及其甲醇制汽油反应性能[J]. 无机材料学报, 2018, 33(11): 1193-1200. |
| [7] | 殷月月, 杨勇, 张良柱, 李永生, 马云峰, 杨莉莉, 黄政仁. 金/钯哑铃状纳米晶的制备及其催化对硝基苯酚还原研究[J]. 无机材料学报, 2018, 33(1): 19-26. |
| [8] | 李建华, 杨冬花, 吕爱凝, 马存存, 李晓峰, 窦 涛. 一步合成核壳结构ZSM-5/EU-1复合分子筛及其表征[J]. 无机材料学报, 2016, 31(5): 492-498. |
| [9] | 杨冬花, 李建华, 王新波, 郭超, 吕爱凝, 董梅, 李晓峰, 窦涛. 双模板剂B-EU-1/ZSM-5复合分子筛的快速合成及催化应用[J]. 无机材料学报, 2016, 31(3): 248-256. |
| [10] | 赵新红, 高向平, 赵江波, 张晓晓, 郝志鑫. 低量结构导向剂改进离子热法高效合成LTA型磷酸铝分子筛[J]. 无机材料学报, 2016, 31(11): 1212-1218. |
| [11] | 刘旭光,马 欣,刘 勇,张宝泉. 无定形晶种引导三次生长制备完备性TAPO-5膜[J]. 无机材料学报, 2015, 30(5): 555-560. |
| [12] | 李良清, 张闻煦, 杨建华, 鲁金明, 殷德宏, 王金渠. 含氟体系下亲水性ZSM-5沸石分子筛膜的制备及其性能[J]. 无机材料学报, 2015, 30(11): 1167-1171. |
| [13] | 郑家军, 张鸿雁, 潘 梦, 李 彪, 张 球, 孔庆岚, 刘芝平, 李瑞丰. 汽相转化法制备纳米晶组成的块状ZSM-5多孔沸石[J]. 无机材料学报, 2015, 30(11): 1161-1166. |
| [14] | 张 球, 谭 薇, 郑家军, 赵强强, 王广帅, 易玉明, 李瑞丰. 气相转移法制备多孔双相沸石复合物[J]. 无机材料学报, 2014, 29(9): 985-990. |
| [15] | 杨冬花, 王新波, 石宝宝, 武正簧, 李晓峰, 窦 涛. 甲醇定向转化制二甲苯的复合分子筛ZSM-5/EU-1的合成及其应用[J]. 无机材料学报, 2014, 29(4): 357-363. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||