为解决该问题, 本研究通过原位生长法, 在改性生物炭表面负载纳米级PB, 将PB的高效配位作用、催化性与生物炭材料的吸附性相结合, 深入研究复合材料对氨氮分子的吸附作用与机制, 探索其在富营养化污水治理中的应用前景。
1 实验方法
1.1 试剂与仪器
实验试剂: 原料竹粉(150 μm (100目))产自于浙江丽水。碳酸氢钾(KHCO3)、36%盐酸(HCl)、30%过氧化氢(H2O2)、氯化铁(FeCl3)、亚铁氰化钾(K4Fe(CN)6)、柠檬酸(C6H8O7)、氯化铵(NH4Cl)、酒石酸钾钠(KNaC4H4O6)、纳氏试剂(HgCl2-KI- KOH)、腐殖酸(HA)、5,5-二甲基-1-呲咯啉-N-氧化物(DMPO)和叔丁醇(C4H10O)均为化学纯, 购自国药集团化学试剂有限公司。实验所有用水均为去离子水, 由江南大学实验物资仓库提供。
实验仪器: 马弗炉, 场发射扫描电子显微镜(SEM, 日立S-4800), 透射电子显微镜(TEM, 日立JEM-2100 plus), X射线衍射仪(XRD, 布鲁克D8 PHASER), 全自动比表面积及微孔物理吸附仪(BET, 麦克ASAP2020 MP), X射线光电子能谱仪(XPS, 岛津Kratos), 热重分析仪(TGA, 梅特勒TGA/1100SF), 紫外可见分光光度计(UV-vis, 岛津UV-2700), 电子自旋共振波谱仪(ESR, 日本电子JES-X3)。
1.2 材料的制备
负载普鲁士蓝: 分别称取1 mmol的FeCl3和K4Fe(CN)6, 将二者分别溶入20 mL、0.05 mmol的柠檬酸水溶液中, 搅拌至完全溶解[17]。将含有FeCl3的柠檬酸溶液加热至55 ℃, 在磁力搅拌下加入1 g BC700生物炭, 待生物炭分散均匀后, 再滴加20 mL K4Fe(CN)6的柠檬酸水溶液(即FeCl3、K4Fe(CN)6与BC700的投料质量比为1 : 1.7 : 2), 55 ℃下加热30 min, 冷却至室温。减压过滤, 取滤渣, 分别使用丙酮和去离子水洗涤, 直至滤液无色, 确保未被负载的PB被完全去除。最后在50 ℃干燥12 h后, 得到PB改性的生物炭复合材料, 命名为BC700-PB。用TGA分析BC700与BC700-PB的灰分率, 进而计算BC700-PB中PB的负载量。
1.3 吸附实验
以氯化铵溶液模拟氨氮废水, 使用HJ 535-2009《纳氏试剂分光光度法》测定氨氮浓度。取100 mL浓度为50 mg/L的氯化铵溶液于锥形瓶中, 加入0.5 g的生物炭吸附剂, 20 ℃磁力搅拌30 min, 过滤取滤液, 使用紫外分光光度计测定并计算其氨氮浓度。通过公式(1, 2)计算吸附效率η及吸附剂的平衡吸附量qe(mg/g)。
式中,C0为溶液中初始氨氮浓度(mg/L); Ce为吸附平衡后溶液中剩余的氨氮浓度(mg/L); V为溶液体积(mL); m为吸附剂质量(g)。
1.4 芬顿催化实验及循环性能
用NH4Cl、腐殖酸(HA)、KCl、MgCl2、AlCl3的混合溶液模拟多组分污水。取100 mL、50 mg/L的模拟污水置于锥形瓶中, 加入5 mL、30% H2O2溶液, 使用盐酸调节pH至pH 2, 加入0.5 g的BC700- PB, 恒温磁力搅拌60 min, 过滤取滤液, 采用纳氏试剂分光光度法测定其氨氮浓度Ce; 采用紫外分光光度法测定腐殖酸的浓度。分别使用pH 10的NaCl/NaOH混合溶液和去离子水作为再生液, 清洗再生吸附氨氮后的BC700-PB。以1 g/100 mL的比例添加再生液, 20 ℃恒温磁力搅拌60 min, 过滤取滤渣, 100 ℃真空干燥120 min后得到再生完成的生物炭材料, 之后多次循环实验步骤1.3, 以测试生物炭材料在两种不同再生液中的循环再生性能。
2 结果与讨论
2.1 BC700-PB的形貌及结构表征
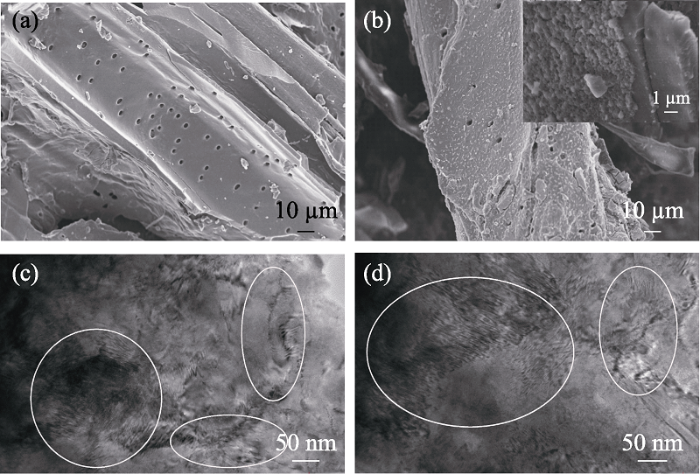
在造孔剂KHCO3作用下,竹粉在700 ℃热解, TGA分析计算BC700的灰分率为4.3%。进一步对其进行形貌观察。从图1(a)的SEM照片可以看出, 添加造孔剂KHCO3后, BC700呈现出多孔结构, 且其表面光滑, 这是由于生物炭经过酸性H2O2溶液浸渍, 大量无机灰分溶解于酸性H2O2溶液中。PB在BC700原位负载后, 根据BC700-PB的灰分率, 可计算PB的负载量为10.6%。图1(b)显示BC700原来光滑的表面出现了大量直径100 nm左右的颗粒, 可归属于PB颗粒。图1(c, d)的TEM照片显示BC700和BC700-PB均具有大量的孔道结构(图内白圈所示)。负载PB后, 可以发现BC700的表面及孔道中都有一定量的PB, 说明PB的原位负载主要是通过形成纳米颗粒后被生物炭复杂的表面形貌和孔道结构所固定, 且负载PB后仍能基本保持生物炭的孔结构[18]。
图1
图1
BC700 (a)和BC700-PB (b)的SEM照片, BC700 (c)和BC700-PB (d)的TEM照片
Fig. 1
SEM images of BC700 (a) and BC700-PB (b), and TEM images of BC700 (c) and BC700-PB (d)
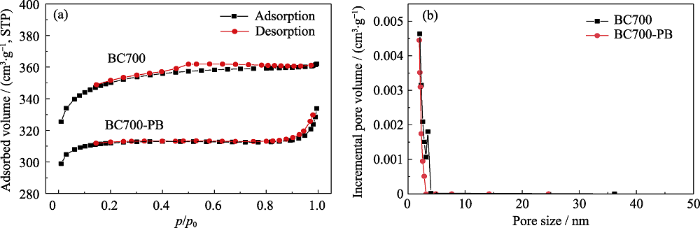
为进一步考察PB的负载对生物炭孔结构的影响, 分别测试BC700和BC700-PB的N2吸附-脱附曲线, 结果如图2所示。从图2(a)看出, 生物炭材料的氮气吸附能力有所下降。通过计算可得, 负载PB后生物炭的比表面积从461.80 m2/g下降至310.86 m2/g,孔容积从0.25 cm3/g下降至0.18 cm3/g。BC700-PB中PB的负载率可以通过O2氛围下的TGA曲线计算获得[4], 改变K4Fe(CN)6与BC700的投料量, 可调控生成的PB在BC700表面的负载量。但较高的负载量会加重生物炭孔道的堵塞, 引起生物炭比表面积和孔容积的下降。为了得到较佳的比表面积与孔容积, 本研究将PB的负载量控制在10.6%。图2(b)的孔径分布图则更加清晰地展现了负载PB降低了BC700的孔体积, 这是由于100 nm左右的PB粒子被生物炭复杂的表面形貌及孔道所固定, 不可避免地造成了孔道堵塞。
图2
图2
BC700和BC700-PB的氮气吸脱附曲线(a)和孔径分布图(b)
Fig. 2
N2 adsorption-desorption curves (a) and pore size distributions (b) of BC700 and BC700-PB
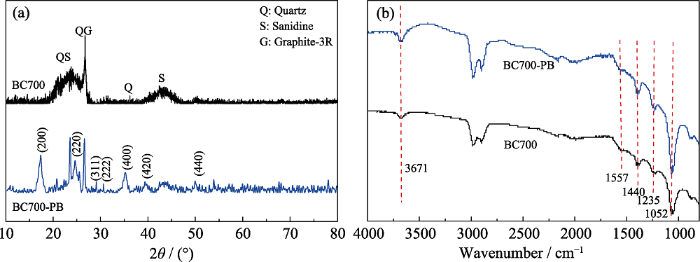
BC700和BC700-PB的XRD图谱如图3(a)所示, 从图中可以看出BC700主要由3R型的石墨(G)、石英(Q)和透长石(S)组成, 分别代表生物炭、SiO2和硅铝酸盐等灰分在高温条件下形成产物的主要晶型。而在原位负载PB后, BC700-PB图谱中出现了(200)、(220)、(311)以及(222)等PB的特征晶面衍射峰[12], 说明PB晶体成功负载在生物炭的表面。而PB良好的结晶性同样有利于其对氨氮的配位作用, 有望提高其对氨氮的吸附选择性。为探究PB与生物炭的相互作用, 采用FT-IR和XPS表征BC700及BC700-PB。图3(b)的红外光谱图显示出生物炭材料在1052、1235、1440、1557和3671 cm-1处的红外伸缩振动峰, 代表生物炭中存在大量的羰基、羟基等含氧官能团[20]。而负载PB后, 这些红外伸缩振动峰并没有明显改变, 说明PB和生物炭之间并没有形成新的化学键, 也未改变生物炭的原有化学性质。
图3
图3
BC700-PB和BC700的XRD图谱(a)和红外光谱图(b)
Fig. 3
XRD patterns (a) and FT-IR spectra (b) of BC700-PB and BC700
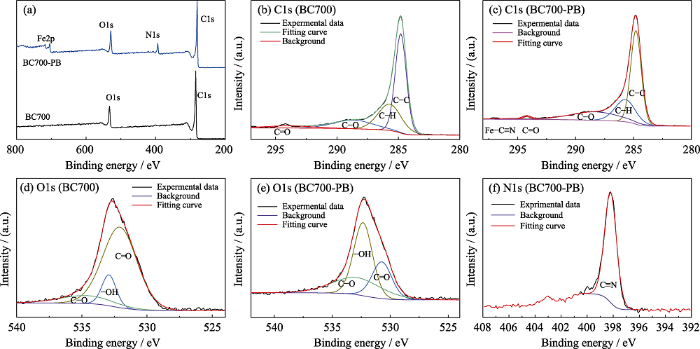
通过对比BC700和BC700-PB的XPS全谱(图4(a))可以发现, BC700的XPS谱图中主要有C1s和O1s的结合能分峰; BC700-PB的C1s分峰拟合显示负载PB后, 出现了Fe-C≡N的结合能峰。此外对N1s的拟合结果表明N仅以C≡N的形式存在, 并未生成新的碳氮键或氮氧键。对氧元素的分峰拟合结果显示, 负载PB引起结合水含量增大, 导致-OH的占比增加, 但是并未生成新的铁氧键, 说明PB与BC700的复合并没有生成新的共价键。
图4
图4
BC700和BC700-PB的XPS全谱(a), BC700(b, d)和BC700-PB (c, e)的C1s(b, c)与O1(d, e)与BC700-PB的N1s(f)XPS谱图
Fig. 4
Full XPS spectra (a) of BC700 and BC700-PB, core-level XPS spectra of the elemental C1s (b, c) and O1s (d, e) of BC700 (b, d) and BC700-PB (c, e), and N1s (f) of BC700-PB
Colorful figures are available on website
2.2 BC700-PB的氨氮吸附性能及理论研究
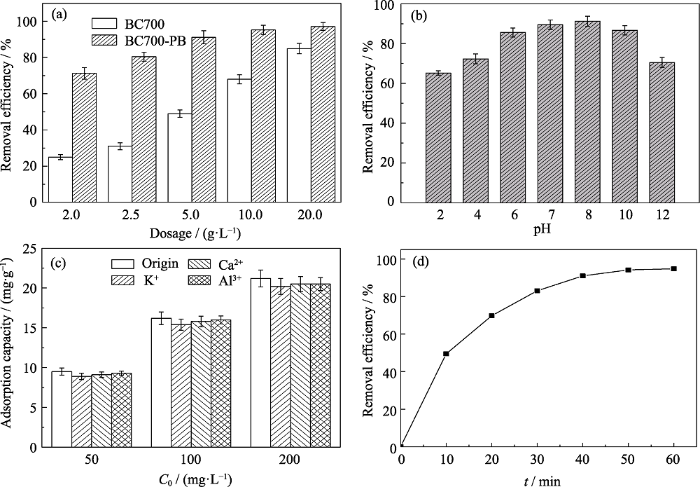
将BC700和BC700-PB置于模拟氨氮废水中, 考察生物炭复合材料的投料量、pH、不同氨氮浓度及反应时间对氨氮的吸附效率, 结果如图5所示。从图5(a)中可以看出, 在氨氮起始浓度较高的条件下(50 mg/L), 当添加2 g/L的BC700-PB时, 去除效果就达到70%, 这意味着溶液中的氨氮浓度已经低于15 mg/L, 达到了污水排放标准的要求; 当BC700-PB投料量增加至5 g/L时, 对氨氮的去除效率提高至80%以上, 且随着BC700-PB投料量进一步增加, 去除效率也逐渐提高, 最高可达95%以上。而同等条件下, 2 g/L的BC700对氨氮的吸附效率只有25%, 并且仅在投料量增大至20 g/L时, 氨氮去除效率才能达到70%以上, 说明负载PB大幅提升了单位时间(60 min)内生物炭对氨氮的吸附速度, 降低了同等去除效果下的吸附剂使用量。
图5
图5
BC700和BC700-PB添加量对吸附氨氮效果的影响(a)(pH 8, C0=50 mg/L, t=60 min), 和体系pH(b)(dosage=5 g/L, C0=50 mg/L, t=60 min)、在不同共存离子(50 mg/L)中污染物初始浓度(c)(dosage=5 g/L, pH 8, t=60 min)以及反应时间(d)(dosage=5 g/L, pH 8, C0=50 mg/L)对BC700-PB吸附氨氮效果的影响
Fig. 5
Effect of dosage on the removal of NH3-N by of BC700 and BC700-PB (pH 8, C0=50 mg/L, t=60 min) (a), effects of pH (dosage=5 g/L, C0=50 mg/L, t=60 min) (b), initial concentration and coexisting ions (C0=50 mg/L, dosage=5 g/L, pH 8, t=60 min) (c), and reaction time(dosage=5 g/L, pH 8, C0=50 mg/L) (d) on the removal of NH3-N by BC700-PB
分别选择K+、Ca2+和Al3+作为不同带电量及离子半径的共存阳离子, 图5(c)显示了BC700-PB在多组分污水中对氨氮的选择性吸附效果。BC700-PB的氨氮吸附容量随着氨氮初始浓度的增加而增大, 说明BC700-PB对氨氮具有较高的去除效率。在其它离子共存下, BC700-PB对氨氮的吸附容量也随氨氮浓度增加而增大, 但其吸附容量都仅比无共存离子时下降了约1 mg/g。Ca2+和Al3+的带电量与粒子半径都比NH+4的大, 因而它们对氨氮的吸附干扰较小。有意思的是, K+的带电量与粒子半径(半径133 pm)均接近于NH+4 (半径143 pm)离子, 但其对氨氮吸附容量的影响却也较小, 传统理论的静电相互作用、离子交换作用和范德华力均无法解释BC700-PB对氨氮的选择性吸附。因此, PB对氨氮的配位作用主导了选择性吸附过程,这是唯一合理的解释[4]。
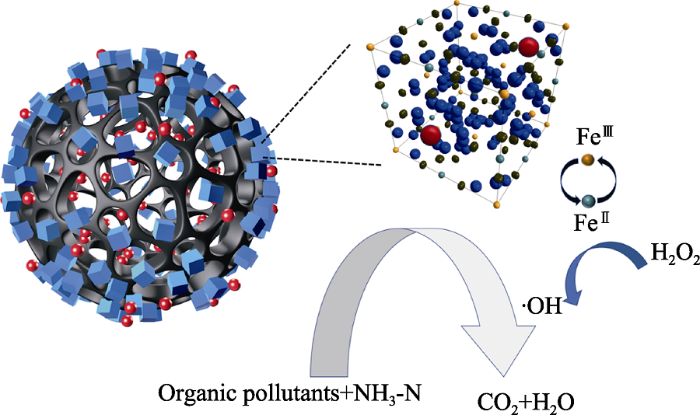
实验进一步考察了BC700-PB对氨氮的吸附效果与时间的关系。如图5(d)所示, 在吸附初期(10 min内), 吸附效率快速增加; 随吸附时间不断延长, 吸附效率逐渐增加, 但增大速率相对初期有所减缓; 吸附至40 min后趋于平缓, 吸附60 min几近平衡且效率在95%以上。据此, 我们推测BC700-PB对氨氮可能的吸附机理(如图6所示)为: 阳离子首先富集在复合材料与溶液接触的界面处, 部分离子通过生物炭的多孔结构进入其表面及内部孔道; 此时, 负载在生物炭表面的PB纳米粒子通过相互作用捕获大量进入复合材料的氨氮, 从而表现出吸附初期快速吸附的效果; PB捕获作用使氨氮在材料表面形成了一定的浓度差, 驱动生物炭进一步吸附更多的氨氮直至PB配位容量的上限和复合材料的吸附容量上限, 表现为其吸附效率缓慢增加并趋于平衡。因为PB对氨氮以外的阳离子没有其他相互作用[12⇓-14], 因此即使存在共存离子, BC700-PB与氨氮间的相互作用力也始终强于其与其它阳离子的作用力, 导致氨氮的吸附速度快于其它阳离子, 并能迅速到达其吸附容量, 最终表现为BC700-PB对氨氮的选择性吸附。
图6
图6
BC700-PB对氨氮的吸附和催化示意图
Fig. 6
Schematic diagram of adsorption and catalysis removal of ammonia nitrogen by BC700-PB
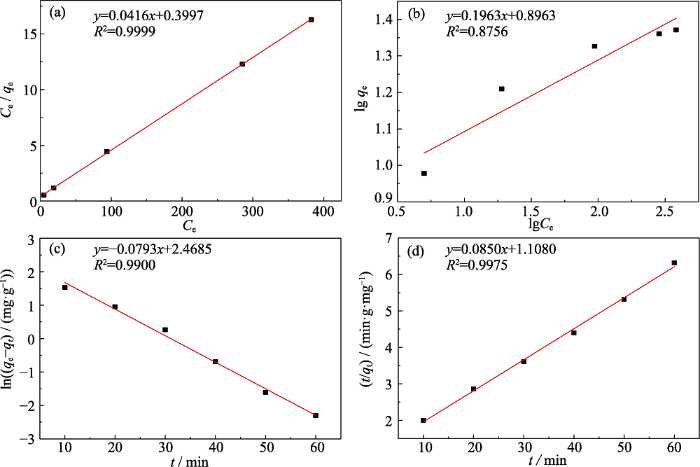
为验证对BC700-PB氨氮吸附机理猜想的合理性, 分别使用Langmuir模型和Freundlich模型拟合吸附数据来研究其氨氮的吸附热力学。从图7(a, b)中可以看出吸附值与Langmuir模型的相关度(R2)更高, 说明氨氮在BC700-PB上的吸附更符合Langmuir模型, 即氨氮在复合材料表面以单分子层的方式均匀吸附, 这不仅证明了PB在BC700表面的均匀负载, 而且证明了氨氮并不是通过吸附的方式与PB相结合, 而可能是通过配位等相互作用进入PB晶胞内部。此外, 通过Langmuir模型计算得到BC700-PB复合材料对氨氮的最大吸附容量为24.4 mg/g, 同样通过类似的方法计算可得BC700的最大吸附容量仅为12.12 mg/g, 增幅为101.3%。
图7
图7
BC700-PB对氨氮的吸附热力学的Langmuir模型(a)和Freundlich模型(b)拟合; BC700-PB对氨氮的吸附动力学的拟一级动力学模型(c)和拟二级动力学模型(d)拟合
Fig. 7
Langmuir (a) and Freundlich models (b) of NH3-N on BC700-PB, and pseudo-first-order kinetic (c) and pseudo-second-order kinetic (d) models of NH3-N on BC700-PB
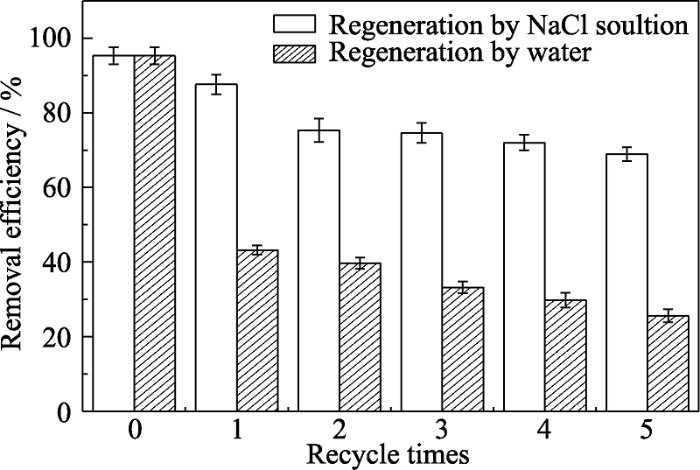
研究表明, 吸附了氨氮的生物炭材料可通过碱性的NaCl溶液清洗再生[7], 而吸附了氨氮的PB仅通过水洗的方法就可以再生[23]。本研究采用两种方法清洗吸附氨氮后的BC700-PB, 再生后用于吸附氨氮, 多次循环后的结果如图8所示。由图可知, 通过碱性NaCl/NaOH溶液再生的BC700-PB的吸附效率在5次循环后仍能达到72%; 而水洗再生的BC700-PB的吸附效率在1次循环后降至45%, 在5次循环后降至28%, 说明碱性NaCl再生的容量恢复更大。这是由于水洗的方法虽然可使PB捕获氨氮分子的能力再生, 但只能使很少一部分生物炭的吸附能力得以再生, 而BC700-PB中PB的含量仅为10.6%, 因此BC700-PB的再生吸附效率较低。
图8
图8
BC700-PB的循环吸附性能(pH 8, dosage=5 g/L, C0= 50 mg/L)
Fig. 8
Recycling removal property of BC700-PB (pH 8, dosage=5 g/L, C0=50 mg/L)
2.3 BC700-PB的芬顿反应性能
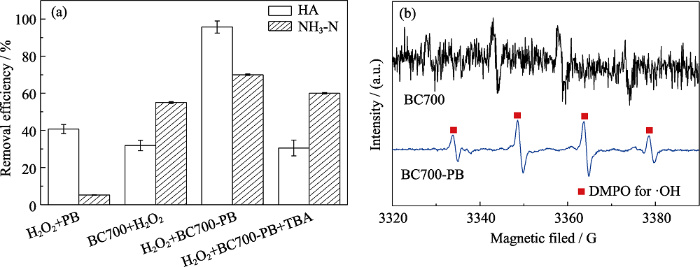
BC700-PB在外加H2O2后, HA的去除效果接近99%, 氨氮的吸附效果也较高, 说明添加H2O2改变了原有仅靠吸附的去除机理, 推测其催化机理如下:
图9
图9
不同吸附剂、添加剂对氨氮和HA双组分污水的吸附效果的影响(pH 2, dosage=5 g/L, C0=50 mg/L) (a)和BC700-PB体系中添加了DMPO的EPR谱图(b)
Fig. 9
Effects of adsorbents and additive on the removal efficiency for mixed solution of NH3-N and HA(pH 2, dosage=5 g/L, C0=50 mg/L) (a), and EPR spectra of DMPO-OH (b) adducts in the systems of BC700-PB
3 结论
本研究通过原位负载的方法将普鲁士蓝纳米粒子与生物炭相结合, 成功制得生物炭/普鲁士蓝复合材料, 通过普鲁士蓝对氨氮的配位作用在多种阳离子共存的污水中对氨氮实现了选择性吸附。主要结论如下:
1)复合材料吸附最佳pH为8, 吸附平衡时间为60 min。对吸附机理研究表明生物炭/普鲁士蓝复合材料的吸附包括了物理吸附和配位吸附, 且对氨氮的吸附符合Langmuir模型及拟二级动力学方程, 理论最大吸附容量为24.4 mg/g, 实现以10.6%的普鲁士蓝负载量提升了101.3%的吸附容量。
2)复合材料可以在外加酸性H2O2溶液的条件下, 催化生成•OH, 从而形成芬顿氧化体系, 实现同步选择性吸附氨氮污染物与催化降解多组分污水中的有机污染物。
因而, 本研究所制备的普鲁士蓝改性生物炭对处理含氨氮化合物的污水有较好的潜在应用价值。
参考文献
Assessment and management of lake eutrophication: a case study in Lake Erhai, China
Spatiotemporal nutrient patterns, composition, and implications for eutrophication mitigation in the Pearl River Estuary, China
Adsorptive removal of phosphate from water using mesoporous materials: a review
Mesoporous materials have significant potential for use as adsorbents for removal of phosphate from water. The chemical and structural properties of materials greatly affect their capacity and rate in the phosphate adsorption process. This paper reviews recent activities in the development of mesoporous materials as phosphate adsorbents. In particular, it mainly focuses on the synthesis, properties and phosphate removal efficiency of various materials with mesoporosity, including metal-coordinated amino-functionalized silicas, ammonium-functionalized silicas, metal-doped mesoporous silicas, metal oxides, metal sulfate and carbon.Copyright © 2017 Elsevier Ltd. All rights reserved.
Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water
Application of biochar-based photocatalysts for adsorption-(photo)degradation/reduction of environmental contaminants: mechanism, challenges and perspective
Biochar for the removal of contaminants from soil and water: a review
Review of organic and inorganic pollutants removal by biochar and biochar-based composites
Phosphate and ammonium adsorption of the modified biochar based on Phragmites australis after phytoremediation
A SAXS study of the pore structure evolution in biochar during gasification in H2O, CO2 and H2O/CO2
Co-modification of biochar and bentonite for adsorption and stabilization of Pb2+ ions
Heavy metals can seriously endanger human health and the ecological environment due to their toxicity, persistence, and bioaccumulative nature. In this study, an alkali-modified biochar-bentonite composite (CaO-Bent-CB) was prepared by alkali modification of corncob residues and bentonite mixture by calcium chloride and calcination at high temperature under anaerobic conditions. The CaO-Bent-CB composite has a specific surface area of 441.1 m2/g, which is significantly higher than CB (132.7 m2/g) and CaO-CB (177.2 m2/g). Meanwhile, the adsorption perfermance of CaO-Bent-CB to lead ions in water was evaluated. The results showed that the removal efficiency reached 98% after 6 h-adsorption, and the adsorption capacity was 109.6 mg/g when the concentration of lead ion was 120 mg/L, the weight ratio of bentonite to corncob residues was 1:5, and the dosage was 1 g/L. The adsorption capacity of CaO-Bent-CB was higher than that of CB (13.4 mg/g), bentonite (72.9 mg/g) and CaO-CB (86.9 mg/g). Moreover, the lead ion contaminated soil was stabilized by CaO-Bent-CB. When the concentration of lead ion in soil is 2200 mg/kg, and the loading of CaO-Bent-CB is 8% of soil, the concentration of lead ions in acid leachate (H2SO4-HNO3, pH=3.2, 12 h-soaking) is 4.5 mg/L, which is lower than the standard value of hazardous waste identification (5 mg/L). The results show that CaO-Bent-CB has a great potential in the water treatment and heavy metals removal in soil.
Prussian blue, an inorganic evergreen
Prussian blue/PVDF catalytic membrane with exceptional and stable Fenton oxidation performance for organic pollutants removal
Historical pigment exhibiting ammonia gas capture beyond standard adsorbents with adsorption sites of two kinds
Prussian blue is a historical pigment synthesized for the first time at the beginning of 18th century. Here we demonstrate that the historical pigment exhibits surprising adsorption properties of gaseous ammonia. Prussian blue shows 12.5 mmol/g of ammonia capacity at 0.1 MPa, whereas standard ammonia adsorbents show only 5.08-11.3 mmol/g. Dense adsorption was also observed for trace contamination in atmosphere. Results also show higher adsorption by Prussian blue analogues with the optimization of chemical composition. The respective capacities of cobalt hexacyanocobaltate (CoHCC) and copper hexacyanoferrate (CuHCF) were raised to 21.9 and 20.2 mmol/g, the highest value among the recyclable adsorbents. Also, CoHCC showed repeated adsorption in vacuum. CuHCF showed regeneration by acid washing. The chemical state of the adsorbed ammonia depends on the presence of the water in atmosphere: NH3, which was stored as in the dehydrated case, was converted into NH4(+) in the hydrated case.
Trace ammonia removal from air by selective adsorbents reusable with water
Ultrafast sequestration of cadmium and lead from water by manganese oxide supported on a macro-mesoporous biochar
Biochar-surface oxygenation with hydrogen peroxide
Biochar was produced from pinewood biomass by pyrolysis at a highest treatment temperature (HTT) of 400 °C. This biochar was then treated with varying concentrations of H2O2 solution (1, 3, 10, 20, 30% w/w) for a partial oxygenation study. The biochar samples, both treated and untreated, were then tested with a cation exchange capacity (CEC) assay, Fourier Transformed Infrared Resonance (FT-IR), elemental analysis, field water-retention capacity assay, pH assay, and analyzed for their capacity to remove methylene blue from solution. The results demonstrated that higher H2O2 concentration treatments led to higher CEC due to the addition of acidic oxygen functional groups on the surface of the biochar, which also corresponds to the resultant lowering of the pH of the biochar with respect to the H2O2 treatment. Furthermore, it was shown that the biochar methylene blue adsorption decreased with higher H2O2 concentration treatments. This is believed to be due to the addition of oxygen groups onto the aromatic ring structure of the biochar which in turn weakens the overall dispersive forces of π-π interactions that are mainly responsible for the adsorption of the dye onto the surface of the biochar. Elemental analysis revealed that there was no general augmentation of the elemental composition of the biochar samples through the treatment with H2O2, which suggests that the bulk property of biochar remains unchanged through the treatment. Copyright © 2015 Elsevier Ltd. All rights reserved.
Synthesis and characterization of Prussian blue modified magnetite nanoparticles and its application to the electrocatalytic reduction of H2O2
In-situ formed Prussian blue nanoparticles supported by porous biochar as highly efficient removal of cesium ions
Biochar catalyzed dichlorination-which biochar properties matter
Environmental-friendly montmorillonite-biochar composites: facile production and tunable adsorption-release of ammonium and phosphate
Biochar modification to enhance sorption of inorganics from water
Biochar can be used as a sorbent to remove inorganic pollutants from water but the efficiency of sorption can be improved by activation or modification. This review evaluates various methods to increase the sorption efficiency of biochar including activation with steam, acids and bases and the production of biochar-based composites with metal oxides, carbonaceous materials, clays, organic compounds, and biofilms. We describe the approaches, and explain how each modification alters the sorption capacity. Physical and chemical activation enhances the surface area or functionality of biochar, whereas modification to produce biochar-based composites uses the biochar as a scaffold to embed new materials to create surfaces with novel surface properties upon which inorganic pollutants can sorb. Many of these approaches enhance the retention of a wide range of inorganic pollutants in waters, but here we provide a comparative assessment for Cd, Cu, Hg, Pb, Zn, NH, NO, PO, CrO and AsO.Copyright © 2017 Elsevier Ltd. All rights reserved.
Pyrolysis of wetland biomass waste: potential for carbon sequestration and water remediation
Management of biomass waste is crucial to the efficiency and sustainable operation of constructed wetlands. In this study, biochars were prepared using the biomass of 22 plant species from constructed wetlands and characterized by BET-N2 surface area analysis, FTIR, TGA, SEM, EDS, and elemental compositions analysis. Biochar yields ranged from 32.78 to 49.02%, with mesopores dominating the pore structure of most biochars. The biochars had a R50 recalcitrance index of class C and the carbon sequestration potential of 19.4-28%. The aquatic plant biomass from all the Chinese constructed wetlands if made into biochars has the potential to sequester 11.48 Mt carbon yr(-1) in soils over long time periods, which could offset 0.4% of annual CO2 emissions from fossil fuel combustion in China. In terms of adsorption capacity for selected pollutants, biochar derived from Canna indica plant had the greatest adsorption capacity for Cd(2+) (98.55 mg g(-1)) and NH4(+) (7.71 mg g(-1)). Whereas for PO4(3-), Hydrocotyle verticillata derived biochar showed the greatest adsorption capacities (2.91 mg g(-1)). The results from this present study demonstrated that wetland plants are valuable feedstocks for producing biochars with potential application for carbon sequestration and contaminant removal in water remediation.Copyright © 2016 Elsevier Ltd. All rights reserved.
Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals
A series of rice straw hydrochars were produced through a microwave-assisted hydrothermal treatment method, characterized and used for the adsorption of three organics and two heavy metals from aqueous solutions. The hydrochars have carbon contents from 37.44% to 43.31%, are rich in oxygen containing functional groups, and the equilibrium of hydrothermal carbonization reactions could be reached rapidly in microwave environment. The hydrochars can effectively adsorb the model pollutants, the maximum adsorption capacities of Congo red, berberine hydrochloride and 2-naphthol at 298 K and initial concentration of 0.5 mg/mL were 222.1, 174.0 and 48.7 mg/g, respectively, and those of Zn and Cu were 112.8 and 144.9 mg/g, respectively. Adsorption thermodynamic parameters were calculated. These results suggest that microwave-assisted hydrothermal treatment is an effective method for the rapid production of hydrochars, and rice straw hydrochars are promising adsorbents for the removal of water pollutants such as organics and heavy metals.Copyright © 2018. Published by Elsevier Ltd.
Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: synergism of bio-char and Fe-Mn binary oxides
This study investigated the hydrogen peroxide (HO) activation potential of Fe-Mn binary oxides modified bio-char (FeMn/bio-char) for the degradation of naphthalene, the dominant PAHs in drinking water. Results showed that FeMn/bio-char exhibited 80.7- and 2.18-times decomposition rates towards HO than that of pure bio-char and Fe-Mn binary oxides, respectively, and consequently the FeMn/bio-char/HO photo-Fenton system presented highest naphthalene removal efficiency. The enhanced catalytic activity could be ascribed to the synergistic effect of the combination of bio-char and Fe-Mn binary oxides, such as promoting the adsorption capacity towards contaminant, increasing concentration of persistent free radicals (PFRs) and introducing Fe-Mn binary oxides as new activator. According to the batch-scale experiments, FeMn/bio-char/HO photo-Fenton system could degrade naphthalene effectively at a wide pH ranges, and 82.2% of naphthalene was degraded under natural pH of 5.6 within 148 min. Free radicals quenching studies and electron spin resonance (ESR) analyses verified that the dominant free radical within FeMn/bio-char/HO photo-Fenton system was hydroxyl radical (•OH). According to the preliminary analysis, the generation of •OH were ascribed to the activation of HO by Fe (II), Mn (II) and PFRs on the catalyst surface. The mainly degradation intermediates of naphthalene were identified by GC-MS analysis. Consequently, the possible degradation pathways were proposed. Moreover, naphthalene degradation experiments were also conducted in river, tap water, industrial wastewater as well as medical wastewater, and the results indicated that the FeMn/bio-char/HO photo-Fenton system was effective in the treatment of naphthalene in natural waters. This study brings a valuable insight for the potential environmental applications of modified bio-char.Copyright © 2019 Elsevier Ltd. All rights reserved.