无机材料学报 ›› 2020, Vol. 35 ›› Issue (12): 1357-1364.DOI: 10.15541/jim20200152 CSTR: 32189.14.10.15541/jim20200152
所属专题: 能源材料论文精选(一):锂离子电池(2020)
颜一垣1( ),鞠江伟2,于美燕1,陈守刚1(
),鞠江伟2,于美燕1,陈守刚1( ),崔光磊2(
),崔光磊2( )
)
收稿日期:2020-03-23
修回日期:2020-05-11
出版日期:2020-12-20
网络出版日期:2020-06-09
作者简介:颜一垣(1994–), 男, 硕士研究生. E-mail: yanyiyuan94@163.com
基金资助:
YAN Yiyuan1( ),JU Jiangwei2,YU Meiyan1,CHEN Shougang1(
),JU Jiangwei2,YU Meiyan1,CHEN Shougang1( ),CUI Guanglei2(
),CUI Guanglei2( )
)
Received:2020-03-23
Revised:2020-05-11
Published:2020-12-20
Online:2020-06-09
About author:YAN Yiyuan(1994–), male, Master candidate. E-mail: yanyiyuan94@163.com
Supported by:摘要:
有机/无机复合电解质被认为是全固态锂电池中最具潜力的固态电解质之一, 但由于无机填料易团聚, 通过提高无机填料含量来改善复合电解质的电导率难有成效。此外, 在全固态锂电池中, 电解质和电极之间松散的固-固接触造成过大的界面阻抗, 限制了全固态锂电池的性能。本研究采用固相法合成具有Li+连续传输通道的自支撑三维多孔Li6.4Al0.1La3Zr1.7Ta0.3O12骨架, 并利用原位聚合的方法构筑一体化电解质/电极固-固界面。此策略指导合成的复合电解质的室温电导率可达1.9×10-4 S·cm-1。同时, 一体化的界面使得Li-Li对称电池的界面阻抗从1540 Ω·cm 2降低至449 Ω·cm 2, 因此4.3 V(vs. Li+/Li)的LiCoO2|Li全固态锂电池展现出良好的电化学性能。
中图分类号:
颜一垣, 鞠江伟, 于美燕, 陈守刚, 崔光磊. 原位聚合三维陶瓷骨架增强全固态锂电池电解质[J]. 无机材料学报, 2020, 35(12): 1357-1364.
YAN Yiyuan, JU Jiangwei, YU Meiyan, CHEN Shougang, CUI Guanglei. In-situ Polymerization Integrating 3D Ceramic Framework in All Solid-state Lithium Battery[J]. Journal of Inorganic Materials, 2020, 35(12): 1357-1364.
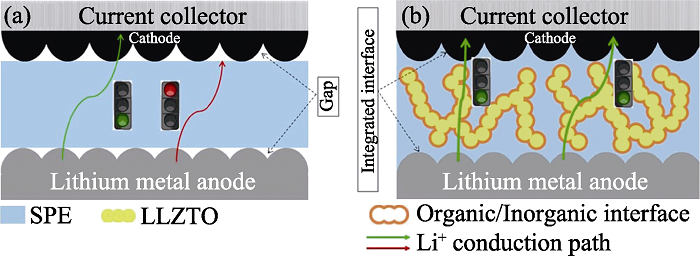
图1 (a)非原位聚合策略和(b)原位聚合策略制备的ASLB内部结构示意图
Fig. 1 Schematic illustration of ASLB structure prepared via (a) ex-situ and (b) in-situ methods with p-LLZTO as ceramic fillers
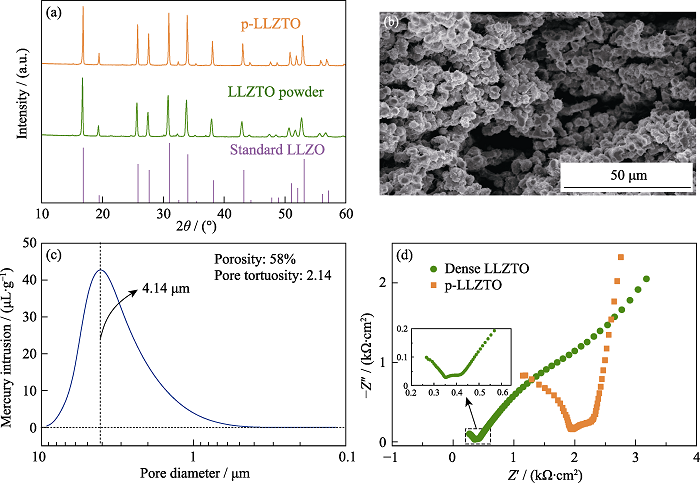
图2 (a)标准LLZO及本实验制备的LLZTO粉末和p-LLZTO的XRD图谱; (b) p-LLZTO的截面SEM照片; (c)p-LLZTO的孔径分布曲线; (d)致密LLZTO和p-LLZTO的室温阻抗图谱(插图: 局部放大的致密LLZTO阻抗谱)
Fig. 2 (a) XRD patterns of standard LLZO, the as-prepared LLZTO powders and p-LLZTO; (b) Cross sectional SEM image of p-LLZTO; (c) Pore size distribution of p-LLZTO; (d) EIS plots of dense LLZTO and p-LLZTO at room temperature with inset showing the partial magnified spectrum of the dense LLZTO
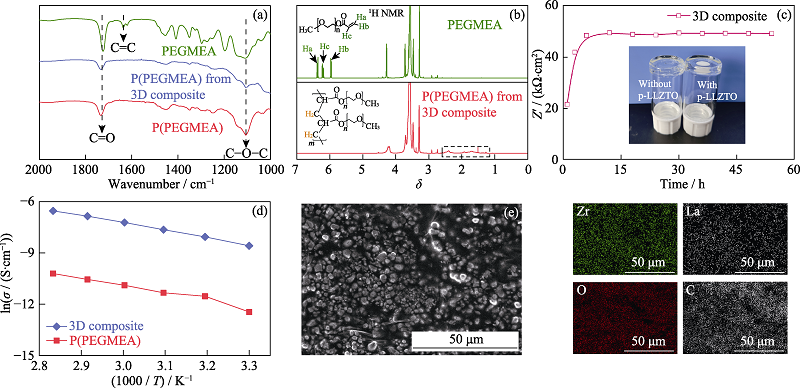
图3 (a)PEGMEA、P(PEGMEA)和3D composite中P(PEGMEA)的红外图谱; (b)PEGMEA和3D composite中P(PEGMEA)的核磁共振氢谱及相关结构式(溶剂为氘代N,N-二甲基甲酰胺); (c)60 ℃条件下steel|3D composite|steel电池欧姆阻抗与加热时间关系曲线, 插图为有/无p-LLZTOP的PEGMEA在小瓶中60 ℃加热24 h后的照片; (d)P(PEGMEA)和3D composite的电导率与温度的关系曲线; (e)3D composite的截面SEM照片及元素分布图
Fig. 3 (a) FT-IR spectra of PEGMEA, P(PEGMEA), and P(PEGMEA) from the 3D composite; (b) 1H NMR spectra of PEGMEA and P(PEGMEA) from the 3D composite(the solvents are deuterated N,N-dimethylformamide) with insets showing the corresponding structural formula of PEGMEA and P(PEGMEA); (c) Thermal evolution of ohmic resistance at 60 ℃ for steel|3D composite|steel symmetrical cell with inset showing the digital image of PEGMEA with/without p-LLZTO after heat-treatment at 60 ℃ for 24 h; (d) Relation between ionic conductivity of electrolyte and temperature for P(PEGMEA) and 3D composite; (e) Cross sectional SEM image and element mapping analysis of the 3D composite
| Electrolyte | Lithium salt | EOa : Li+ | Conductivity of polymer/(S·cm-1) | Conductivity of composite/(S·cm-1) | Promotion factor | Ref. |
|---|---|---|---|---|---|---|
| PEO/LATP particles | LiClO4 | 15 : 1 | 1.3×10-6 | 9.5×10-6 | 7.5 | [ |
| PEO/LLZO fibers | LiTFSIb | - | 2.5×10-6 | 2.7×10-5 | 11 | [ |
| PEO/LATPc fibers | LiTFSI | 8 : 1 | 3.2×10-6 | 4.9×10-5 | 15 | [ |
| PEO/3D LLZO | LiTFSI | 10 : 1 | 1.8×10-6 | 8.5×10-5 | 47 | [ |
| PEO/3D LLTOd | LiTFSI | 10 : 1 | 2.2×10-6 | 8.8×10-5 | 40 | [ |
表1 不同固态电解质的室温电导率$(\sigma_{Li^+})$
Table 1 Conductivities $(\sigma_{Li^+})$ of different solid electrolytes at room temperature
| Electrolyte | Lithium salt | EOa : Li+ | Conductivity of polymer/(S·cm-1) | Conductivity of composite/(S·cm-1) | Promotion factor | Ref. |
|---|---|---|---|---|---|---|
| PEO/LATP particles | LiClO4 | 15 : 1 | 1.3×10-6 | 9.5×10-6 | 7.5 | [ |
| PEO/LLZO fibers | LiTFSIb | - | 2.5×10-6 | 2.7×10-5 | 11 | [ |
| PEO/LATPc fibers | LiTFSI | 8 : 1 | 3.2×10-6 | 4.9×10-5 | 15 | [ |
| PEO/3D LLZO | LiTFSI | 10 : 1 | 1.8×10-6 | 8.5×10-5 | 47 | [ |
| PEO/3D LLTOd | LiTFSI | 10 : 1 | 2.2×10-6 | 8.8×10-5 | 40 | [ |
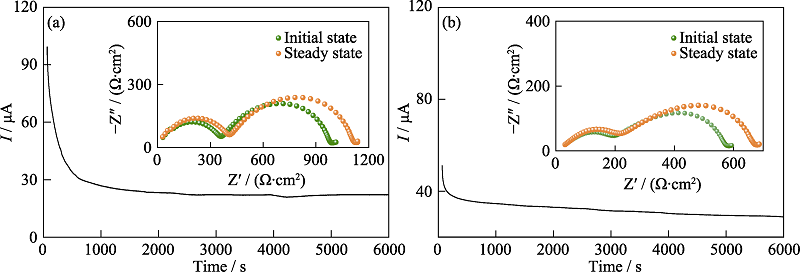
图S2 (a) Li|P(PEGMEA)|Li和(b)Li|3D composite|Li电池室温下极化过程电流随时间变化曲线
Fig. S2 Current variation with time during polarization of (a) Li|P(PEGMEA)|Li and (b) Li|3D composite|Li symmetrical cell at room temperature
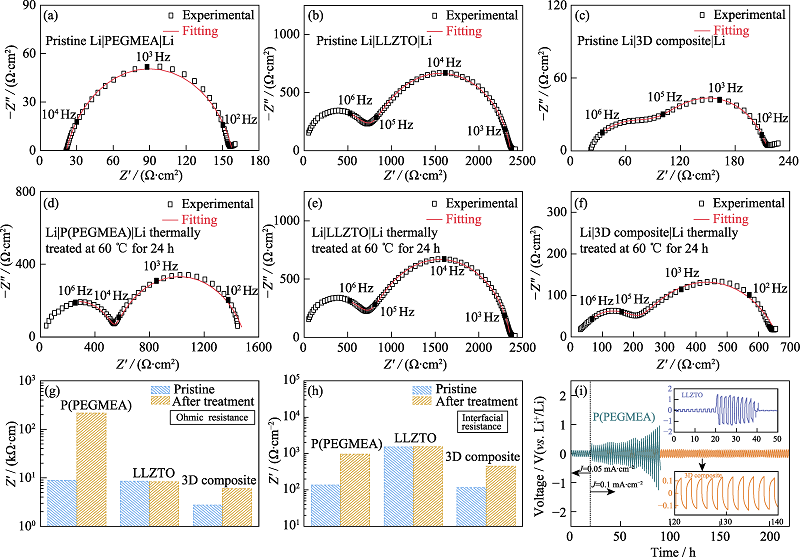
图4 热处理(a~c)前(d~f)后基于(a, d)PEGMEA、(b, e)LLZTO和(c, f)3D composite的Li-Li对称电池的EIS图谱; 基于不同电解质的Li-Li电池处理前后(g)欧姆阻抗和(h)界面阻抗对比; (i)P(PEGMEA)和3D composite的Li-Li电池室温下的直流恒流循环曲线(上插图为LLZTO的Li-Li电池室温下的直流恒流循环曲线, 下插图为3D composite的Li-Li电池的局部放大极化曲线, 电流密度为0.1 mA·cm-2)
Fig. 4 EIS plots of (a-c) pre- and (d-f) post-treated Li-Li symmetrical batteries based on (a, d) PEGMEA, (b, e) LLZTO, (c, f) 3D composites; (g) Ohmic and (h) interfacial resistance comparison of pre- and post-treated Li-Li symmetrical cells; (i) DC galvanostatic cycle of Li-Li symmetrical batteries based on P(PEGMEA) and the 3D composite under room temperature at 0.1 mA·cm-2 with insets showing D.C. galvanostatic cycle of Li-Li symmetrical battery based on LLZTO(up) and the magnified profile of Li|3D composite|Li(down)
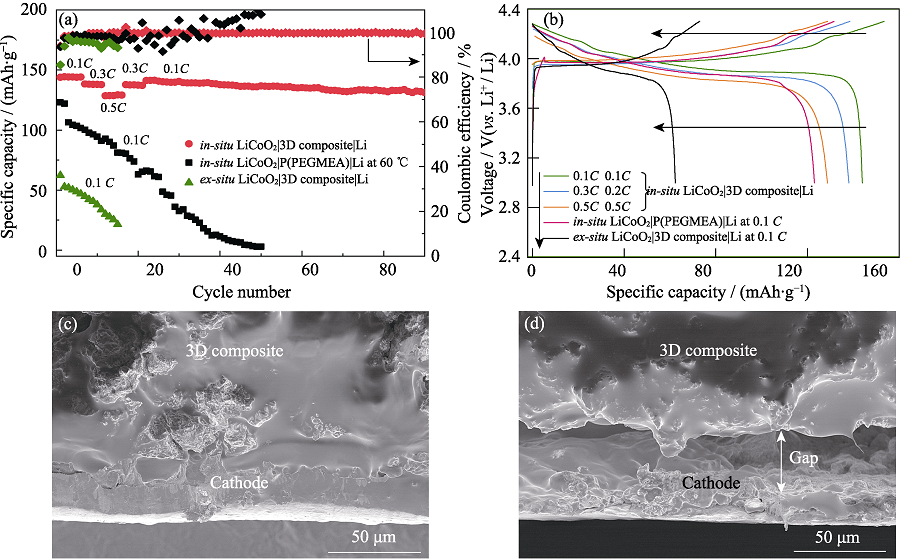
图5 (a)原位聚合LiCoO2|3D composite|Li、原位聚合LiCoO2|P(PEGMEA)|Li和非原位聚合LiCoO2|3D composite|Li ASLBs的循环性能; (b)原位聚合LiCoO2|3D composite|Li、原位聚合LiCoO2|P(PEGMEA)|Li和非原位聚合LiCoO2|3D composite|Li ASLBs的充放电曲线; (c)原位聚合和(d)非原位聚合LiCoO2|3D composite|Li ASLBs拆解后的LiCoO2/3D composite界面的截面SEM照片
Fig. 5 (a) Cycle performances of in-situ LiCoO2|3D composite|Li, in-situ LiCoO2|P(PEGMEA)|Li, ex-situ LiCoO2|3D composite|Li ASLBs; (b) Charge-discharge curves of in-situ LiCoO2|3D composite|Li, in-situ LiCoO2|P(PEGMEA)|Li, ex-situ LiCoO2|3D composite|Li ASLBs; Cross-sectional SEM images of the LiCoO2/3D composite interface from the disassembled (c) in-situ and (d) ex-situ LiCoO2|3D composite|Li ASLBs
| [1] |
GAO Z, SUN H, FU L, et al. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Advanced Materials, 2018,30(17):e1705702.
DOI URL PMID |
| [2] |
BACHMAN J C, MUY S, GRIMAUD A, et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chemical Reviews, 2016,116(1):140-162.
DOI URL PMID |
| [3] | ZHENG F, KOTOBUKI M, SONG S, et al. Review on solid electrolytes for all-solid-state lithium-ion batteries. Journal of Power Sources, 2018,389:198-213. |
| [4] | ZHANG B, TAN R, YANG L, et al. Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Materials, 2018,10:139-159. |
| [5] | CHEN R, QU W, GUO X, et al. The pursuit of solid-state electrolytes for lithium batteries: from comprehensive insight to emerging horizons. Materials Horizons, 2016,3(6):487-516. |
| [6] | FAN L, WEI S, LI S, et al. Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Advanced Energy Materials, 2018,8(11):1702657. |
| [7] | YUE L, MA J, ZHANG J, et al. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Materials, 2016,5:139-164. |
| [8] | MANTHIRAM A, YU X, WANG S. Lithium battery chemistries enabled by solid-state electrolytes. Nature Reviews Materials, 2017,2(4):16103 |
| [9] | GAO Y, WANG D, LI Y C, et al. Salt-based organic-inorganic nanocomposites: towards a stable lithium metal/Li10GeP2S12 solid electrolyte interface. Angew. Chem. Int. Ed., 2018,57(41):13608-13612. |
| [10] | BUANNIC L, ORAYECH B. Dual substitution strategy to enhance Li+ ionic conductivity in Li7La3Zr2O12 solid electrolyte. Chemistry of Materials, 2017,29(4):1769-1778. |
| [11] | ZHANG Z, SHAO Y, LOTSCH B, et al. New horizons for inorganic solid state ion conductors. Energy & Environmental Science, 2018,11(8):1945-1976. |
| [12] | CHENG X B, ZHAO C Z, YAO Y X, et al. Recent advances in energy chemistry between solid-state electrolyte and safe lithium- metal anodes. Chem, 2019,5(1):74-96. |
| [13] |
ZHA W, CHEN F, YANG D, et al. High-performance Li6.4La3Zr1.4Ta0.6O12/poly(ethylene oxide)/succinonitrile composite electrolyte for solid-state lithium batteries. Journal of Power Sources, 2018,397:87-94.
DOI URL |
| [14] |
ZHU P, YAN C, DIRICAN M, et al. Li0.33La0.557TiO3 ceramic nanofiber- enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries. Journal of Materials Chemistry A, 2018,6(10):4279-4285.
DOI URL |
| [15] | WAN Z, LEI D, YANG W, et al. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder. Advanced Functional Materials, 2019,29(1):1805301. |
| [16] | CHEN L, LI Y, LI S P, et al. PEO/garnet composite electrolytes for solid-state lithium batteries: from “ceramic-in-polymer” to “polymer- in-ceramic”. Nano Energy, 2018,46:176-184. |
| [17] | XIE H, YANG C, FU K K, et al. Flexible, scalable, and highly conductive garnet-polymer solid electrolyte templated by bacterial cellulose. Advanced Energy Materials, 2018,8(18):1703474. |
| [18] | BAE J, LI Y, ZHANG J, et al. A 3D nanostructured hydrogel- framework-derived high-performance composite polymer lithium-ion electrolyte. Angew. Chem. Int. Ed., 2018,57(8):2096-2100. |
| [19] | BAE J, LI Y, ZHAO F, et al. Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries. Energy Storage Materials, 2018,15:46-52. |
| [20] |
LIU Y, SUN Q, ZHAO Y, et al. Stabilizing the interface of NASICON solid electrolyte against Li metal with atomic layer deposition. ACS Applied Materials & Interfaces, 2018,10(37):31240-31248.
DOI URL PMID |
| [21] |
JU J, WANG Y, CHEN B, et al. Integrated interface strategy toward room temperature solid-state lithium batteries. ACS Applied Materials & Interfaces, 2018,10(16):13588-13597.
DOI URL PMID |
| [22] | ZHAO Q, LIU X, STALIN S, et al. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nature Energy, 2019,4(5):365-373. |
| [23] |
DUAN H, YIN Y X, SHI Y, et al. Dendrite-free Li-metal battery enabled by a thin asymmetric solid electrolyte with engineered layers. Journal of the American Chemical Society, 2018,140(1):82-85.
DOI URL PMID |
| [24] | PARANJAPE N, MANDADAPU P C, WU G, et al. Highly- branched cross-linked poly(ethylene oxide) with enhanced ionic conductivity. Polymer, 2017,111:1-8. |
| [25] | BAN X, ZHANG W, CHEN N, et al. A high-performance and durable poly(ethylene oxide)-based composite solid electrolyte for all solid-state lithium battery. The Journal of Physical Chemistry C, 2018,122(18):9852-9858. |
| [26] | GONG Y, FU K, XU S, et al. Lithium-ion conductive ceramic textile: a new architecture for flexible solid-state lithium metal batteries. Materials Today, 2018,21(6):594-601. |
| [27] |
LI D, CHEN L, WANG T, et al. 3D fiber-network-reinforced bicontinuous composite solid electrolyte for dendrite-free lithium metal batteries. ACS Applied Materials & Interfaces, 2018,10(8):7069-7078.
DOI URL PMID |
| [28] |
LI Z, HUANG H M, ZHU J K, et al. Ionic conduction in composite polymer electrolytes: case of PEO:Ga-LLZO composites. ACS Applied Materials & Interfaces, 2019,11(1):784-791.
DOI URL PMID |
| [29] | WANG Q, WEN Z, JIN J, et al. A gel-ceramic multi-layer electrolyte for long-life lithium sulfur batteries. Chem. Commun. (Camb), 2016,52(8):1637-1640. |
| [30] |
HAN X, GONG Y, FU K K, et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nature Materials, 2017,16(5):572-579.
DOI URL PMID |
| [31] | JU J, CHEN F, XIA C. Ionic conductivity of impregnated samaria doped ceria for solid oxide fuel cells. Electrochimica Acta, 2014,136:422-429. |
| [32] | WU B, WANG S, LOCHALA J, et al. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy & Environmental Science, 2018,11(7):1803-1810. |
| [33] |
HU J L, TIAN J, Li C L. Nanostructured carbon nitride polymer- reinforced electrolyte to enable dendrite-suppressed lithium metal batteries. ACS Applied Materials & Interfaces, 2017,9:11615-11625.
DOI URL PMID |
| [34] | HU J L, YAO Z G, CHEN K Y, et al. High-conductivity open framework fluorinated electrolyte bonded by solidified ionic liquid wires for solid-state Li metal batteries. Energy Storage Materials, 2020,28:37-46. |
| [1] | 郭宇翔, 黄立强, 王刚, 王宏志. 双锂盐凝胶复合电解质的制备及其在锂金属电池中的应用[J]. 无机材料学报, 2023, 38(7): 785-792. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||