无机材料学报 ›› 2019, Vol. 34 ›› Issue (8): 817-826.DOI: 10.15541/jim20180487 CSTR: 32189.14.10.15541/jim20180487
收稿日期:2018-10-16
修回日期:2019-01-07
出版日期:2019-08-20
网络出版日期:2019-05-13
作者简介:朱萌萌(1993-), 女, 硕士研究生. E-mail: <email>zhu_m19930821@126.com</email>
ZHU Meng-Meng,LI Guo-Hua( ),ZHANG Xue-Ming,ZHAI Jia-Xin,GAN Si-Ping,SONG Xiao
),ZHANG Xue-Ming,ZHAI Jia-Xin,GAN Si-Ping,SONG Xiao
Received:2018-10-16
Revised:2019-01-07
Published:2019-08-20
Online:2019-05-13
摘要:
纳米级氧化亚铜具有高效的催化性能, 但较差的稳定性使其应用受限。本研究采用简单可控的抗坏血酸液相还原及气氛焙烧法, 制备了一种兼具高催化活性与催化稳定性的Cu2O/BNNSs-OH负载型催化剂, 其中以聚乙烯吡咯烷酮(PVP)与水相变提供的“推-拉”作用剥离的氮化硼纳米片(BNNSs)为载体, 液相还原反应体系pH=11时, 抗坏血酸向Cu 2+滴定制备的Cu2O纳米颗粒(2~7 nm)为活性组分。通过扫描电子显微镜(SEM)、高分辨透射电子显微镜(HRTEM)、原子力显微镜(AFM)、X射线衍射仪(XRD)、X射线光电子能谱仪(XPS)、傅里叶变换红外光谱仪(FT-IR)及拉曼(Raman)光谱仪等对样品的形貌和结构进行表征, 结果表明: Cu2O纳米粒子不但高度分散于载体表面, BNNSs对Cu2O还有一定的稳定作用, 避免其被氧化成CuO。将Cu2O/BNNSs-OH应用于对硝基苯酚催化还原反应中, 该催化剂表现出同贵金属类似的高催化活性, 5次重复利用后的转化率仍高达90%。
中图分类号:
朱萌萌, 李国华, 张雪明, 翟佳欣, 甘思平, 宋潇. 氮化硼纳米片负载纳米Cu2O及其催化还原对硝基苯酚[J]. 无机材料学报, 2019, 34(8): 817-826.
ZHU Meng-Meng, LI Guo-Hua, ZHANG Xue-Ming, ZHAI Jia-Xin, GAN Si-Ping, SONG Xiao. Boron Nitride Nanosheets Supported Cu2O Nanoparticles: Synthesis and Catalytic Reduction for 4-nitrophenol[J]. Journal of Inorganic Materials, 2019, 34(8): 817-826.
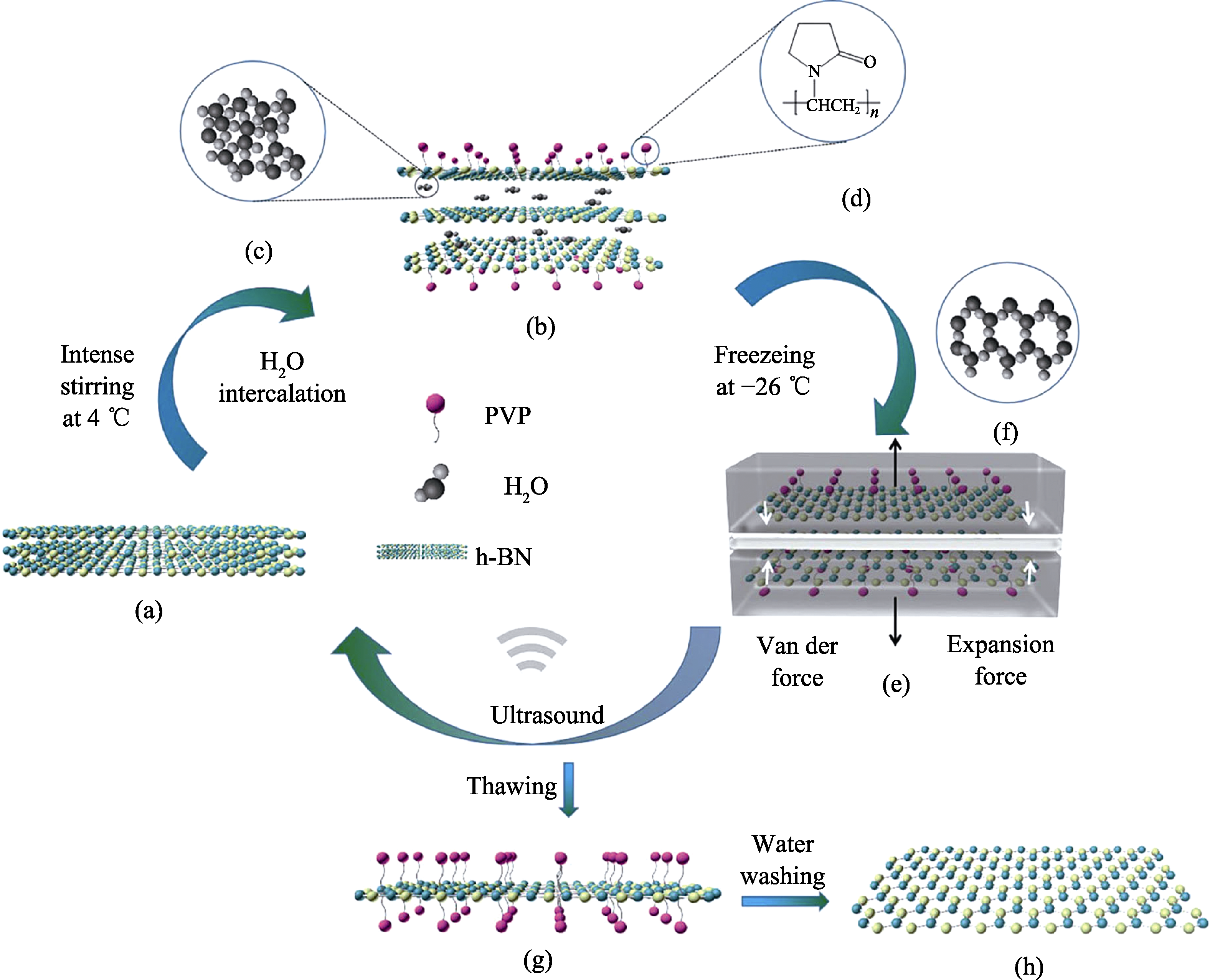
图1 冻融法剥离的机理示意图
Fig. 1 Gentle water freezing-thawing exfoliation of h-BN triggered by freezing expansion force and against reaggregation by PVP coating
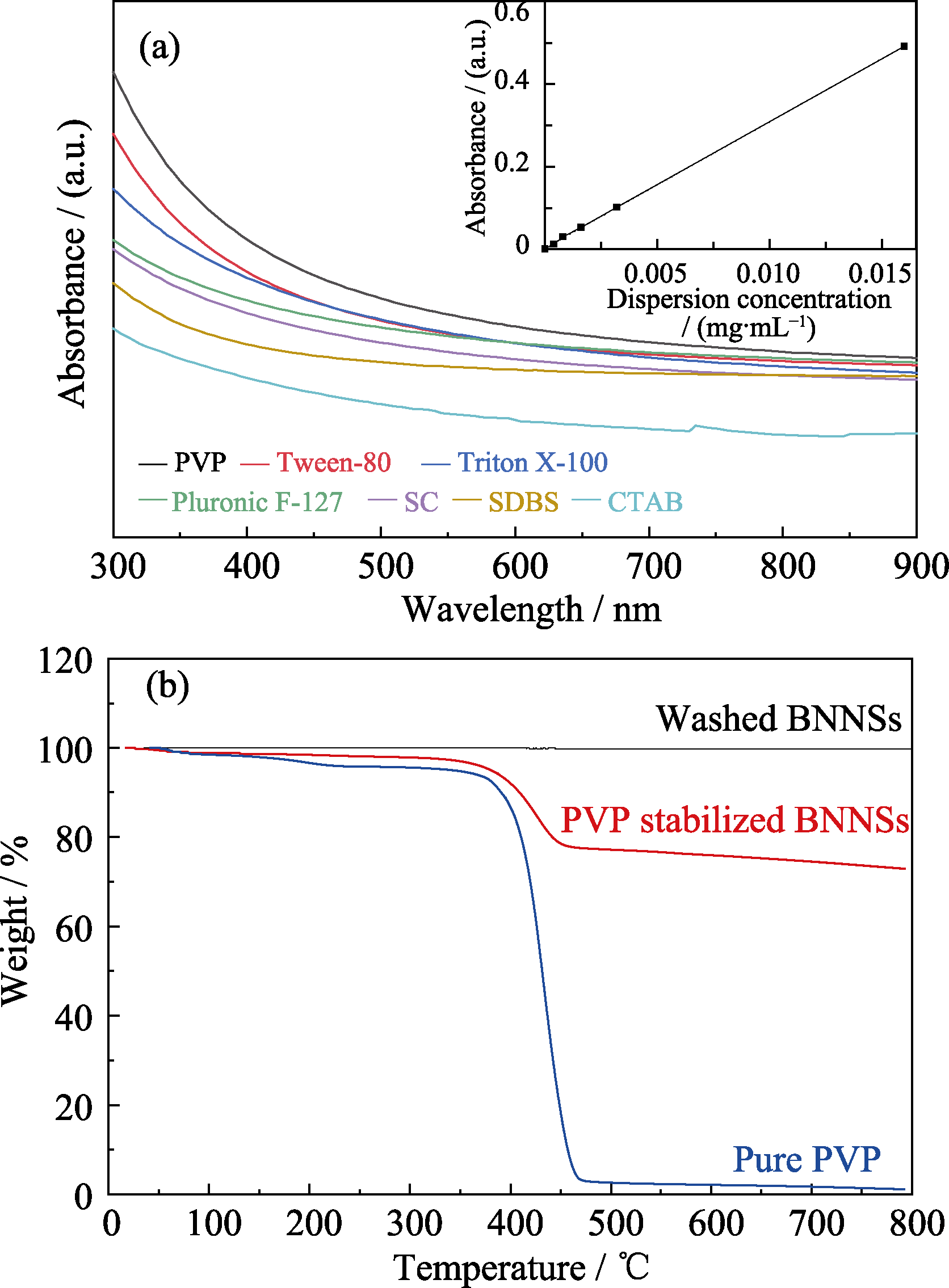
图2 不同表面活性剂剥离的BNNSs紫外-可见吸收光谱图(a), BNNSs、PVP/ BNNSs、纯PVP的TGA曲线(b)
Fig. 2 Absorption spectra for h-BN dispersions stabilized with various surfactants (a), TGA curves of washed BNNSs, PVP stabilized BNNSs, and pure PVP (b)
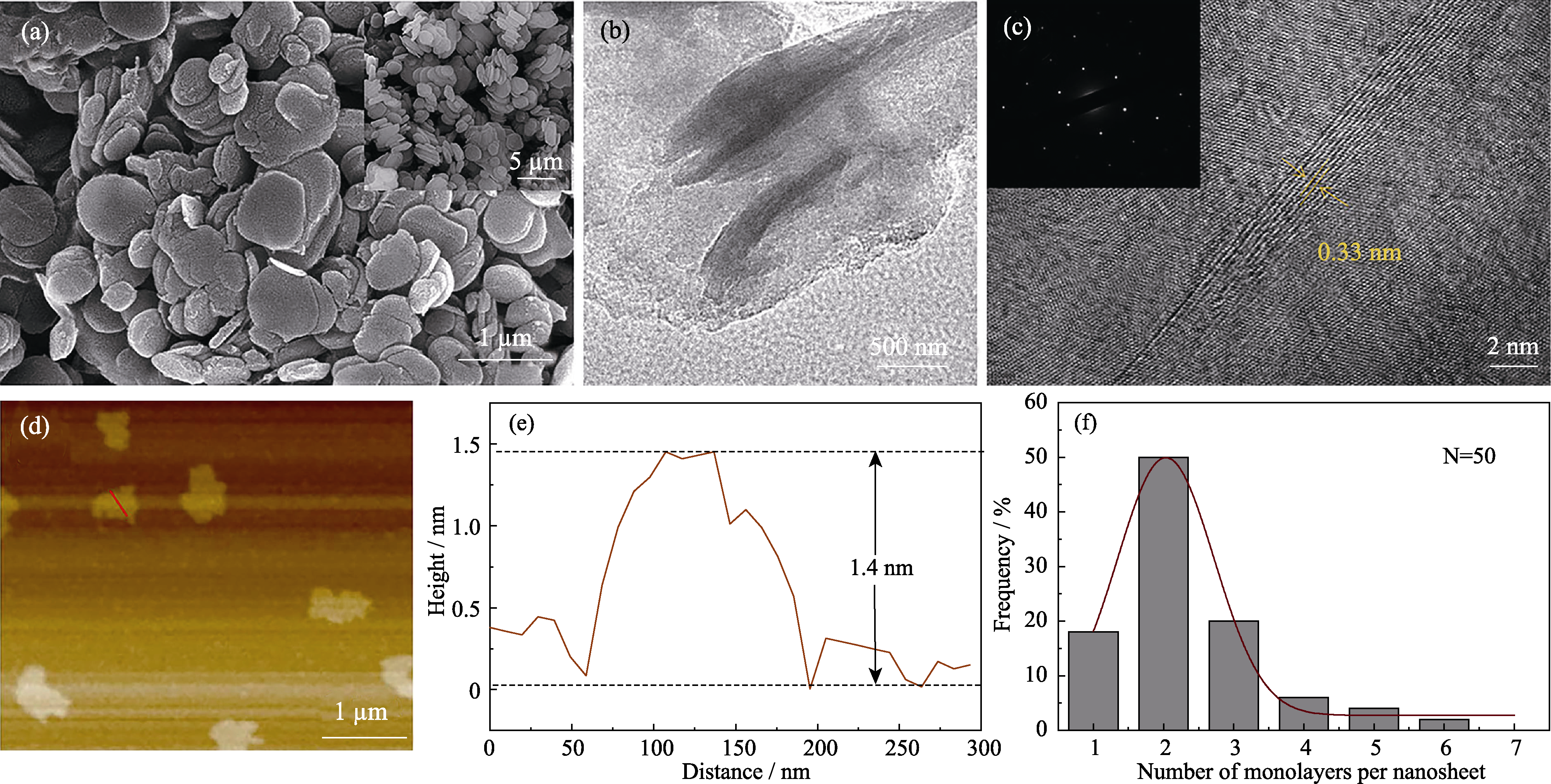
图3 BNNSs的SEM ((a)右上角为块状h-BN放大图片), TEM (b)和HRTEM照片((c)左上角为SEAD图案), 及其AFM图(d)、高度轮廓图(e)和厚度统计图(f)
Fig. 3 SEM image (a) with inset showing bulk h-BN, TEM image (b), HRTEM image (c) with inset showing corresponding SAED pattern, AFM image (d), and the corresponding height profile of random nanosheet along the red trace (e) and statistical analyse on the number of monolayers per sheet (f) of BNNSs
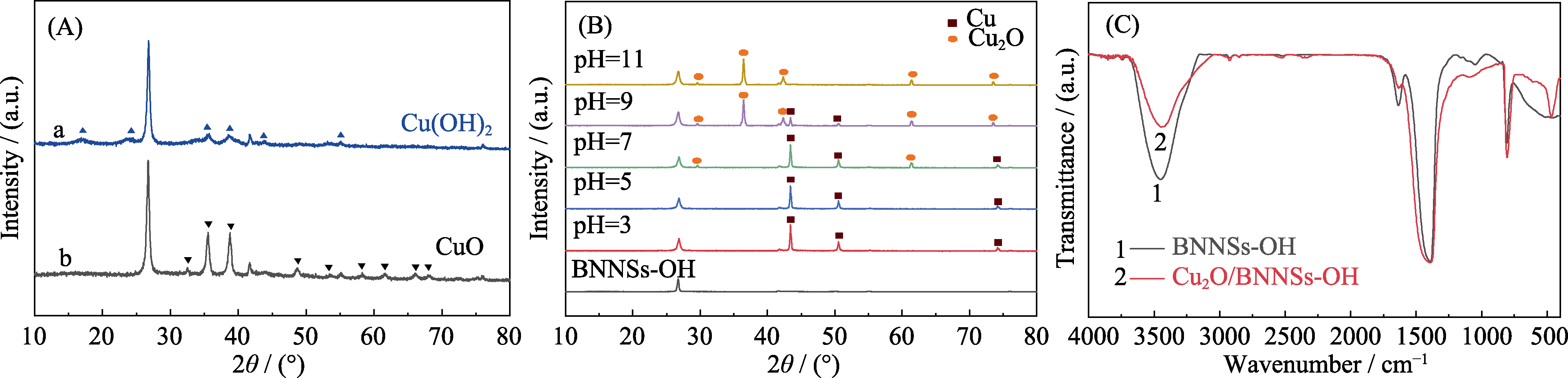
图5 加入抗坏血酸前混合溶液不同pH(a: pH 5-7, b: pH 9-11)下得到前驱体的XRD谱(A), 抗坏血酸还原反应体系不同pH下制备的样品的XRD图谱(B), Cu2O/BNNSs-OH的红外光谱图(C, pH11)
Fig. 5 XRD patterns of precursors (A) before adding VC at pH 5-7 (a) and pH 9-11 (b), specimens (B) in ascorbic acid solution reduction system at different pH, and FT-IR spectra of the as-obtained samples at pH 11 (C)
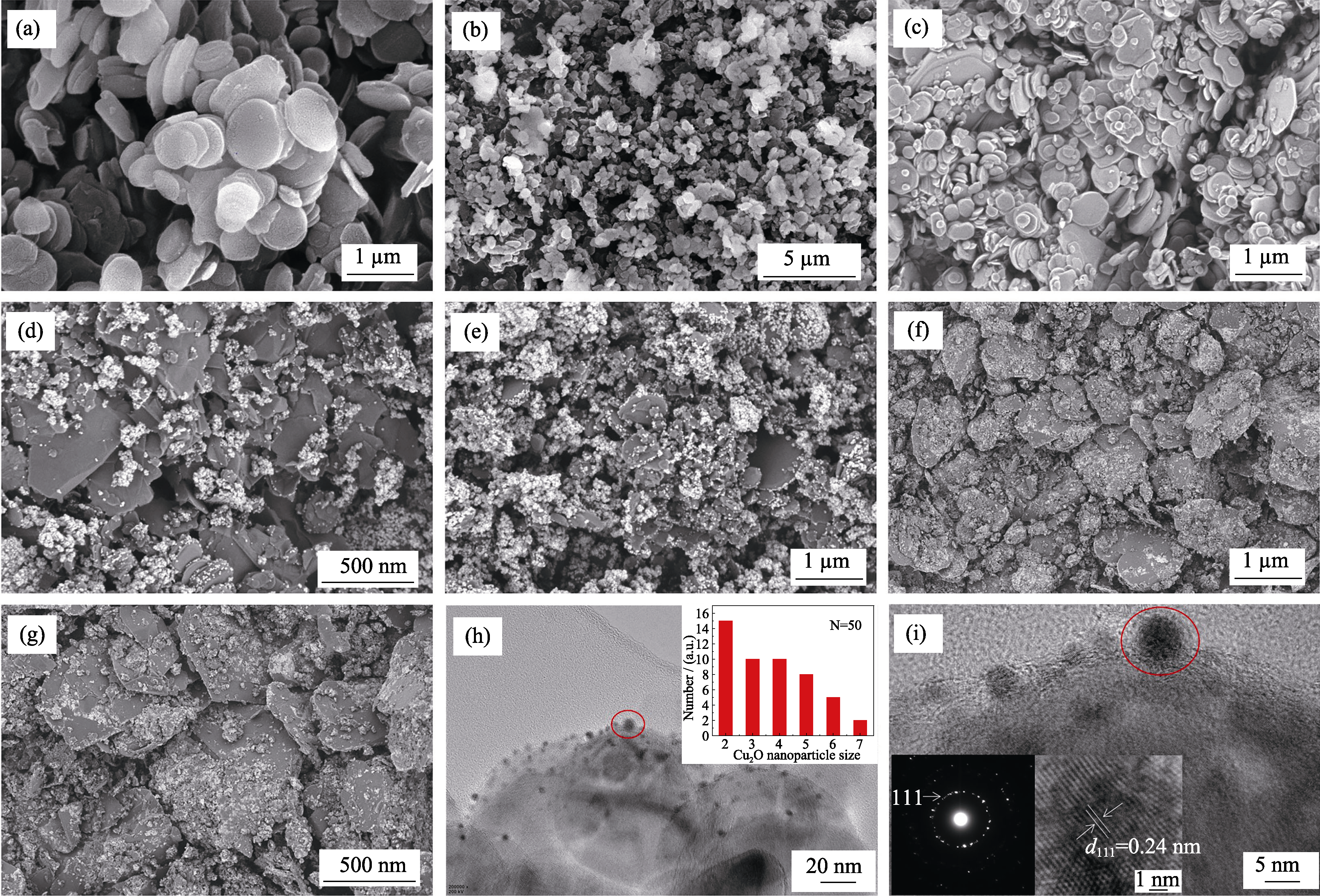
图7 羟基化氮化硼纳米片(a), CuO/BNNSs-OH(b)在不同pH抗坏血酸还原反应体系下制备样品的SEM照片((c) pH 3, (d) pH 5, (e) pH 7, (f) pH 9, (g) pH 11), Cu2O/BNNSs-OH的HRTEM照片(h~i) (h)右上角为Cu2O NPs粒径统计图, (i)左下角为SEAD图案及晶格条纹图案, pH 11
Fig. 7 SEM images of BNNSs-OH (a), CuO/BNNSs-OH (b), products prepared in ascorbic acid solution reduction system at different pH((c) pH 3, (d) pH 5, (e) pH 7, (f) pH 9, (g) pH 11), HRTEM images of Cu2O/BNNSs-OH (h-i) with inset in (h) showing the corresponding size distributions of Cu2O NPs with inset in (i) showing the corresponding selected SAED pattern and lattice fringe pattern at pH 11
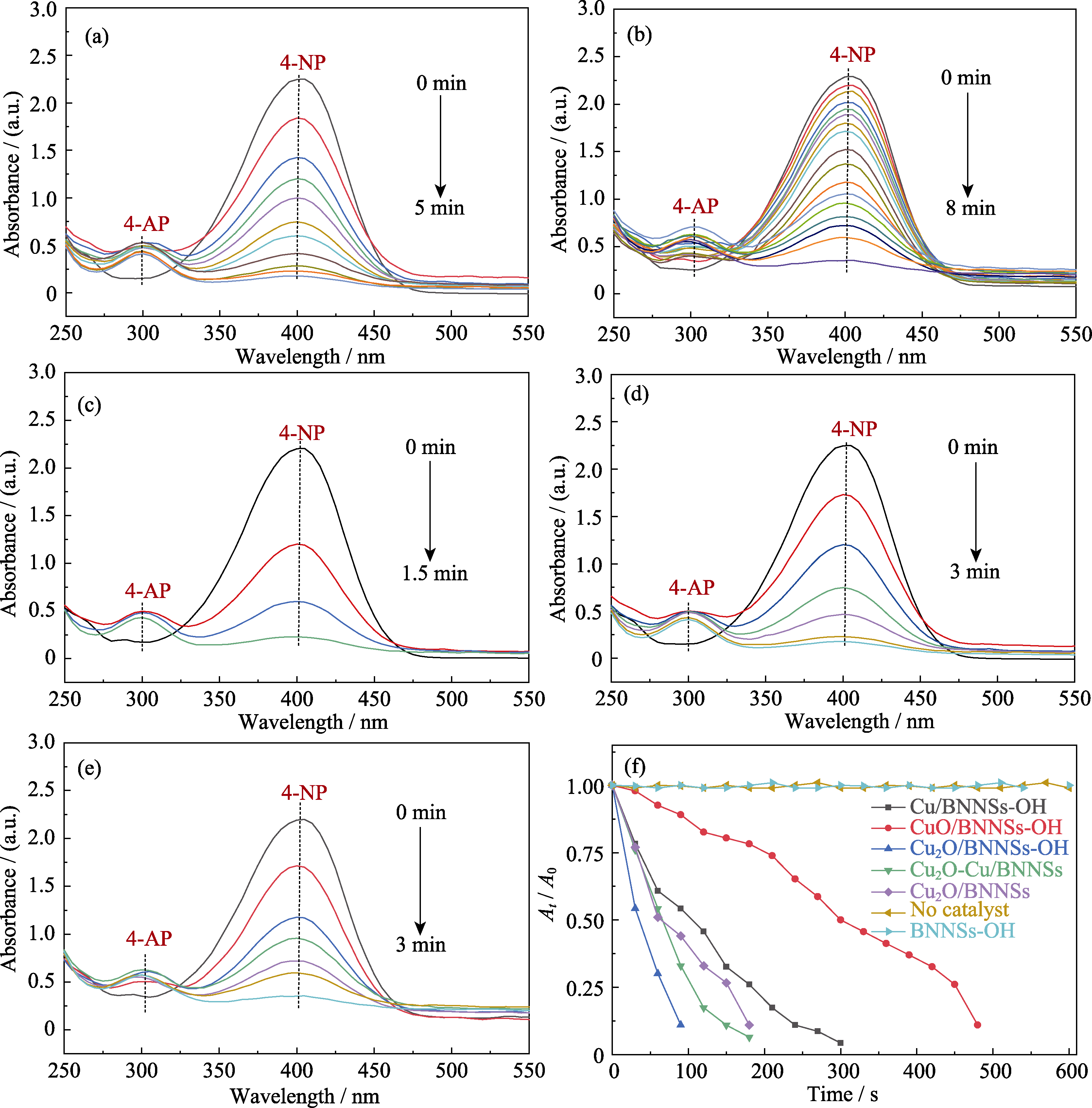
图9 Cu/BNNSs-OH(a)、CuO/BNNSs-OH(b)、Cu2O/BNNSs-OH(c)、Cu2O-Cu/BNNSs-OH(d)以及Cu2O/BNNSs(e)催化还原4-NP紫外-可见吸收光谱图, At/A0与化学反应时间T的关系图(f)
Fig. 9 UV-Vis absorption spectra of Cu/BNNSs-OH (a), CuO/BNNSs-OH (b), Cu2O/BNNSs-OH (c), Cu2O-Cu/BNNSs-OH (d), and Cu2O/BNNSs (e) in contrast to the reduction of 4-NP as a function of reaction time with excess amount of NaBH4 over various catalysts (f)
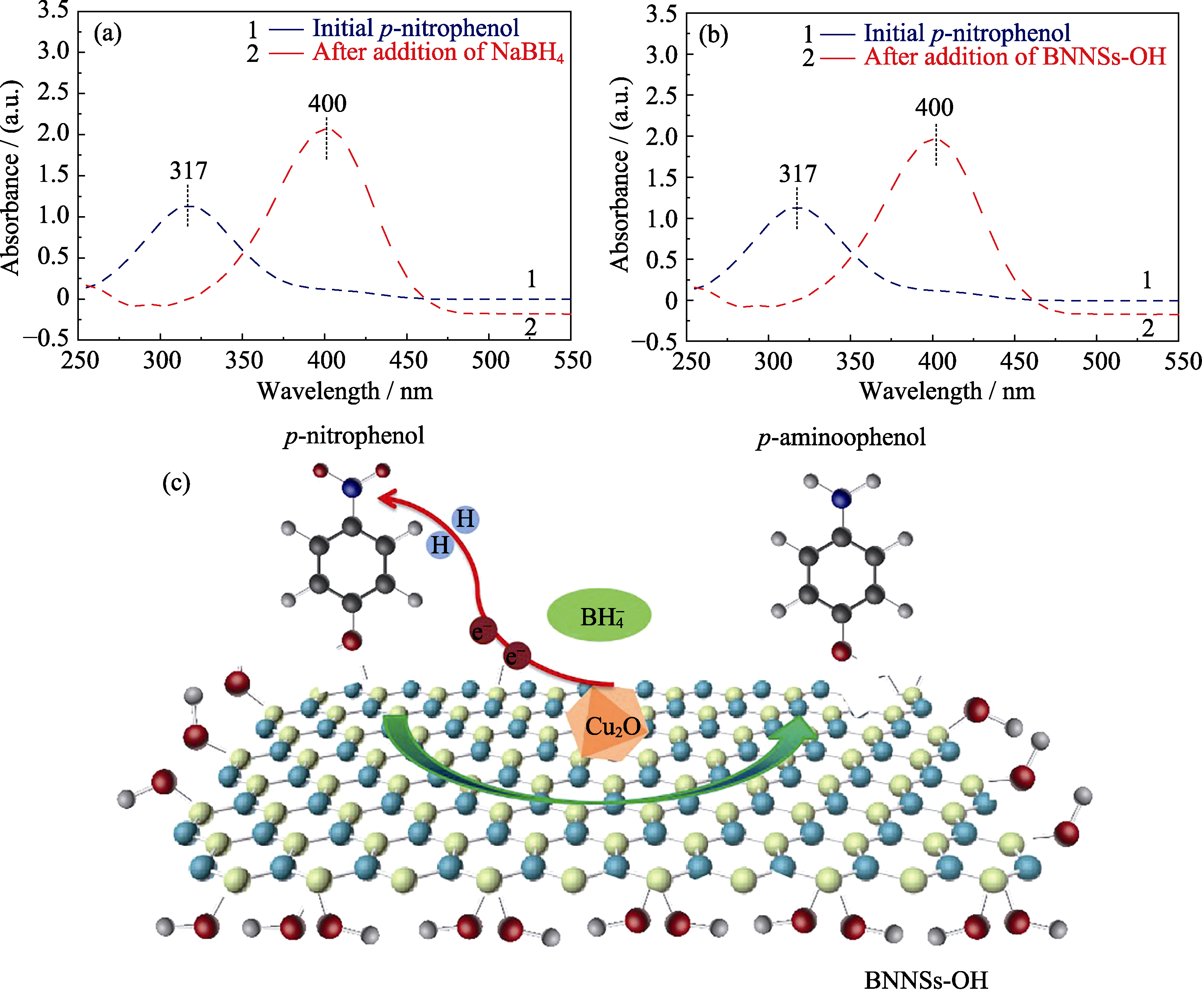
图10 加入NaBH4(a)、BNNSs-OH(b)前后4-NP的紫外-可见光谱图, 及Cu2O/BNNSs-OH催化还原4-NP的机理示意图
Fig. 10 UV-Vis absorption spectra of 4-NP solution before and after NaBH4 (a) and BNNSs-OH (b) additions, and schematic of the reduction of 4-NP to 4-AP over the Cu2O/ BNNSs-OH (c)
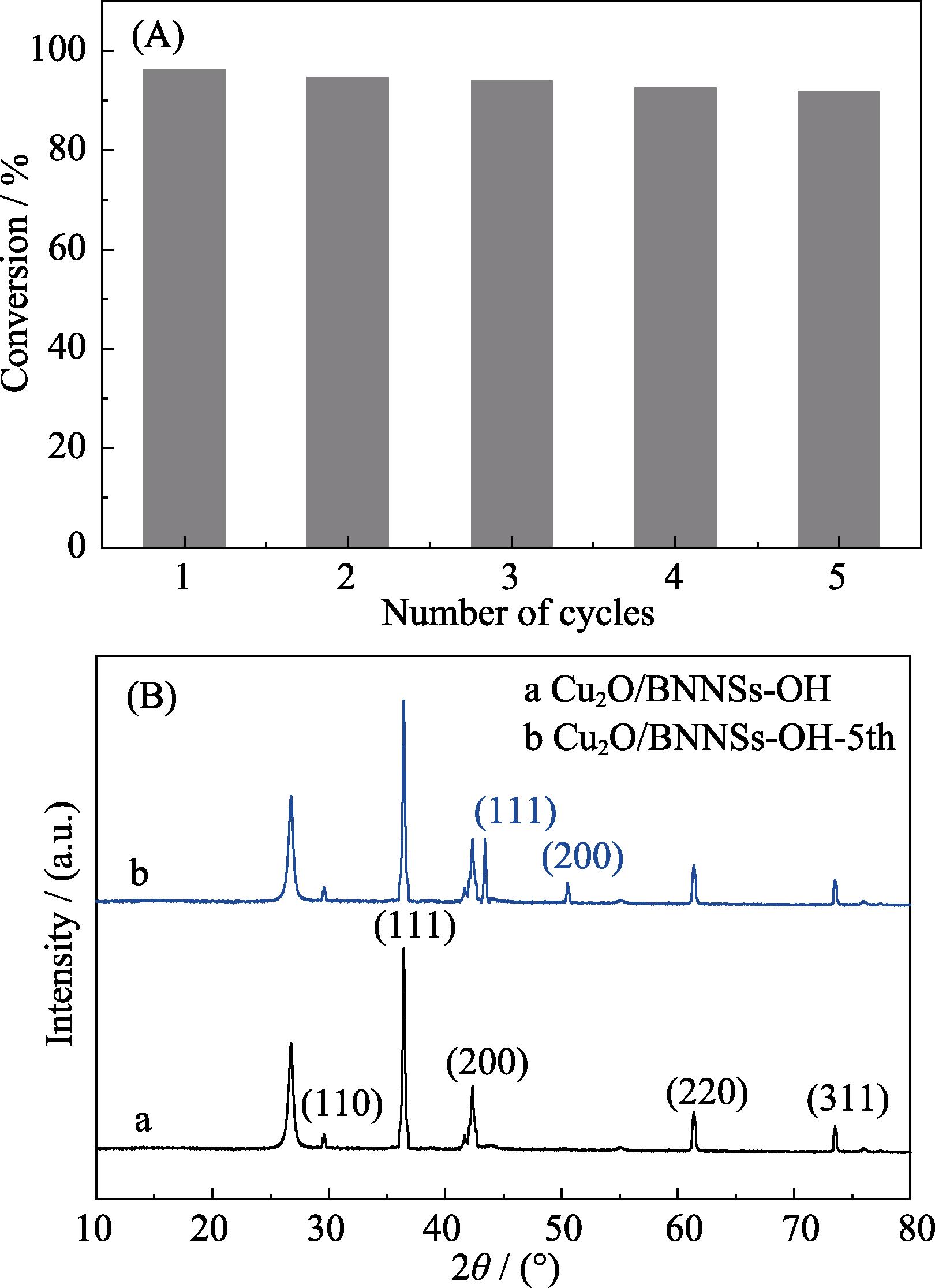
图11 Cu2O/BNNSs-OH催化还原4-NP循环利用图(A), 5次循环前后Cu2O/BNNSs-OH催化剂XRD图谱(B)
Fig. 11 Reusability of Cu2O/BNNSs-OH catalyst for the reduction of 4-NP with NaBH4 (A), XRD patterns of Cu2O/BNNSs- OH catalyst before and after five usages (B)
| [1] | RATH P C, SAIKIA D, MISHRA M , et al. Exceptional catalytic performance of ultrafine Cu2O nanoparticles confined in cubic mesoporous carbon for 4-nitrophenol reduction. Applied Surface Science, 2018,427:1217-1226. |
| [2] | NIU H, LIU S, CAI Y , et al. MOF derived porous carbon supported Cu/Cu2O composite as high performance non-noble catalyst. Microporous and Mesoporous Materials, 2016,219:48-53. |
| [3] | ROCHA M, COSTA P, PEREIRA C , et al. L-serine-functionalized montmorillonite decorated with Au nanoparticles: a new highly efficient catalyst for the reduction of 4-nitrophenol. Journal of Catalysis, 2018,361:143-155. |
| [4] | MAHAM M, NASROLLAHZADEH M, SAJADI S M , et al. Biosynthesis of Ag/reduced graphene oxide/Fe3O4 using Lotus garcinii leaf extract and its application as a recyclable nanocatalyst for the reduction of 4-nitrophenol and organic dyes. Journal of Colloid & Interface Science, 2017,497:33-42. |
| [5] | ZOU P P, WANG M S, ZHAO L , et al. One-step synthesis of Pt@three-dimensional graphene composite hydrogel: an efficient recyclable catalyst for reduction of 4-nitrophenol. Applied Organometallic Chemistry, 2016,30(8):722-725. |
| [6] | COCCIA F, TNUCCI L, BOSCO D , et al. One-pot synthesis of lignin-stabilised platinum and palladium nanoparticles and their catalytic behaviour in oxidation and reduction reaction. Green Chemistry, 2012,14(4):1073-1078. |
| [7] |
GACEM N, DIAO P . Effect of solvent polarity on the assembly behavior of PVP coated rhodium nanoparticles. Colloids and Surfaces A, 2013,417:32-38.
DOI URL |
| [8] | YANG X F, WANG A, QIAO B , et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Accounts of Chemical Research, 2013,46(8):1740-1748. |
| [9] | OH S D, KIM M R, CHOI S H , et al. Radiolytic synthesis of Pd-M (M=Ag, Au, Cu, Ni and Pt) alloy nanoparticles and their use in reduction of 4-nitrophenol. Journal of Industrial and Engineering Chemistry, 2008,14(5):687-692. |
| [10] | MUNNIK P, DE JONGH P E, DE JONG K P . Recent developments in the synthesis of supported catalysts. Chemical Reviews, 2015,46(38):6687-6714. |
| [11] | YAN X, WANG X, TANG Y , et al. Unusual loading-dependent sintering-resistant properties of gold nanoparticles supported within extra-large mesopores. Chemistry of Materials, 2013,25(9):1556-1563. |
| [12] | NAG A, RAIDOGIA K, HEMBRAM K P , et al. Graphene analogues of BN: novel synthesis and properties. ACS Nano, 2010,4(3):1539-1544. |
| [13] | LASKOWSKI R, BLAHA P, SCHWARZ K . Bonding of hexagonal BN to transition metal surfaces: an ab initio density-functional theory study. Physical Review B Condensed Matter, 2008,78(78):1436-1446. |
| [14] | HUANG C, CHEN C, YE X , et al. Stable colloidal boron nitride nanosheet dispersion and its potential application in catalysis. Journal of Materials Chemistry A, 2013,1(39):12192-12197. |
| [15] | ZHENG M, LIU Y, GU Y , et al. Synthesis and characterization of boron nitride sponges as a novel support for metal nanoparticles. Science in China Series B: Chemistry, 2008,51(3):205-210. |
| [16] | LIANG H L . Research on the physical properties of fully hydrogenated boron nitride films. Jinan: Shandong University. 2012:1-24. |
| [17] | LI C, WANG T L, WU Y Z , et al. Fabrication of two-dimensional nanosheets via water freezing expansion exfoliation. Nanotechnology, 2014,25(49):1-6. |
| [18] | GUARDIA L, PAREDES J I, ROZADA R , et al. Production of aqueous dispersions of inorganic graphene analogues by exfoliation and stabilization with non-ionic surfactants. RSC Advances, 2014,4(27):14115-14127. |
| [19] | LIU Q, KAZUAKI N, KENSUKE K , et al. Effects of reaction parameters on the preparation of submicron Cu particles by liquid phase reduction method and the study of reaction mechanism. Powder Technology, 2013,241(3):98-104. |
| [20] | SONG H, LI T, ZHANG J , et al. Highly anisotropic Sb2Se3 nanosheets: gentle exfoliation from the bulk precursors possessing 1D crystal structurep. Advanced Materials, 2017,29(29):1-7. |
| [21] | SMITH R J, KIBG P J, LOTYA M , et al. Large-scale exfoliation of inorganic layered compounds in aqueous surfactant solutions. Advanced Materials, 2011,23(34):3944-3948. |
| [22] |
MA P, SPENCER J T . Non-covalent stabilization and functionalization of boron nitride nanosheets (BNNSs) by organic polymers: formation of complex BNNSs-containing structures. Journal of Materials Science, 2015,50(1):313-323.
DOI URL |
| [23] |
GAO W, ZHAO Y, YIN H . Lateral size selection of liquid exfoliated hexagonal boron nitride nanosheets. RSC Advances, 2018,8:5976-5983.
DOI URL |
| [24] | HUMINIC G, HUMINIC A . Application of nanofluids in heat exchangers: a review. Renewable & Sustainable Energy Reviews, 2012,16(8):5625-5638. |
| [25] | PARK K S, LEE D Y, KIM K J , et al. Observation of a hexagonal BN surface layer on the cubic BN film grown by dual ion beam sputter deposition. Applied Physics Letters, 1997,70(3):315-317. |
| [26] | WAGNER C D, RIGGS W M, Davis L E , et al. Muilenber, handbook of X-ray Photoelectron Spectroscopy. erkin Elmer Corporation Physical Electronics Division, USA, 1979: 1-190. |
| [27] | ESPINOS J P, MORALES J, BARRANCO A , et al. Interface effects for Cu, CuO, and Cu2O deposited on SiO2 and ZrO2. XPS determination of the valence state of copper in Cu/SiO2 and Cu/ZrO2 Catalysts. Journal of Physical Chemistry B, 2002,106(27):6921-6929. |
| [28] | SUN Q, LI Y, SUN X , et al. Improved photoelectrical performance of single-crystal TiO2 nanorod arrays by surface sensitization with copper quantum dots. ACS Sustainable Chemistry & Engineering, 2013,1(7):798-804. |
| [29] | YAN X Y, TONG X L, ZHANG Y F , et al. Cuprous oxide nanoparticles dispersed on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. Chemical Communications, 2012,48(13):1892-1894. |
| [30] | MICHIKAZU H, TAKESHI K, MUTSUKO K , et al. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chemical Communication, 1998,3:357-358. |
| [31] | HUANG J, VOGEHR S, TANG S , et al. Highly catalytic Pd-Ag bimetallic dendrites. Journal of Physical Chemistry C, 2010,114(35):15005-15010. |
| [1] | 马慧, 陶疆辉, 王艳妮, 韩玉, 王亚斌, 丁秀萍. 硅钛杂化介孔球负载金纳米粒子及其催化性能调控[J]. 无机材料学报, 2022, 37(4): 404-412. |
| [2] | 耿仁杰, 俄松峰, 李朝威, 李涛涛, 吴隽, 姚亚刚. 高结晶度氮化硼纳米片的制备及其与聚乙烯醇复合薄膜的性能[J]. 无机材料学报, 2019, 34(4): 401-406. |
| [3] | 殷月月, 杨勇, 张良柱, 李永生, 马云峰, 杨莉莉, 黄政仁. 金/钯哑铃状纳米晶的制备及其催化对硝基苯酚还原研究[J]. 无机材料学报, 2018, 33(1): 19-26. |
| [4] | 吴 霜, 刘 波, 邱志澈, 陈士伟, 张娟楠, 刘小林, 顾 牡, 黄世明, 倪 晨. LuTaO4:Ln3+(Ln=Eu,Tb)透明薄膜制备改进与其发光性能研究[J]. 无机材料学报, 2016, 31(4): 372-376. |
| [5] | 张 浩, 张高校, 吴相伟, 温兆银. PVP溶胶-凝胶法制备锂稳定Na-β-Al2O3纳米粉体[J]. 无机材料学报, 2013, 28(9): 916-920. |
| [6] | 朱丁,刘恒,姚亚东,李大成. 纳米结构V2O5空心微球的制备与表征[J]. 无机材料学报, 2008, 23(1): 43-48. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||