无机材料学报 ›› 2026, Vol. 41 ›› Issue (1): 87-95.DOI: 10.15541/jim20250044 CSTR: 32189.14.jim20250044
邬博宇1( ), 张深根1,2(
), 张深根1,2( ), 张生杨1, 刘波1, 张柏林1,2(
), 张生杨1, 刘波1, 张柏林1,2( )
)
收稿日期:2025-02-05
修回日期:2025-04-13
出版日期:2026-01-20
网络出版日期:2025-06-05
通讯作者:
张深根, 教授. E-mail: zhangshengen@ncu.edu.cn;作者简介:邬博宇(1997-), 男, 博士研究生. E-mail: wuboyu@aol.com
基金资助:
WU Boyu1( ), ZHANG Shengen1,2(
), ZHANG Shengen1,2( ), ZHANG Shengyang1, LIU Bo1, ZHANG Bolin1,2(
), ZHANG Shengyang1, LIU Bo1, ZHANG Bolin1,2( )
)
Received:2025-02-05
Revised:2025-04-13
Published:2026-01-20
Online:2025-06-05
Contact:
ZHANG Genshen, professor. E-mail: zhangshengen@ncu.edu.cn;About author:WU Boyu (1997-), male, PhD candidate. E-mail: wuboyu@aol.com
Supported by:摘要: 氮氧化物(NOx)作为我国主要的大气污染物, 通常采用氨气选择性催化还原(NH3-SCR)技术实现其超低排放。低温NH3-SCR具有能耗低、成本低等优势, 但在120 ℃条件下, MnOx基催化剂普遍存在稳定性不足和SO2、H2O中毒的问题。为提高MnOx基催化剂在低温、稀薄烟气条件下的脱硝性能, 本研究采用沉淀-焙烧分解法制备了CeO2/MnOx催化剂。通过一系列表征手段, 系统研究了CeO2对催化剂结构、表面性质及低温NH3-SCR性能的影响。结合第一性原理计算, 从微观层面揭示了CeO2对催化机理的影响及其反应活化能降低的内在原因。结果表明, 添加CeO2细化了催化剂微观颗粒尺寸, 降低了主晶相MnO2的占比, 显著提升了催化剂的弱酸位点浓度, 提高了Mn3+/Mn和Oα/O比值, 改善了催化剂的表面酸性和氧化还原性能。其中, Mn10Ce3和Mn10Ce5催化剂中Mn : Ce物质的量比为10 : 3和10 : 5, 两者均在120 ℃获得了98%以上的NO转化率和较好的稳定性, 且添加CeO2使团聚态的MnOx得到分散和Mn4+分布浓度降低, 这在一定程度上阻碍了高价态Mn4+对NH3和NO的过度氧化, 从而抑制了N2O的形成, 提升了催化剂的N2选择性。第一性原理计算进一步证实, 添加CeO2可降低反应路径中各中间态的活化能, 从而降低反应温度并提高低温NH3-SCR效率。
中图分类号:
邬博宇, 张深根, 张生杨, 刘波, 张柏林. CeO2对MnOx催化剂低温脱硝性能的影响及其机理研究[J]. 无机材料学报, 2026, 41(1): 87-95.
WU Boyu, ZHANG Shengen, ZHANG Shengyang, LIU Bo, ZHANG Bolin. Effect of CeO2 on Low-temperature Denitrification Performance of MnOx Catalysts and Its Mechanism[J]. Journal of Inorganic Materials, 2026, 41(1): 87-95.
| Site | NH3@Ol | NH3@Oα |
|---|---|---|
| 1 | -1.28 eV | -1.61 eV |
| 2 | -1.53 eV | -1.97 eV |
| 3 | -1.60 eV | -2.06 eV |
| 4 | -1.10 eV | -1.84 eV |
表1 NH3在CeO2/MnO2表面不同吸附位点的吸附能
Table 1 NH3 adsorption energies at different sites on CeO2/MnO2 surface
| Site | NH3@Ol | NH3@Oα |
|---|---|---|
| 1 | -1.28 eV | -1.61 eV |
| 2 | -1.53 eV | -1.97 eV |
| 3 | -1.60 eV | -2.06 eV |
| 4 | -1.10 eV | -1.84 eV |
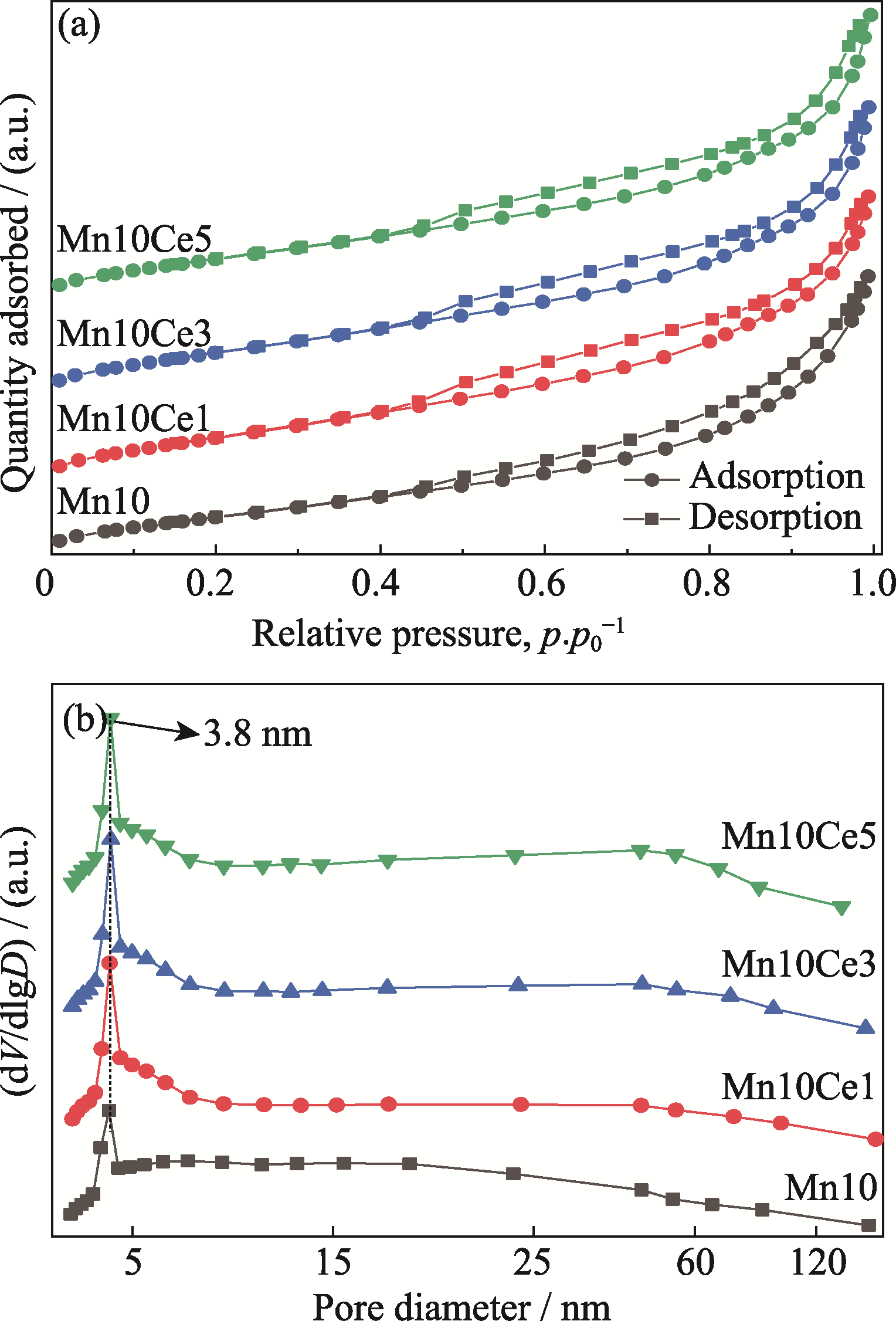
图S1 催化剂样品的(a) N2吸附-脱附等温线和(b) BJH孔径分布曲线
Fig. S1 (a) N2 adsorption-desorption isotherms and (b) corresponding BJH pore diameter distributions of the prepared catalysts
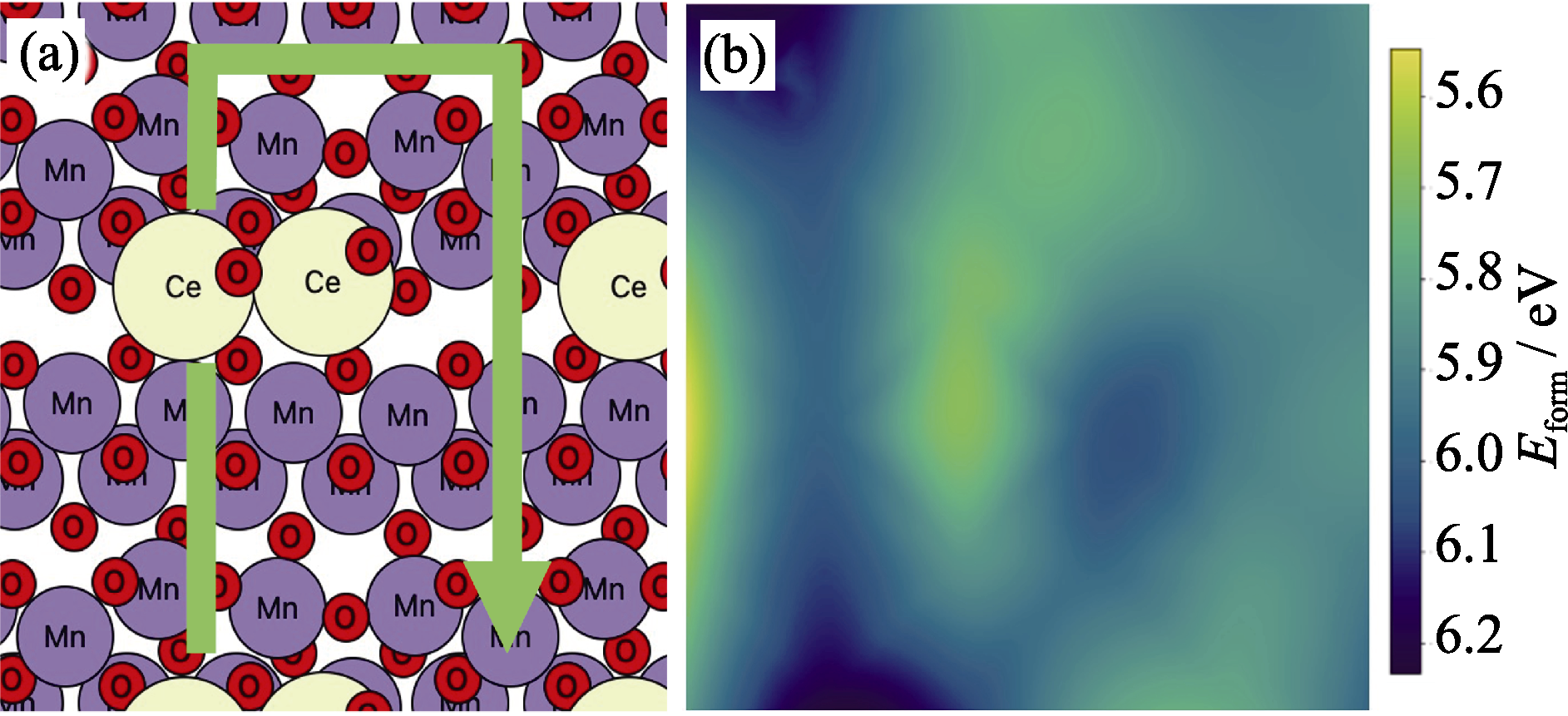
图S2 (a) CeO2二聚体在MnO2表面的最佳构型和(b) CeO2/MnO2催化剂表面模型形成能
Fig. S2 (a) Optimal configuration of CeO2 dimer on MnO2 surface and (b) formation energy of CeO2/MnO2 model
| Catalyst | SBET/(m2·g−1) | Pore volume/ (cm3·g−1) | Average pore size/nm |
|---|---|---|---|
| Mn10 | 34.7 | 0.078 | 7.9 |
| Mn10Ce1 | 35.4 | 0.067 | 6.8 |
| Mn10Ce3 | 37.3 | 0.074 | 7.2 |
| Mn10Ce5 | 38.3 | 0.079 | 7.5 |
表S1 催化剂样品的比表面积、孔容和平均孔径
Table S1 Specific surface area, pore volume and average pore size of the prepared catalysts
| Catalyst | SBET/(m2·g−1) | Pore volume/ (cm3·g−1) | Average pore size/nm |
|---|---|---|---|
| Mn10 | 34.7 | 0.078 | 7.9 |
| Mn10Ce1 | 35.4 | 0.067 | 6.8 |
| Mn10Ce3 | 37.3 | 0.074 | 7.2 |
| Mn10Ce5 | 38.3 | 0.079 | 7.5 |
| Catalyst | NH3-TPD | NO-TPD | ||||
|---|---|---|---|---|---|---|
| Peak I | Peak II | Total | Peak I | Peak II | Total | |
| Mn10 | 64.4 | 35.3 | 99.7 | 1.9 | 0.4 | 2.3 |
| Mn10Ce1 | 66.1 | 17.9 | 84.0 | 1.9 | 1.0 | 2.9 |
| Mn10Ce3 | 72.6 | 45.0 | 117.6 | 2.3 | 1.9 | 4.2 |
| Mn10Ce5 | 100.6 | 30.5 | 131.1 | 2.1 | 1.0 | 3.1 |
表S2 催化剂样品的NH3和NO吸附量(μmol·g−1)
Table S2 Adsorption capacity for NH3 and NO of the prepared catalysts (μmol·g−1)
| Catalyst | NH3-TPD | NO-TPD | ||||
|---|---|---|---|---|---|---|
| Peak I | Peak II | Total | Peak I | Peak II | Total | |
| Mn10 | 64.4 | 35.3 | 99.7 | 1.9 | 0.4 | 2.3 |
| Mn10Ce1 | 66.1 | 17.9 | 84.0 | 1.9 | 1.0 | 2.9 |
| Mn10Ce3 | 72.6 | 45.0 | 117.6 | 2.3 | 1.9 | 4.2 |
| Mn10Ce5 | 100.6 | 30.5 | 131.1 | 2.1 | 1.0 | 3.1 |
| Catalyst | Mn | Ce | O | Mn3+/Mn | Ce3+/Ce | Oα/O |
|---|---|---|---|---|---|---|
| Mn10 | 36.4 | - | 63.6 | 19.4 | - | 26.4 |
| Mn10Ce1 | 30.1 | 4.4 | 65.5 | 21.3 | 5.7 | 29.0 |
| Mn10Ce3 | 21.9 | 11.9 | 66.2 | 22.4 | 7.9 | 35.9 |
| Mn10Ce5 | 19.6 | 15.6 | 64.8 | 23.2 | 6.4 | 29.3 |
表S3 催化剂样品表面元素分析(%, 原子分数)
Table S3 Surface elements analysis of the prepared catalysts (%, atom fraction)
| Catalyst | Mn | Ce | O | Mn3+/Mn | Ce3+/Ce | Oα/O |
|---|---|---|---|---|---|---|
| Mn10 | 36.4 | - | 63.6 | 19.4 | - | 26.4 |
| Mn10Ce1 | 30.1 | 4.4 | 65.5 | 21.3 | 5.7 | 29.0 |
| Mn10Ce3 | 21.9 | 11.9 | 66.2 | 22.4 | 7.9 | 35.9 |
| Mn10Ce5 | 19.6 | 15.6 | 64.8 | 23.2 | 6.4 | 29.3 |
| Catalyst | Peak I | Peak II | Total |
|---|---|---|---|
| Mn10 | 3.3 | 16.4 | 19.7 |
| Mn10Ce1 | 3.2 | 16.9 | 20.1 |
| Mn10Ce3 | 4.4 | 15.2 | 19.6 |
| Mn10Ce5 | 4.1 | 14.2 | 18.3 |
表S4 催化剂样品H2-TPR测试的H2消耗量相对值
Table S4 H2 relative consumption of the prepared catalysts by H2-TPR test
| Catalyst | Peak I | Peak II | Total |
|---|---|---|---|
| Mn10 | 3.3 | 16.4 | 19.7 |
| Mn10Ce1 | 3.2 | 16.9 | 20.1 |
| Mn10Ce3 | 4.4 | 15.2 | 19.6 |
| Mn10Ce5 | 4.1 | 14.2 | 18.3 |
| Catalyst | NO conversion @120 ℃/% | T90/℃ | NO inlet/ppm |
|---|---|---|---|
| Mn10Ce3 | 99.1 | 110 | 100 |
| Mn10Ce5 | 98.2 | 110 | 100 |
| MnOx-CeO2[S4] | - | 120 | 400 |
| NbmCeOx[S5] | - | 200 | 1000 |
| Mn-Ce/Al2O3[S6] | ~98 | 100 | 500 |
| MnOx-CeO2-TiO2[S7] | ~28 | 220 | 3000 |
| CeO2/Mn-Fe-O[S8] | ~98 | - | 500 |
表S5 本研究制备催化剂的NH3-SCR性能与文献数据对比
Table S5 Comparison of NH3-SCR performance of the samples prepared in this study with existing literature
| Catalyst | NO conversion @120 ℃/% | T90/℃ | NO inlet/ppm |
|---|---|---|---|
| Mn10Ce3 | 99.1 | 110 | 100 |
| Mn10Ce5 | 98.2 | 110 | 100 |
| MnOx-CeO2[S4] | - | 120 | 400 |
| NbmCeOx[S5] | - | 200 | 1000 |
| Mn-Ce/Al2O3[S6] | ~98 | 100 | 500 |
| MnOx-CeO2-TiO2[S7] | ~28 | 220 | 3000 |
| CeO2/Mn-Fe-O[S8] | ~98 | - | 500 |
| [1] |
ZHANG K Y, LUO N, HUANG Z S, et al. Recent advances in low-temperature NH3-SCR of NOx over Ce-based catalysts: performance optimizations, reaction mechanisms and anti- poisoning countermeasures. Chemical Engineering Journal, 2023, 476: 146889.
DOI URL |
| [2] |
YAN Z D, SHAN W P, SHI X Y, et al. The way to enhance the thermal stability of V2O5-based catalysts for NH3-SCR. Catalysis Today, 2020, 355: 408.
DOI URL |
| [3] |
HAN L P, CAI S X, GAO M, et al. Selective catalytic reduction of NOx with NH3 by using novel catalysts: state of the art and future prospects. Chemical Reviews, 2019, 119(19): 10916.
DOI URL |
| [4] |
NIU Z R, GAO F Y, WU W J, et al. Preparation and optimization of Mn-based catalysts for low-temperature NH3-SCR: component selection, synthesis strategy and influencing factors. Separation and Purification Technology, 2025, 357: 130103.
DOI URL |
| [5] |
XU G Y, GUO X L, CHENG X X, et al. A review of Mn-based catalysts for low-temperature NH3-SCR: NOx removal and H2O/SO2 resistance. Nanoscale, 2021, 13(15): 7052.
DOI URL |
| [6] |
CHEN Y L, SHU S, WANG S X, et al. Mn-HAP SCR catalyst: preparation and sulfur resistance. Journal of Inorganic Materials, 2022, 37(10): 1065.
DOI |
| [7] |
WANG M M, REN S, XING X D, et al. Catalytic performance of CeO2-NPs and α-MnO2 mixed oxides catalysts for low-temperature NH3-SCR of NO. Journal of the Energy Institute, 2022, 103: 54.
DOI URL |
| [8] |
WANG Y L, QIAN X Y, SHEN C Y, et al. Graphene based mesoporous manganese-cerium oxides catalysts: preparation and low-temperature catalytic reduction of NO. Journal of Inorganic Materials, 2024, 39(1): 81.
DOI URL |
| [9] |
LI T Y, LI W J, WEY M Y. Strategies for designing hydrophobic MnCe-montmorillonite catalysts against water vapor for low- temperature NH3-SCR. Fuel, 2023, 350: 128857.
DOI URL |
| [10] |
ZHANG P F, LU H F, ZHOU Y, et al. Mesoporous MnCeOx solid solutions for low temperature and selective oxidation of hydrocarbons. Nature Communications, 2015, 6: 8446.
DOI |
| [11] |
XIONG S C, CHEN J J, HUANG N, et al. The poisoning mechanism of gaseous HCl on low-temperature SCR catalysts: MnOx-CeO2 as an example. Applied Catalysis B: Environmental, 2020, 267: 118668.
DOI URL |
| [12] |
XIE Q, AN D Q, ZHOU L S, et al. Deactivation induced by metal sulfate over MnCeOx catalyst in NH3-SCR reaction at low temperature. Journal of Rare Earths, 2024, 42(6): 1056.
DOI URL |
| [13] |
CHEN W B, XU S Z. Unraveling the complete mechanism of the NH3-selective catalytic reduction of NO over CeO2. ACS Catalysis, 2023, 13(23): 15481.
DOI URL |
| [14] |
MA J J, YANG Y Y, GAO M Y, et al. Preparation and activity of CeO2 nanoparticles in synthesis of polycarbonates from CO2. Journal of Inorganic Materials, 2025, 40(1): 70.
DOI URL |
| [15] |
GONÇALVES P R G, DE ABREU H A, DUARTE H A. Stability, structural, and electronic properties of hausmannite (Mn3O4) surfaces and their interaction with water. The Journal of Physical Chemistry C, 2018, 122(36): 20841.
DOI URL |
| [16] |
ZHANG B L, ZHANG S G, LIU B. Effect of oxygen vacancies on ceria catalyst for selective catalytic reduction of NO with NH3. Applied Surface Science, 2020, 529: 147068.
DOI URL |
| [17] |
WANG C Z, LI Q Y, ZHANG Z L, et al. Enhancement strategies for NH3-SCR performance of MnCeOx nanowire aerogel catalyst: synergistic modulation of phosphotungstic acid and structure. Separation and Purification Technology, 2025, 358: 130361.
DOI URL |
| [18] |
LI W M, LIU H D, CHEN Y F. Fabrication of MnOx-CeO2-based catalytic filters and their application in low-temperature selective catalytic reduction of NO with NH3. Industrial & Engineering Chemistry Research, 2020, 59(28): 12657.
DOI URL |
| [19] |
LUO N, GAO F Y, LIU H H, et al. Hierarchical structured Ti-doped CeO2 stabilized CoMn2O4 for enhancing the low- temperature NH3-SCR performance within highly H2O and SO2 resistance. Applied Catalysis B: Environmental, 2024, 343: 123442.
DOI URL |
| [20] |
LI Y S, SUN Z X, ZHANG Z P, et al. Enhancing reactive oxygen release-replenishment and Lewis acid acidity by Nb doping in NbmCeOx for simultaneous elimination of NOx and toluene. Applied Catalysis B: Environment and Energy, 2025, 364: 124842.
DOI URL |
| [21] |
HAO S J, CAI Y D, WEI W, et al. Distinct effect of preparation methods on reaction efficiency of Mn-Ce/Al2O3 catalysts in low- temperature NH3-SCR. Journal of Rare Earths, 2024, 42(10): 1865.
DOI URL |
| [22] |
SHI W, LIU J J, ZHU Y, et al. Extruded monolith MnOx-CeO2-TiO2 catalyst for NH3-SCR of low temperature flue gas from an industry boiler: deactivation and recovery. Journal of Rare Earths, 2023, 41(9): 1336.
DOI URL |
| [23] |
ZHANG Y Y, HAI G T, HUANG Z S, et al. Ce-doping rather than CeO2 modification and their synergistic effect: promotion from Ce species in the electrocatalytic oxidation of 5-hydroxymethylfurfural over NiFe-LDH. Advanced Energy Materials, 2024, 14(38): 2401449.
DOI URL |
| [24] |
HUANG X B, WANG P, TAO J Z, et al. CeO2 modified Mn-Fe-O composites and their catalytic performance for NH3-SCR of NO. Journal of Inorganic Materials, 2020, 35(5): 573.
DOI URL |
| [25] |
YE L M, LU P, CHEN X B, et al. The deactivation mechanism of toluene on MnOx-CeO2 SCR catalyst. Applied Catalysis B: Environmental, 2020, 277: 119257.
DOI URL |
| [26] |
ZHANG B L, DENG L F, LIEBAU M, et al. Tar induced deactivation and regeneration of a commercial V2O5-MoO3/TiO2 catalyst during selective catalytic reduction of NO with NH3. Fuel, 2022, 316: 123324.
DOI URL |
| [27] |
WANG F M, CAI Q, GAO J Q, et al. Synthesis of hollow structured MnCeOx@PrCeOx catalyst for low temperature NH3-SCR with enhanced SO2 resistance. Applied Surface Science, 2025, 685: 162067.
DOI URL |
| [28] |
LIU B, LIU J, XIN L, et al. Unraveling reactivity descriptors and structure sensitivity in low-temperature NH3-SCR reaction over CeTiOx catalysts: a combined computational and experimental study. ACS Catalysis, 2021, 11(13): 7613.
DOI URL |
| [29] |
LI J C, ZHANG C, FANG D L, et al. The inhibition mechanism of N2O generation in NH3-SCR process by water vapor. Journal of Hazardous Materials, 2025, 485: 136881.
DOI URL |
| [30] |
CHEN L, YAO X J, CAO J, et al. Effect of Ti4+ and Sn4+ co-incorporation on the catalytic performance of CeO2-MnOx catalyst for low temperature NH3-SCR. Applied Surface Science, 2019, 476: 283.
DOI URL |
| [1] | 刘江平, 管鑫, 唐振杰, 朱文杰, 罗永明. 含氮挥发性有机化合物催化氧化的研究进展[J]. 无机材料学报, 2025, 40(9): 933-943. |
| [2] | 张瑞阳, 王壹, 欧博文, 周莹. α-Ni(OH)2表面羟基协同Ni3+位点催化氧化甲醛机理研究[J]. 无机材料学报, 2023, 38(10): 1216-1222. |
| [3] | 高娃, 熊宇杰, 吴聪萍, 周勇, 邹志刚. 基于超薄纳米结构的光催化二氧化碳选择性转化[J]. 无机材料学报, 2022, 37(1): 3-14. |
| [4] | 郑燕宁, 季军荣, 梁雪玲, 赖正杰, 陈启帆, 廖丹葵. 氮掺杂中空碳球氧化物模拟酶性能研究[J]. 无机材料学报, 2021, 36(5): 527-534. |
| [5] | 许云青,王海增. EDTA辅助水热法制备不同形貌的氟化镁钠[J]. 无机材料学报, 2019, 34(9): 933-937. |
| [6] | 鱼银虎, 汪涛, 廖秋平, 缪润杰, 潘剑锋, 张度宝. 低温固相反应合成纳米级TiB2-TiC复合粉体[J]. 无机材料学报, 2016, 31(3): 324-328. |
| [7] | 鱼银虎, 汪 涛, 张洪敏, 张度宝, 潘剑锋. PTFE促发TiC陶瓷粉体低温固相合成研究[J]. 无机材料学报, 2015, 30(3): 272-276. |
| [8] | 豆志河, 张廷安, 文 明, 史冠勇, 赫冀成. 燃烧合成法制备NdB6超细粉体及反应机理[J]. 无机材料学报, 2014, 29(7): 711-716. |
| [9] | 史晓睿, 王 群, 吕羚源, 李 洋, 俞 潇, 陈 刚. 碲化铜纳米材料的液相可控合成及其电导率[J]. 无机材料学报, 2012, 27(4): 433-438. |
| [10] | 穆云超, 梁宝岩, 郭基凤. 金刚石表面形成Ti3SiC2的反应机理[J]. 无机材料学报, 2012, 27(10): 1099-1104. |
| [11] | 徐秀华, 肖汉宁, 郭文明, 高朋召, 彭苏华. 常压固相反应合成 LaB6 粉末及其反应机理[J]. 无机材料学报, 2011, 26(4): 417-421. |
| [12] | 吴 皓1,2, 陈 诚1, 蒋丹宇2, 李 强1. 纳米铌镁酸铅机械化学法低温快速合成[J]. 无机材料学报, 2010, 25(5): 541-545. |
| [13] | 刘学建,李会利,黄政仁,王士维,江东亮. 高温固相反应工艺制备AlON粉体[J]. 无机材料学报, 2009, 24(6): 1159-1162. |
| [14] | 徐顺建,乔冠军,王红洁,李涤尘,卢天健. 微孔碳陶瓷化反应机理的研究[J]. 无机材料学报, 2009, 24(2): 291-296. |
| [15] | 李江鸿,张红波,熊翔,肖鹏,赵磊,黄伯. 含钽树脂先驱体转变生成TaC的过程研究[J]. 无机材料学报, 2007, 22(5): 973-978. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||