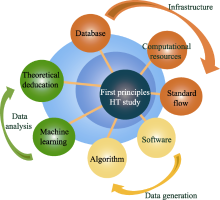
 Fig. 1
Schematic diagram of first principles high-throughput (HT) study on materials
Fig. 1
Schematic diagram of first principles high-throughput (HT) study on materials
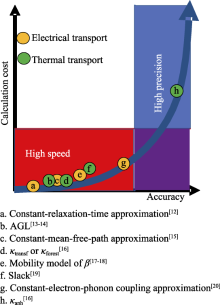 Fig. 2
Relationship between calculation cost and accuracy
Fig. 2
Relationship between calculation cost and accuracy
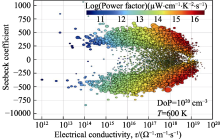 Fig. 3
Electrical properties in MP with a constant relaxation time approximation[
Fig. 3
Electrical properties in MP with a constant relaxation time approximation[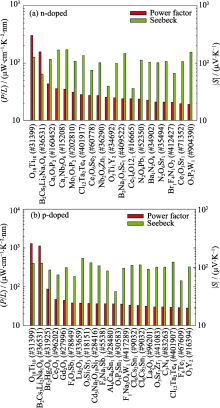 Fig. 4
n- (a) and p-doped (b) average normalized power factor P/L and Seebeck coefficient[
Fig. 4
n- (a) and p-doped (b) average normalized power factor P/L and Seebeck coefficient[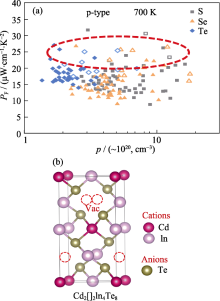 Fig. 5
Power factor of chalcogenides with diamond-like structures (a) and vacancy-containing ternary chalcogenides (b)[
Fig. 5
Power factor of chalcogenides with diamond-like structures (a) and vacancy-containing ternary chalcogenides (b)[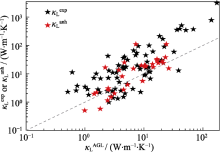 Fig. 6
Relationship between κLAGLandκLanh/κLexp(Data from Ref.[13])
Fig. 6
Relationship between κLAGLandκLanh/κLexp(Data from Ref.[13])
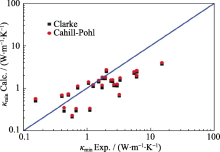 Fig. 7
Relationship between calculated κL and experimental κL[
Fig. 7
Relationship between calculated κL and experimental κL[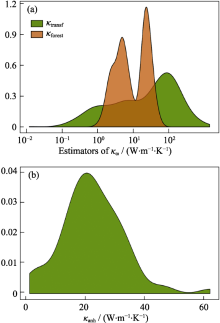 Fig. 8
Three prediction models of the κL in half-Heusler compounds[16](a) Frequency densities of the estimators of thermal conductivity at 300 Kκtransfand κforest;and (b) distribution of κanhover the 75 thermodynamically stable half-Heuslers
Fig. 8
Three prediction models of the κL in half-Heusler compounds[16](a) Frequency densities of the estimators of thermal conductivity at 300 Kκtransfand κforest;and (b) distribution of κanhover the 75 thermodynamically stable half-Heuslers
 Fig.1
A 128-member binary library[
Fig.1
A 128-member binary library[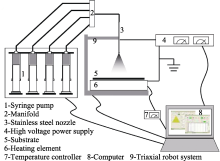 Fig.2
Schematic diagram of combinatorial electrostatic atomization system “M-ist Combi”[
Fig.2
Schematic diagram of combinatorial electrostatic atomization system “M-ist Combi”[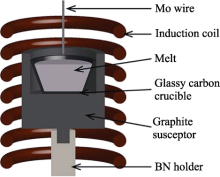 Fig. 3
Schematic illustration for the Czochralski crystal growth[
Fig. 3
Schematic illustration for the Czochralski crystal growth[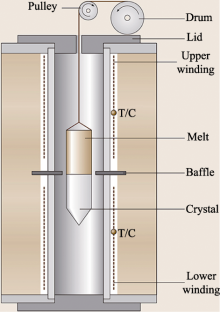 Fig.4
Schematic illustration for the vertical Bridgman crystal growth[
Fig.4
Schematic illustration for the vertical Bridgman crystal growth[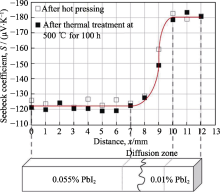 Fig.5
Variation of Seebeck coefficient values along n-type 0.01/0.055% PbI2 doped (Pb0.95Sn0.05Te)0.92(PbS)0.08 functionally graded materials[
Fig.5
Variation of Seebeck coefficient values along n-type 0.01/0.055% PbI2 doped (Pb0.95Sn0.05Te)0.92(PbS)0.08 functionally graded materials[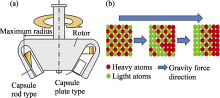 Fig.6
(a) Scheme of a rotor with capsules for sedimentation experiment and (b) mechanism of sedimentation of atoms in the strong acceleration field[
Fig.6
(a) Scheme of a rotor with capsules for sedimentation experiment and (b) mechanism of sedimentation of atoms in the strong acceleration field[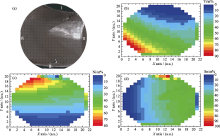 Fig.7
(a) Optical image of the annealed Ti-Ni-Sn thin film materials library; (b-d) color-coded results of the high-throughput EDX measurements of the material library[
Fig.7
(a) Optical image of the annealed Ti-Ni-Sn thin film materials library; (b-d) color-coded results of the high-throughput EDX measurements of the material library[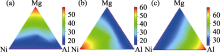 Fig.8
EDX measurements of the concentration of (a)magnesium, (b)nickel and (c)aluminum of an Mg-Ni-Al ternary thin film library[
Fig.8
EDX measurements of the concentration of (a)magnesium, (b)nickel and (c)aluminum of an Mg-Ni-Al ternary thin film library[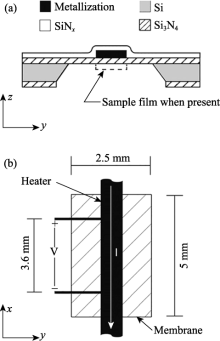 Fig.9
Layout of the nanocalorimeter cell[
Fig.9
Layout of the nanocalorimeter cell[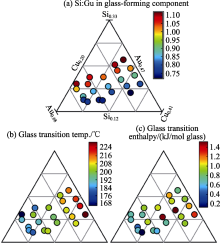 Fig.10
Composition trends over the sample library, (a) Si:Cu ratio for the glass-forming component, (b) glass transition temperature and (c) the total enthalpy of this glass reaction[
Fig.10
Composition trends over the sample library, (a) Si:Cu ratio for the glass-forming component, (b) glass transition temperature and (c) the total enthalpy of this glass reaction[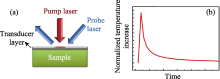 Fig.11
(a) Schematic of the pump and probe laser measurement setup and (b)temperature increase as a function of time after a single-pulse[
Fig.11
(a) Schematic of the pump and probe laser measurement setup and (b)temperature increase as a function of time after a single-pulse[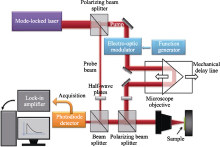 Fig.12
Time-domain thermoreflectance experimental setup[
Fig.12
Time-domain thermoreflectance experimental setup[|
|