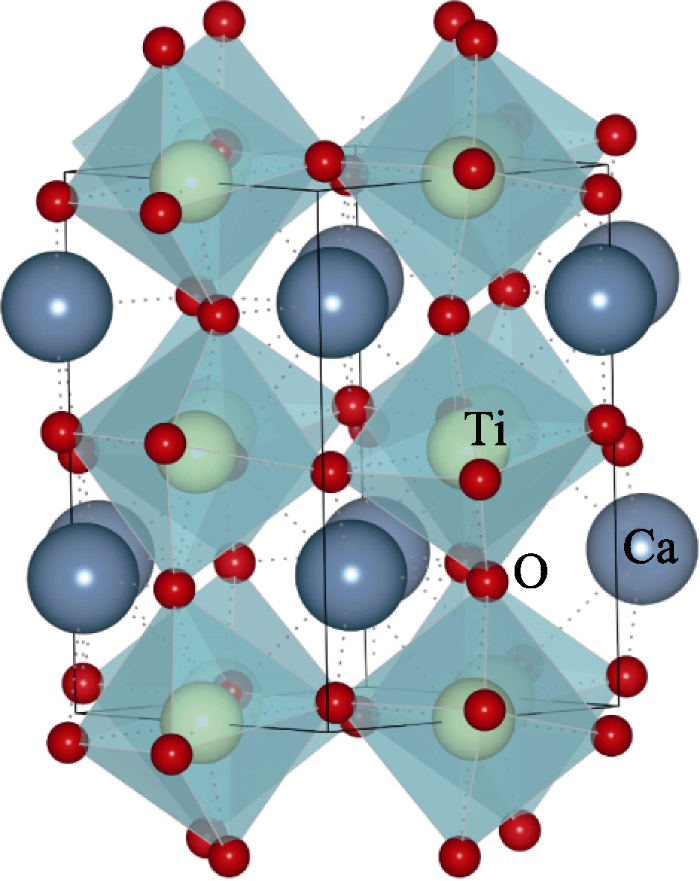
1839年, 德国矿物学家古斯塔夫·罗斯(Gustav Rose)首次发现了钛酸钙。CaTiO3是典型的ABO3型钙钛矿结构, 在1380 K以下为正交结构, 空间群为Pnma; 在1380~1500 K转变为另一种正交结构, 空间群为Cmcm; 1500 K时转变为四方结构, 空间群为I4/mcm; 1580 K以上转变成立方结构, 空间群为Pm$\bar{3}$m[1]。图1为CaTiO3在室温下的结构示意图。离子半径较大的Ca2+与8个O2-配位形成Ca-O十二面体, 而半径较小的Ti4+与O2-形成Ti-O八面体。由于CaTiO3受到八面体和十二面体配位的几何约束, 其结构比较稳定[2]。同价或异价的离子置换Ca2+或Ti4+时, 会使CaTiO3产生新的氧化还原性质与表面性质, 从而改变CaTiO3的物理化学性质。因此, CaTiO3在微波通信、薄膜电容器、光催化、非易失性存储器、光电化学电池、硬盘读取磁头、自旋电子器件、激光以及固定放射性废料等方面有广泛应用[3⇓⇓⇓⇓⇓⇓⇓-11]。但是由于电导率较差、热导率较高, CaTiO3作为热电材料一直未被关注。
图1
图1
室温下CaTiO3的晶体结构示意图
Fig. 1
Schematic diagram of crystal structure for CaTiO3 at room temperature
热电性能通常由无纲量热电优值
1 实验方法
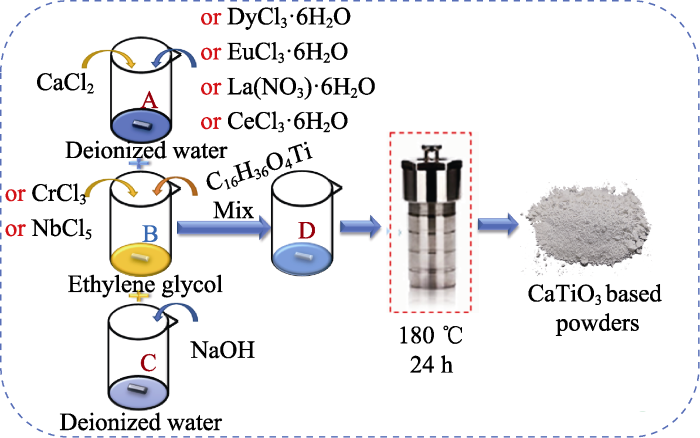
粉体制备流程如图2所示, 将CaCl2粉体溶于去离子水中形成溶液A, 钛酸四丁酯溶于乙二醇中形成溶液B, NaOH粉体溶于去离子水中形成溶液C。混合溶液A、B、C后得到前驱体溶液D, 倒入高压反应釜中, 在180 ℃干燥箱保温24 h。将得到的固液混合物经过滤、洗涤、干燥后得到CaTiO3粉体。其中, 按照化学计量比, 分别将CrCl3粉体或NbCl5粉体溶于溶液B中, EuCl3·6H2O粉体、DyCl3·6H2O粉体、CeCl3·6H2O粉体或La(NO3)·6H2O粉体溶于溶液A中, 分别获得CaTi0.8Cr0.2O3、CaTi0.8Nb0.2O3、Eu0.2Ca0.8TiO3、Dy0.2Ca0.8TiO3、Ce0.2Ca0.8TiO3、La0.2Ca0.8TiO3粉体, 分别标记为Cr20、Nb20、Eu20、Dy20、Ce20和La20。将上述粉体采用研钵研磨后放入石墨模具, 进行真空热压烧结, 在1500 ℃保温2 h, 得到块体材料。其中, 升温速率为20 ℃/min(<800 ℃), 10 ℃/min(800~1500 ℃)。
图2
图2
水热法合成CaTiO3的示意图
Fig. 2
Schematic diagram of synthesis of CaTiO3 by hydrothermal method
2 结果与讨论
2.1 材料的物相分析
图3
图3
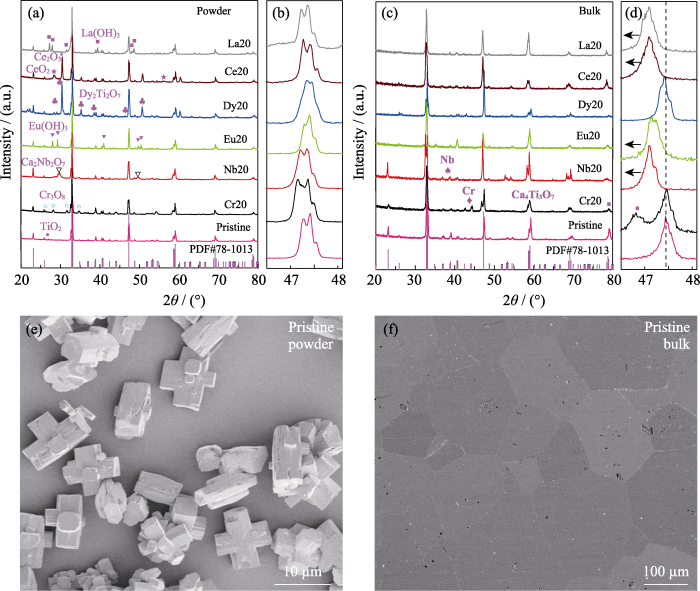
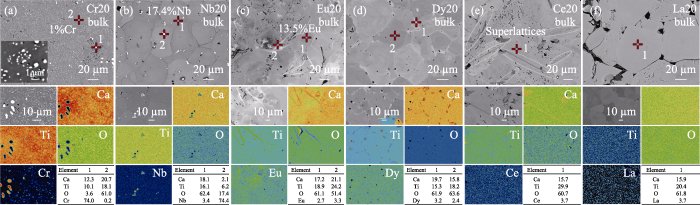
不同元素掺杂的CaTiO3(a, b)粉体与(c, d)块体的XRD图谱; (e)纯CaTiO3粉体的SEM照片与(f)块体的EPMA背散图片
Fig. 3
XRD patterns of CaTiO3 (a, b) powders and (c, d) bulks doped with different elements; (e) SEM image of powder and (f) BES image of bulk for the pristine CaTiO3 sample
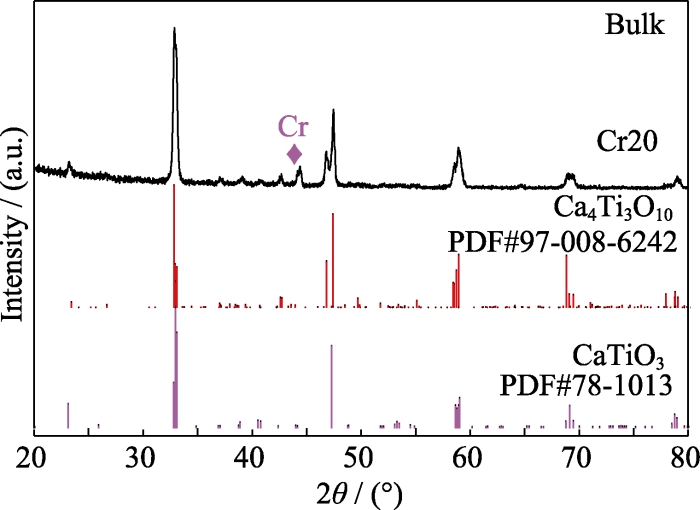
从图3(b)的局部放大图可以看出, Cr和Nb掺杂的CaTiO3粉体的XRD衍射峰与纯CaTiO3的峰位相比, 峰位向左发生偏移, 说明大离子半径的Cr和Nb进入到了CaTiO3晶格中(表1); 而Eu、Dy、Ce和La掺杂的CaTiO3粉体的XRD衍射峰并未发生偏移, 说明掺杂元素并未进入到CaTiO3晶格内, 而是以氧化物或氢氧化物等杂质存在。粉体经过高温烧结后, Eu、Dy、Ce、La等元素的氧化物或氢氧化物杂相基本消失, 而Cr和Nb掺杂的块体样品分别析出了Cr相和Nb相, 如图3(c)所示。图3(d)中, 与纯CaTiO3的峰位相比, Nb、Eu、Ce和La掺杂的CaTiO3块体样品的XRD衍射峰都向左发生了偏移, 表明掺杂元素成功替代了CaTiO3的晶格原子, 导致晶格膨胀。对比图3(b, d)可以发现, Cr20粉体的XRD峰位发生了偏移, 而块体的XRD峰位却未发生偏移, 表明烧结后的基体晶格中不含Cr或者仅含少量的Cr元素。基体中Cr析出后, ABO3中的B位元素含量变少, 即B位的Ti与Cr含量变少, 导致块体的XRD衍射峰出现Ca4Ti3O10杂相, 如图S1所示。Dy20块体的XRD结果并没有出现杂相的衍射峰, 衍射峰位也未发生偏移, 表明Dy元素掺杂并未造成明显的晶格膨胀或收缩。
表1 不同原子的原子半径与离子半径
Table 1
| Atom | Atomic radius/pm | Ionic radius/pm |
|---|---|---|
| Ca | 174 | 99 (M2+) |
| Ti | 132 | 68 (M4+) |
| Cr | 118 | 84 (M3+) |
| Nb | 134 | 70 (M5+) |
| Dy | 177.3 | 90.8 (M3+) |
| Ce | 182.4 | 103.4 (M3+) |
| La | 187.7 | 106 (M3+) |
如图3(e)所示, 本工作中所制备的纯CaTiO3粉体晶粒呈三维交叉立方体状, 大小约10 μm。在水热合成粉体过程中, NaOH作为碱性物质提供OH-, 并与Ca2+或Ti4+结合, 形成氢氧化物。同时, 在高温高压环境下, 形成的氢氧化物在水中的溶解度增大, 溶液中溶质的扩散速度加快, 导致热力学上发生结合反应, 形成三维交叉立方体状晶粒。通过调节溶液的pH也可获得不同尺寸和形貌的粉体颗粒。例如, 矩形棱镜、六边形板、薄片状、棒状、立方体和空心立方体等不同形貌的CaTiO3晶粒[30]。这是因为溶液的pH可以影响溶质的溶解度和晶体在不同晶向的生长速度, 并且可以改变溶液中生长基元的结构, 从而形成不同形貌和大小的晶粒。如图3(f)所示, 与粉体的晶粒尺寸相比, 块体CaTiO3晶粒长大至50~200 μm, 这主要是由在热压过程中晶粒的重新聚集和长大而造成[31]。
2.2 材料的微观结构及成分分析
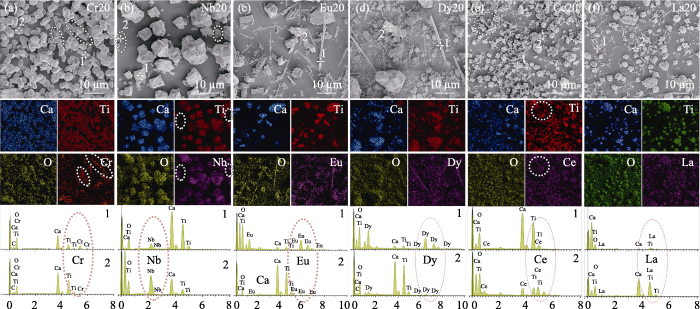
由各个样品的SEM照片(图4)可以看出, 掺杂的元素不同, 粉体颗粒的形貌不同。Cr20粉体中的Cr3O8杂质呈长条状; Nb20粉体中的Ca2Nb2O7杂质呈立方体状; Eu20粉体中的Eu(OH)3杂质呈针状; Dy20粉体中的Dy2Ti2O7杂质呈针状; Ce20粉体中的CeO2、CeO杂质呈条状或片状; La20粉体中的La(OH)3杂质呈长条状。这是因为在水热过程中, Cr、Nb、Eu、Dy、Ce与La元素具有不同的化学性质, 可能同时与Ca2+和Ti4+产生相互作用析出, 也可能只与Ca2+或Ti4+产生作用析出, 甚至直接生成氢氧化物或氧化物, 形成杂质相。
图4
图4
(a)Cr20、(b)Nb20、(c)Eu20、(d)Dy20、(e)Ce20与(f)La20粉体的SEM照片以及对应的元素分布图与EDS能谱图
Fig. 4
SEM images, element mappings, and corresponding EDS spectra of (a) Cr20, (b) Nb20, (c) Eu20, (d) Dy20, (e) Ce20, and (f) La20 powders
将粉体在1500 ℃高温烧结, 由于Cr3O8、Eu2O3(由Eu(OH)3受热分解生成)、La2O3(由La(OH)3受热分解生成)、Dy2Ti2O7、Ca2Nb2O7等杂质的高温稳定性不如CaTiO3, 因此更趋向于生成CaTiO3基块体材料[22]。根据固相反应法合成原理, 反应物分子在高温下的热运动加快, 更容易与周围的分子相互作用, 从而促进化学反应发生, 并使反应物逐渐形成热稳定性更高的CaTiO3化合物。图5为高温烧结后六种元素掺杂的CaTiO3块体的电子探针显微分析(EPMA)图。图5(a)表明, Cr20样品中析出的Cr相中含有少量的Ca、Ti和O元素, 这可能是由析出颗粒过小等因素造成。基体中Cr的原子分数仅有1%, 与理论20%相差甚远, 这与其XRD峰位未发生偏移的结果一致(图3(d))。图5(b)表明, 在Nb20块体中析出了微米级的Nb相, 而且基体中的Nb含量高达17.4%, 略低于理论掺杂浓度20%。图5(c)为Eu20块体的EPMA背散射分析图。其中Eu元素在基体中分布不均匀, 其含量约为13.5%。在Eu20基体中观察到明亮的针状条纹, 成分分析结果表明, 条纹中的氧元素含量较低, 这预示条纹中的氧空位浓度可能较高, 使Eu20样品整体表现出导电性而非绝缘。图5(d)为Dy20块体的EPMA分析图。Dy元素在基体中分布比较均匀。在样品晶界处出现了明亮的区域, 成分分析结果表明, 明亮区域中Ca含量明显高于Ti含量, 说明Dy元素可能取代了Ti的位置而生成第二相, 也可能生成了富Ca的第二相。在基体中Ca含量比Ti含量少, 说明Dy元素主要取代了Ca的位置, 这种第二相富集在晶界处的结构可能有助于降低晶格热导率。
图5
图5
(a)Cr20、(b)Nb20、(c)Eu20、(d)Dy20、(e)Ce20与(f)La20块体的EPMA分析图以及对应元素分布图与点分析结果
Fig. 5
EPMA images, element mappings, and corresponding chemical compositions of (a) Cr20, (b) Nb20, (c) Eu20, (d) Dy20, (e) Ce20, and (f) La20 bulks
Unit in tables: % (in atom)
2.3 材料的热电性能分析
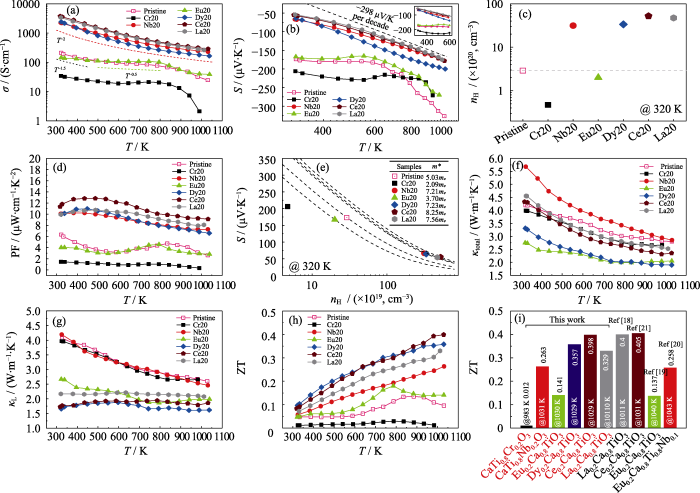
图6
图6
Cr20、Nb20、Eu20、Dy20、Ce20、La20等块体的(a)电导率、(b)塞贝克系数(插图为在300~600 K范围内的放大图)、(d)功率因子、(f) 总热导率、(g) 晶格热导率、(h)热电优值随温度的变化曲线; Cr20、Nb20、Eu20、Dy20、Ce20、La20等块体在320 K的(c)载流子浓度、(e)Pisarenko曲线及(i)与文献[18⇓⇓-21]报道的ZT性能比较
Fig. 6
Temperature-dependence of (a) electrical conductivity, (b) Seebeck coefficient with inset showing enlarged plots in temperature range of 300-600 K, (d) power factor, (f) total thermal conductivity, (g) lattice thermal conductivity, and (h) ZT of Cr20, Nb20, Eu20, Dy20, Ce20, and La20 bulks, and their (c) carrier concentration at 320 K, (e) Pisarenko curves and (i) ZT compared to literature[18⇓⇓-21]
如图6(c)所示, 纯CaTiO3的载流子浓度为2.9×1020 cm-3, 主要由氧空位提供。由于CaTiO3的特性与掺杂元素无关, 载流子浓度并不随温度变化而变化, 并且迁移率较低, 通常在10 cm2·V-1·s-1以下。因此, 高的载流子浓度预示着较高的电导率。与纯CaTiO3(2.9×1020 cm-3)相比, Cr20块体的载流子浓度降低至4.7×1019 cm-3, 这主要是由于Cr相析出降低了基体中的施主原子浓度与氧空位浓度, 从而导致载流子浓度降低[32]。因此, Cr20块体的电导率极低, 在320 K时仅为34 S·cm-1(图6(a)), 功率因子仅为1.45 μW·cm-1·K-2(图6(d))。Eu20块体的载流子浓度为2.0×1020 cm-3, 稍低于CaTiO3, 这主要是由于Eu掺杂后, 在价带顶引入了Eu4f轨道, 在一定程度减小了CaTiO3的带隙, 但Eu4f轨道局域化较高并不会参与能带之间的电荷转移。同时, Eu取代Ca已被证实为同价取代, 并未提供额外电 子[19],因此其载流子浓度主要由氧空位提供, 导致其电导率不高, 如图6(c)所示, 在320 K时仅为144 S·cm-1(图6(a)), 功率因子仅为4 μW·cm-1·K-2 (图6(d))。
图6(b)为不同元素掺杂样品的塞贝克系数随温度的变化曲线。其中, 插图为塞贝克系数在300~600 K范围内的放大图。Nb20、Dy20、Ce20与La20块体的塞贝克系数在低温下(<600 K)与温度成正比, 趋势可用简并费米气体模型来解释:
其中, kB为玻尔兹曼常数(1.38×10-23 J·K-1); e为电荷量; n为载流子浓度(cm-3)。当温度高于600 K时, Nb20、Dy20、Ce20与La20样品的塞贝克系数变化斜率接近每10倍温度-298 μV·K-1(-298 μV·K-1 per temperature decade), 这一趋势可用非简并半导体模型来解释:
其中, A为传输因子; h为普朗克常数(6.626×10-34 J·s)。假设运输因子A为常数, 根据公式(2, 3)可得:
因此, 塞贝克系数S以–3/2·ln10·kB/e
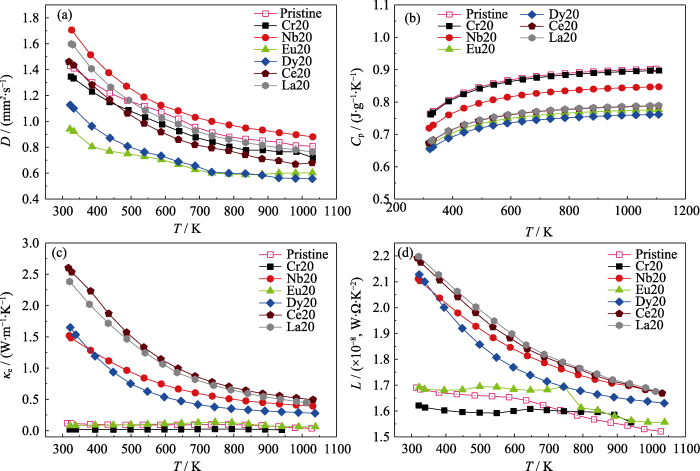
为进一步研究不同元素掺杂对塞贝克系数的影响规律, 利用单抛物线能带模型绘制了温度为320 K时样品的Pisarenko曲线, 如图6(e)所示。掺杂Nb、Dy、Ce与La元素可以有效提升CaTiO3的有效质量, 有助于增大塞贝克系数, 使Ce、La、Dy与Nb元素掺杂CaTiO3保持较高的功率因子, 如图6(d)所示。图6(f)为不同元素掺杂样品的热导率随温度的变化曲线。其中, κtotal=D·Cp·ρ, D为热扩散系数(图S2(a)), Cp为根据德拜模型计算的比热容(图S2(b)), ρ为样品的密度(表S2)。图6(g)为不同元素掺杂的样品的晶格热导率κL随温度的变化曲线。其中κL通过κtotal减去电子热导率κe得到(图S2(c)), 而κe = L·T, 其中L为洛伦兹常数, 通过
Cr20、Nb20样品分别析出了高热导率的Cr相(300 K 时93.90 W·m-1·K-1)与Nb相(300 K 时 53.70 W·m-1·K-1[35])。尽管析出Cr相的尺寸在500 nm以下, 对声子有一定的散射作用[36], 但最终Cr20样品的晶格热导率相比纯CaTiO3略微降低, 如图6(g)所示。对Nb20样品来讲, 尽管基体中原子分数17.4%的Nb造成点缺陷散射声子, 但仅仅抵消了微米级Nb带来的热导率升高, 最终导致晶格热导率在整个测试温度范围内与纯CaTiO3晶格热导率基本接近。减小微米级Nb相析出, 例如略微降低烧结温度(图S3和图S4), 功率因子会进一步提升, 进而改善热电性能。Eu20块体表现出较低的总热导率与
晶格热导率(图6(f, g)), 在320 K时总热导率由纯CaTiO3的4.20 W·m-1·K-1降低至2.74 W·m-1·K-1, 降低幅度高达35%左右, 在1031 K降低幅度仍然高达27%左右。这主要是由于Eu和Ca的质量差较大造成了质量场波动与应力场波动增强了声子散射。如能在基体中注入额外的施主原子, 将会提升材料的功率因子, 从而获得更为优异的热电性能。
在Dy20样品中, Dy原子取代造成了质量场波动与应力场波动, 并且在晶界处富集了第二相, 两者共同作用可有效降低晶格热导率。在320 K时总热导率由纯CaTiO3的4.20 W·m-1·K-1降低至 3.31 W·m-1·K-1(Dy20), 如图6(f)所示。对应的晶格热导率由4.06 W·m-1·K-1(纯CaTiO3)降低至 1.65 W·m-1·K-1(Dy20), 降低幅度高达52%。在1030 K时Dy20的晶格热导率1.61 W·m-1·K-1比Ce20的1.85 W·m-1·K-1降低了13%, 比La20的2.07 W·m-1·K-1降低了22%。
图6(h)为不同元素掺杂样品的ZT随温度的变化曲线。与纯CaTiO3相比, Nb20、Eu20、Dy20、Ce20与La20块体的ZT在整个温度区间内均有明显改善。在1030 K左右, ZT分别为0.263、0.141、0.357、0.398和0.329, 比纯CaTiO3的0.096分别提升了180%、56%、296%、342%、265%。而Cr20的ZT在整个温度测试区间内则远远低于纯CaTiO3。其中, Dy元素可同时取代A位与B位, 导致了超低的晶格热导率, 有望解耦电和热传输性能, 获得更高的ZT。图6(i)为本工作样品的ZT与文献的对比数据。Eu20样品在1031 K时的ZT超过文献, 表明强还原烧结可提高氧空位浓度从而提升ZT。同时, 本文中单掺Nb的性能也超越了文献中Eu和Nb双掺杂的性能, 表明Nb元素是一种潜在的CaTiO3有效掺杂元素。减少微米级Nb相的析出, 可以进一步降低热导率, 有望获得与La20样品相当的热电性能。
3 结论
本研究采用水热法结合热压烧结法分别制备Cr、Nb、Eu、Dy、Ce和La掺杂的CaTiO3热电块体材料。其中, Cr20样品中析出的纳米级Cr相(<500 nm)略微降低样品的热导率, 而微米级Nb相则使Nb20样品的总热导率大幅增加。Eu元素作为同价取代Ca位, 导致Eu20的功率因子较纯CaTiO3没有明显提升, 而热导率在320 K时为2.74 W·m-1·K-1, 相比纯样品降低了近35%, 在1031 K时的降幅仍高达27%。Dy、Ce与La元素在掺杂基体中均匀分布, 既能作为施主原子为基体提供载流子, 又能充当点缺陷有效散射声子而降低晶格热导率, 从而改善CaTiO3基块体的热电性能。其中, Dy20的晶格热导率在整个温度测试范围内最低, 在1030 K时为1.61 W·m-1·K-1, 相比纯样、La20、Ce20样品分别降低了52%、13%、22%。同时, Dy20还保持了较高的功率因子(6.59 μW·cm-1·K-2 at 1031 K), 并获得了0.35的高ZT。调控Dy含量与晶界处富集第二相的含量, 可以分别调控载流子浓度与晶格热导率, 解耦电和热传输性能, 有望刷新目前CaTiO3基热电材料的ZT纪录(0.405)。
补充材料
本文相关补充材料可登录
不同元素掺杂对CaTiO3微观结构及热电性能的影响
李建波1, 田 震1, 蒋全伟1, 于砺锋1,康慧君1,2, 曹志强1,2, 王同敏1,2
(1. 大连理工大学 材料科学与工程学院, 辽宁省凝固控制与数字化制备技术重点实验室, 大连 116024; 2. 大连理工大学宁波研究院, 宁波 315000)
S1 试剂
表S1 实验试剂一览表
Table S1
| Chemical composition | Purity | Production factories |
|---|---|---|
| CaCl2 | ≥ 99.99% | Aladdin |
| DyCl3·6H2O | ≥ 99.99% | Aladdin |
| EuCl3·6H2O | ≥ 99.99% | Aladdin |
| La(NO3)3·6H2O | ≥ 99.99% | Aladdin |
| CeCl3·7H2O | ≥ 99.99% | Aladdin |
| CrCl3 | ≥ 99.99% | Aladdin |
| NbCl5 | ≥ 99.9% | Aladdin |
| C16H36O4Ti | ≥ 99% | Aladdin |
| NbCl5 | ≥ 99.9% | Aladdin |
| NaOH | ≥ 99% | Aladdin |
| C2H6O2 | ≥ 95% | Aladdin |
S2 材料表征
采用X射线衍射仪(XRD, Empyrean, PANalytical, 荷兰)对粉体和块体材料进行物相分析, 选用Cu Kα射线, 波长为0.15406 nm, 扫描角度为20°~80°。利用场发射扫描电子显微镜(SEM, NOVA NanoSEM 450, 美国), 在二次电子模式下观察粉体的微观形貌和晶粒大小, 加速电压为15 kV。利用电子探针分析仪(EPMA, JXA-8530F Plus, JEOL, 日本)在背散射模式下观察样品的微观形貌, 使用配备的波谱仪(WDS)分析样品的元素分布。采用Seebeck系数/电阻率测量仪(LSR-3, Linseis, 德国)同时测量Seebeck系数和电阻率。采用霍尔测试系统(同济大学)测试样品的室温载流子浓度nH和迁移率μH; 采用激光热导仪(LFA457 MicroFlash, Netzsch, 德国)测量样品的热扩散系数D, 通过密度天平(ME204E)以阿基米德法测量样品的密度ρ, 采用德拜模型计算热容Cp, 运用公式κ = DCpρ 计算样品的热导率。
图S1
图S2
图S2
纯CaTiO3、Cr20、Nb20、Eu20、Dy20、Ce20与La20样品的(a)扩散系数、(b)比热容、(c) 电子热导率和(d) 洛伦兹常数随温度的变化曲线
Fig. S2
Temperature-dependent (a) thermal diffusion, (b) specific heat, (c) electrical thermal conductivity, and (d) Lorenz constant for pristine for Pristine CaTiO3, Cr20, Nb20, Eu20, Dy20, Ce20, and La20 samples
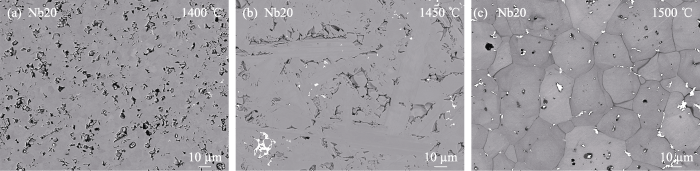
图S3
图S3
CaTi0.8Nb0.2O3(Nb20)经过(a)1400、(b)1450、(c)1500 ℃温度烧结后块体的EPMA背散射图
Fig. S3
EPMA backscattering images of the CaTi0.8Nb0.2O3 (Nb20) bulk sintered at (a) 1400, (b) 1450, and (c) 1500 ℃, respectively
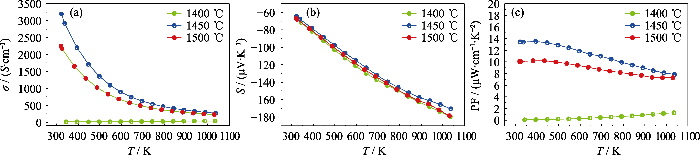
图S4
图S4
经过1400、1450、1500 ℃温度烧结后块体的(a)电导率、(b)塞贝克系数、(c)功率因子随温度的变化曲线
Fig. S4
Temperature-dependence of the (a) electrical conductivity, (b) Seebeck coefficient, and (c) power factor of CaTi0.8Nb0.2O3 (Nb20) sintered at (a) 1400, (b) 1450, and (c) 1500 ℃, respectively
表S2 所有块体的化学成分、简称、测量密度、理论密度以及致密度
Table S2
| Nominal chemical composition | Composition sample code | Measured density/ (g·cm-3) | Theoretical density/(g·cm-3) | Relative density/% |
|---|---|---|---|---|
| CaTiO3 | Pristine | 3.85 | 4.04 | 95.2 |
| CaTi0.8Cr0.2O3 | Cr20 | 3.89 | 4.06 | 95.8 |
| CaTi0.8Nb0.2O3 | Nb20 | 4.07 | 4.30 | 94.6 |
| Eu0.2Ca0.8TiO3 | Eu20 | 4.39 | 4.70 | 93.4 |
| Dy0.2Ca0.8TiO3 | Dy20 | 4.48 | 4.76 | 94.1 |
| Ce0.2Ca0.8TiO3 | Ce20 | 4.40 | 4.63 | 95.0 |
| La0.2Ca0.8TiO3 | La20 | 4.19 | 4.62 | 90.7 |
参考文献
Phase transitions in perovskite at elevated temperatures: a powder neutron diffraction study
ABO3-based photocatalysts for water splitting
Preparation and microwave dielectric properties of CaTiO3added Mg0.95Ni0.05-Ti0.98Zr0.02O3 composite ceramics for high frequency applications
Mg0.95Ni0.05Ti0.98Zr0.02O3 and CaTiO3 were prepared separately using solid state reaction method. The effect of CaTiO3 addition on the microwave dielectric properties of Mg0.95Ni0.05Ti0.98Zr0.02O3 was investigated to get low loss and temperature stable ceramics in (1 – x)Mg0.95Ni0.05Ti0.98Zr0.02O3-xCaTiO3 series. Mg0.95Ni0.05Ti0.98Zr0.02O3 formed as the major phase along with Mg0.95Ni0.05Ti2O5 phase that formed as minor secondary phase for the composition with x = 0. Microwave dielectric properties ∈r ~ 17.1, Qufo of 195,855 GHz and τf of –46.3 ppm/°C were obtained for the composition with x = 0. The positive τf value of CaTiO3, tuned the τf value of Mg0.95Ni0.05Ti0.98Zr0.02O3 through zero and ∈r ~ 28.4, Qufo ~ 108,775 GHz and τf ~ 3.1 ppm/°C were attained for x = 0.15 in this study. This composition is the best choice for high frequency applications.
Effects of CaTiO3 addition on the microwave dielectric properties and antenna properties of BiVO4 ceramics
Frequency and temperature dependent electrical characteristics of CaTiO3nano-ceramic prepared by high-energy ball milling
Understanding oxygen vacancies in disorder-engineered surface and subsurface of CaTiO3 nanosheets on photocatalytic hydrogen evolution
Studies on synthesis, characterization and applications of nano CaTiO3powder
A review on CaTiO3 photocatalyst: activity enhancement methods and photocatalytic applications
Inorganic waste forms for efficient immobilization of radionuclides
Dielectric and MLCC property of modified (Sr,Ca)TiO3based energy storage ceramic
Preparation and properties of CaTiO3:Pr3+/TiO2-mica fluorescent pearlescent pigments
Low lattice thermal conductivity and enhanced thermoelectric performance of SnTe via chemical electroless plating of Ag
Entropy engineering in CaZn2Sb2-YbMg2Sb2 Zintl alloys for enhanced thermoelectric performance
Thermoelectric transport coefficients of n-doped CaTiO3, SrTiO3 and BaTiO3: a theoretical study
The under-pressure behaviour of mechanical, electronic and optical properties of calcium titanate and its ground state thermoelectric response
CaTiO3 linear dielectric ceramics with greatly enhanced dielectric strength and energy storage density
Synthesis, structural refinement and optical behavior of CaTiO3 powders: a comparative study of processing in different furnaces
Processing bulk insulating CaTiO3 into a high-performance thermoelectric material
A squeeze on the perovskite structure improves the thermoelectric performance of europium calcium titanates
Synergistic effects of Eu and Nb dual substitution on improving the thermoelectric performance of the natural perovskite CaTiO3
Emerging homogeneous superlattices in CaTiO3bulk thermoelectric materials
Homogenous superlattices consisting of homogenous structural CeδCa1−δTiO3 and CaTi1−δCeδO3 alternate layers were obtained through a variable-valence Ce doping, providing multi-quantum well interfaces between the alternate layers.
Eu3+-site occupation in CaTiO3 perovskite material at low temperature
Thermoelectric properties of non-doped and Y-doped SrTiO3 polycrystals synthesized by polymerized complex process and hot pressing
Sr1-xLaxTiO3 nanoparticles: synthesis, characterization and enhanced thermoelectric response
Effect of sintering temperature on thermoelectric properties of La-doped SrTiO3ceramics prepared by Sol-Gel process and spark plasma sintering
A large thermoelectric figure of merit of La-doped SrTiO3 prepared by combustion synthesis with post-spark plasma sintering
Broadening the temperature range for high thermoelectric performance of bulk polycrystalline strontium titanate by controlling the electronic transport properties
Record high thermoelectric performance in bulk SrTiO3 via nano-scale modulation doping
High-figure-of-merit thermoelectric La-doped A-site-deficient SrTiO3 ceramics
Hydrothermal synthesis of size- and shape-controlled CaTiO3 fine particles and their photocatalytic activity
Hot pressing sintering process and sintering mechanism of W-La2O3-Y2O3-ZrO2
Analysis of nanoprecipitates in a Na-doped PbTe-SrTe thermoelectric material with a high figure of merit
Thermopower of Sr1-xLaxTiO3 ceramics
The thermopower η of Sr1−xLaxTiO3 ceramics was investigated up to x=0.5 and in the temperature range between 150 K and 1200 K. In addition, the carrier concentration n was determined by Hall measurements and by a chemical Ti3+-analysis. For low temperatures and high n, η depends linearly on temperature and on n−2/3, as expected from a degenerate quasi free electron gas. In the case of high temperatures and low n, the absolute value of η rises with 1.5⋅ln10⋅k/e per decade of temperature and with ln10⋅k/e per decade of carrier concentration, as expected from a classical broad-band semiconductor obeying the Boltzmann statistics. In the range of degeneration an effective mass meff of 4.2 electron masses can be deduced without the assumption of a transport factor Ae. In the classical range Ae=3 can be evaluated, requiring only a temperature and lanthanum independent meff. Thus, the thermopower of Sr1−xLaxTiO3 ceramics can be described by a constant effective mass and a constant transport factor within a wide range of temperature and lanthanum content. Furthermore, the transition from degeneration to classical behavior can be described as a function of temperature and electron density, e.g., at room temperature it takes place at about x≊0.2 (i.e., n≊3.4⋅1021/cm3).
Characterization of Lorenz number with Seebeck coefficient measurement
In analyzing zT improvements due to lattice thermal conductivity (κL) reduction, electrical conductivity (σ) and total thermal conductivity (κTotal) are often used to estimate the electronic component of the thermal conductivity (κE) and in turn κL from κL = ∼ κTotal − LσT. The Wiedemann-Franz law, κE = LσT, where L is Lorenz number, is widely used to estimate κE from σ measurements. It is a common practice to treat L as a universal factor with 2.44 × 10−8 WΩK−2 (degenerate limit). However, significant deviations from the degenerate limit (approximately 40% or more for Kane bands) are known to occur for non-degenerate semiconductors where L converges to 1.5 × 10−8 WΩK−2 for acoustic phonon scattering. The decrease in L is correlated with an increase in thermopower (absolute value of Seebeck coefficient (S)). Thus, a first order correction to the degenerate limit of L can be based on the measured thermopower, |S|, independent of temperature or doping. We propose the equation: L=1.5+exp−|S|116 (where L is in 10−8 WΩK−2 and S in μV/K) as a satisfactory approximation for L. This equation is accurate within 5% for single parabolic band/acoustic phonon scattering assumption and within 20% for PbSe, PbS, PbTe, Si0.8Ge0.2 where more complexity is introduced, such as non-parabolic Kane bands, multiple bands, and/or alternate scattering mechanisms. The use of this equation for L rather than a constant value (when detailed band structure and scattering mechanism is not known) will significantly improve the estimation of lattice thermal conductivity.
Laser nitriding of niobium for application to superconducting radio-frequency accelerator cavities