吸附法的操作简单且效率高, 是去除废水中铜离子的重要手段[5]。常用的吸附剂包括活性炭、沸石、高分子材料以及水合硅酸钙。其中, 水合硅酸钙因可通过离子交换和表面络合固定重金属离子表现出显著的吸附能力而备受关注[6-7]。然而, 合成高质量的水合硅酸钙一般需要昂贵的原料, 且使用后的恢复再生过程极其复杂, 如若处理不当, 极易造成二次污染[8]。2018年, 有研究者对吸附铜离子后的水合硅酸钙进行了300 ℃的热处理, 将其转化为光催化材料[9]。Chen等[10]利用水合硅酸钙吸附了Cu2+、Zn2+、Ni2+等典型重金属, 并成功将其转化为固碳催化材料。此外, 共价有机框架(COFs)和金属有机框架(MOFs)纳米材料在通过吸附-催化策略去除溶液中有机/无机污染物的过程里也表现出巨大的应用潜力[11]。这些工作证实了将“金属污染物”转化为“催化材料”的可行性。在异相催化领域, 载体材料会对活性物质的结构及催化性能产生重要影响[12]。作为载体的水合硅酸钙, 在“金属污染物”转化为“催化材料”过程中起到的桥接作用会显著影响最终催化材料的性能。一方面, 水合硅酸钙的吸附量决定了其对废水中铜离子的利用率以及后续铜基催化剂中活性物质负载量; 另一方面, 吸附的铜离子在水合硅酸钙表面的分布也会决定后续催化物质的分散性、结晶性甚至形态, 从而影响催化性能。
本工作针对所制备样品进行一系列的表征、性能测试以及拟合分析, 有望探索出“金属污染物”到“催化材料”转化过程中影响水合硅酸钙桥接作用的控制因素。
1 实验方法
1.1 实验试剂
氢氧化钠(NaOH)、氢氧化钙(Ca(OH)2)、氯化铜(CuCl2∙2H2O)、硝酸锌(Zn(NO3)2∙6H2O)、硝酸铅(Pb(NO3)2)、盐酸(HCl)和罗丹明B(RhB, AR)、过氧硫酸氢钾(PMS, 4.5%活性氧)、五水硫代硫酸钠(AR)由国药化学试剂有限公司提供。4-硝基苯酚(4-NP, AR, 99%)、硼氢化钠(NaBH4, 98%)、对苯醌(BQ)购买自上海阿拉丁生化科技股份有限公司。叔丁醇(TBA)、叠氮化钠(NaN3)、乙醇(EtOH)购买自泰坦科技。聚乙烯亚胺(PEI, 50%, 支链型, 分子量750 kDa)购自Honeywell Fluka。粉煤灰来自嘉兴市某发电厂, 其主要化学成分(质量分数)为:SiO2(48.25%)、Al2O3(24.04%)、Fe2O3(6.41%)、CaO(11.25%)、TiO2 (1.12%)、MgO(1.47%)、Na2O(1.54%)、SO3(2.72%)。
1.2 样品制备
5 g粉煤灰和8 gNaOH混合后在600 ℃马弗炉中热处理2 h, 冷却至室温后, 加入适量的去离子水搅拌, 静置过夜, 过滤沉淀物后得到SiO2浓度为 6 mg/mL的脱硅溶液。100 mL脱硅溶液与0.1 g改性剂PEI的混合物加热至60 ℃后, 加入50 mL质量浓度1.5%的Ca(OH)2悬浮液, 使n(Ca)∶n(Si)=1。随后, 将温度升高到80 ℃, 继续反应2 h。反应结束后依次用蒸馏水和乙醇清洗, 真空烘干得到PCSH。在相同条件下, 不添加改性剂得到CSH。
将0.2 g CSH (或PCSH)分散于100 mL的1 g/L Cu(II)溶液中吸附30 min后过滤, 并用去离子水和乙醇进行清洗, 得到CSH-Cu (或PCSH-Cu)。将其分散在20 mL的1.25 mol/L NaOH溶液中, 60 ℃反应2 h后, 清洗干燥收集得到CSH-Cu-c (或PCSH- Cu-c)。
1.3 样品表征
用电感耦合等离子体质谱仪(ICP, 725 ICP-OES, Agilent)测量脱硅溶液的硅离子浓度。用布鲁克Advance D8衍射仪测量样品的X射线衍射(XRD)图谱, 扫描速度为2 (°)/min, 扫描范围为10°~80°。利用扫描电子显微镜(FESEM, Magellan 400)观察样品的形貌并进行SAED分析。使用Micromeritics ASAP 2460在77 K下通过N2吸附-解吸等温线测量Brunauer-Emmett-Teller(BET)表面积和Barrett-Joyner- Halenda(BJH)孔径尺寸。在布鲁克EQUINOX55 FT-IR光谱仪上采用KBr颗粒测试方法记录傅里叶变换红外图谱(FT-IR)。此外, 利用X射线光电子能谱(XPS, Thermo Scientific KAlpha)表征样品表面的元素和价态。
1.4 性能测试
1.4.1 吸附批实验
用0.1 mol/L HCl和0.1 mol/L NaOH调节测试溶液的pH。所有的吸附试验都在25 ℃下250 r/min转速的摇床上进行, 吸附剂的用量保持为0.5 g/L。实验一式三份进行, 取平均值。所取样经针式过滤器过滤后, 用HACH2800水质分析仪分析清液中Cu(II)浓度。根据公式(1)计算样品对Cu(II)的吸附能力:
其中, qe为平衡吸附量(mg/g), C0和Ce分别为Cu(II)的初始浓度和平衡浓度(mg/L), V是溶液的体积(L), M代表吸附实验中吸附剂的质量(g)。为探讨干扰金属离子对样品吸附Cu(II)的影响, 设置了样品在Cu(II)初始浓度固定(CCu= 20 mg/L), 并且Zn(II)与Pb(II)浓度相同(CZn=CPb=10, 20, 30 mg/L)的三元金属溶液中的吸附试验。
1.4.2 催化性能测试
RhB降解试验 将40 mg催化剂在50 mL浓度为20 mg/L的RhB溶液(pH 7.0±0.3)中搅拌均匀, 加入50 μL PMS溶液 (0.12 g/mL)后开始反应。在特定时间取样, 并用少量五水合硫代硫酸钠固体快速淬灭。所取样经针式过滤器过滤后, 采用752N型紫外分光光度计测量其在554 nm处的吸光度来确定残余RhB浓度。
4-NP降解试验 将10 mg催化剂在60 mL浓度为0.1 mmol/L (0.0139 g/L)的4-NP溶液中搅拌均匀(pH (11.0±0.3))。随后加入3 mL浓度为0.1 mol/L 的NaBH4溶液开始反应。所取溶液过滤后测量其在400 nm处的吸光度来确定残余4-NP浓度。残余浓度(C)与吸光度(A)的计算公式为:
其中, A0、C0分别为污染物溶液的初始吸光度和初始浓度。
2 结果与讨论
2.1 样品的表征
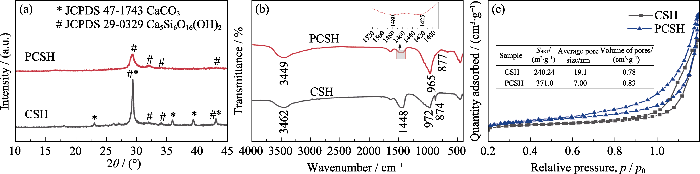
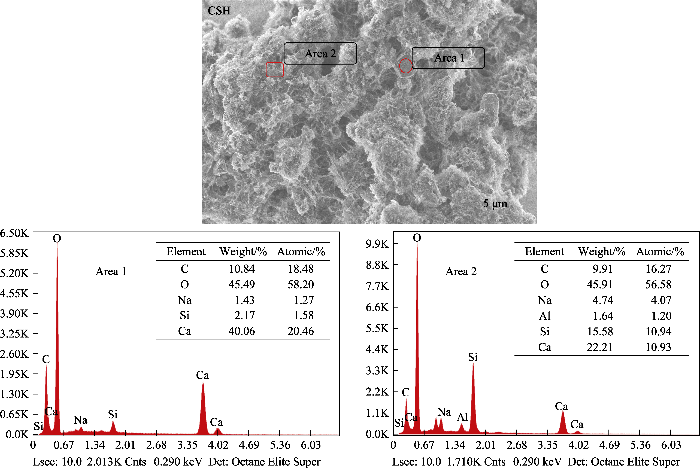
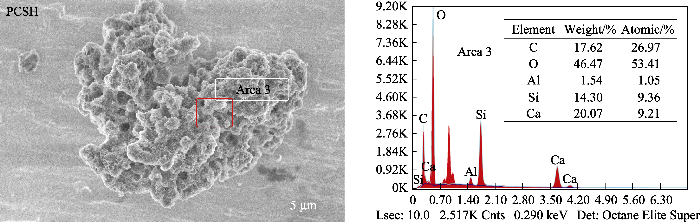
图1(a)为样品的XRD图谱。CSH样品在2θ= 29.5°、32.1°、34.1°、43.3°处出现了一系列对应于水合硅酸钙Ca5Si6O16(OH)2(JCPDS 29-0329)的宽衍射峰; 在2θ=23.1°、29.4°、36.0°、39.4°和43.2°处出现的特征峰对应于方解石CaCO3(JCPDS 47-1743)。PCSH中的特征峰均对应于水合硅酸钙, 表明引入PEI对CaCO3的形成具有显著的抑制作用, 可有效提高产品的纯度。此外, PCSH中衍射峰的强度较弱, 峰形比CSH相的宽化, 这意味着样品的结晶度较低, 其主要成分为无定形水合硅酸钙。
图1
图1
CSH与PCSH的(a) XRD图谱, (b) FT-IR图谱以及(c) N2吸附脱附曲线
Fig. 1
(a) XRD patterns, (b) FT-IR spectra and (c) N2 adsorption-desorption isotherms of CSH and PCSH
样品的FT-IR图谱如图1(b)所示。CSH样品在3462 cm-1处的峰归因于水中羟基的伸缩振动, 并且其Si-O-Ca振动峰出现在972 cm-1处[16]。PCSH样品由于存在PEI的N-H伸缩振动峰, 使3449 cm-1处峰的强度和宽度增加; 其在1480 cm-1处新形成的峰应归于SiO-···H···NH2+氢键系统中NH3+的对称变形[17], 证明PEI通过氢键作用与水合硅酸钙成功接枝[18]。此外, PCSH的Si-O-Ca伸缩振动峰转移至965 cm-1, 说明引入PEI对水合硅酸钙结构产生了影响。此外, CSH样品在1448和874 cm-1处出现了CO32-的特征峰, 而PCSH样品中这两个峰的强度非常微弱。
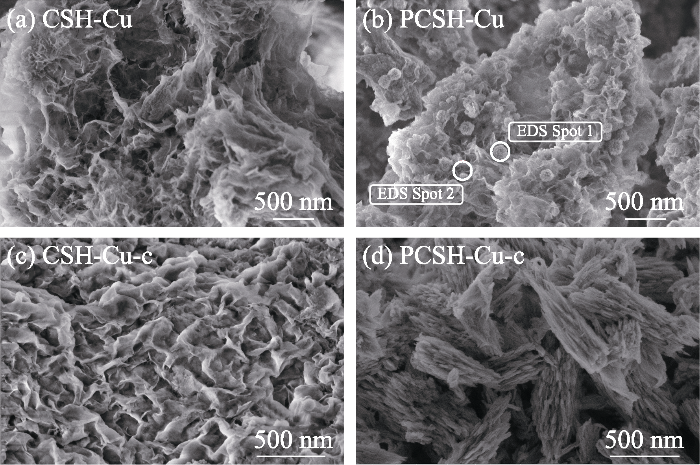
图2
图2
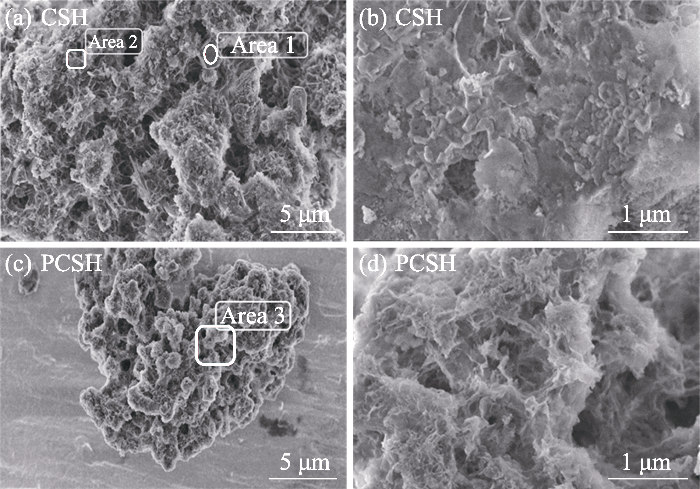
(a, b) CSH与 (c, d) PCSH的不同放大倍率SEM照片
Fig. 2
Different magnification SEM images of (a, b) CSH and (c, d) PCSH
上述表征结果充分证明, 在水合硅酸钙合成过程中加入PEI, 显著影响了杂质含量及产物的微观形貌。长支链型大分子聚合物PEI不仅与硅酸根离子产生氢键作用影响其活度, 同时其具有的空间位阻效应还会在合成反应中对水合硅酸钙晶体的成核生长以及层状结构的分散与堆积产生极大影响, 进而诱导形成小尺寸的高度多孔层状结构[19]。此外, PEI支链结构上的大量络合性氨基对游离Ca2+离子的识别结合则有效抑制了CaCO3的形成。总之, 加入PEI提高了水合硅酸钙纯度, 并通过调控显微结构引入了更为丰富的吸附位点。考虑到PCSH中残余PEI对Cu(II)离子的强选择性螯合作用, 其对Cu(II)有望展示出高吸附能力[20]。
2.2 样品的吸附性能
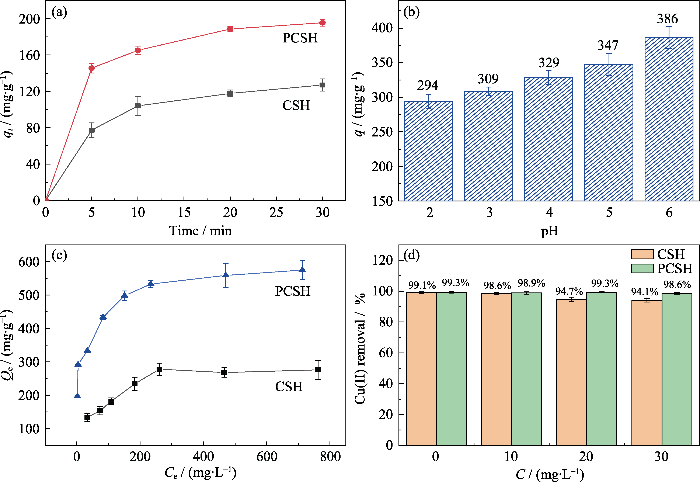
图3(a)为样品吸附量随时间的变化曲线。吸附实验在Cu(II)溶液为100 mg/L、吸附剂用量为0.5 g/L、初始pH 6的条件下进行。所有样品的吸附量在5~20 min内迅速增加, 这是由高浓度差与大量未反应活性位点共同导致。当接触时间从20 min延长到30 min, 由于溶液与吸附剂表面之间的Cu(II)浓度差逐渐减小, 样品吸附量的上升趋势也随之变缓。当接触30 min时吸附达到平衡, CSH与PCSH的吸附量分别达到127和195 mg/g, 去除率分别为64%和97%, PCSH显示出显著的吸附优势。
图3
图3
样品吸附性能表征
Fig. 3
Adsorption characteristics of samples
(a) Variation of adsorption capacity of samples with time; (b) Effect of pH on the adsorption capacity of PCSH; (c) Adsorption isotherms of samples; (d) Variation of adsorption capacity of samples with initial concentration of Zn(II)-Pb(II)
图3(b)显示了pH对PCSH吸附能力的影响, Cu(II)的初始浓度为300 mg/L, 吸附剂用量为0.5 g/L, 吸附4 h后取样。在低pH (pH 2~4)下, PCSH的吸附性能变差, 这是由于在低pH下PCSH的官能团(-NH2)与氢离子反应发生质子化, 削弱了PCSH与Cu(II)离子的络合作用, 此时的吸附途径主要为依靠比表面积与孔结构的离子交换。在pH 5~6范围, PCSH表面官能团被激活, 活性官能团与金属离子开始产生配位作用, 从而使PCSH的吸附性能明显增加。此外, 由于pH>6.0时溶液中产生明显的沉淀现象, 后续实验的pH设定为5.0。
Cu(II)的初始浓度范围在100~1000 mg/L之间, 调整初始pH为5.0±0.3, 吸附剂用量为0.5 g/L, 吸附4 h后取样。如图3(c)所示, 所有样品对Cu(II)的平衡吸附量在低初始浓度时均迅速上升, 而随着浓度提升, 样品吸附量上升速率逐渐减缓。CSH与PCSH分别在初始浓度为900和1000 mg/L时接近吸附上限, 吸附量分别为276与575 mg/g。对样品的吸附等温线分别利用Langmuir模型(公式3)和Freundlich模型(公式4)进行拟合:
其中, Ce为平衡浓度(mg/L), qe为平衡吸附量(mg/g), qm为饱和吸附量(mg/g), KF为Freundlich吸附常数(mg/g), n为吸附强度, KL为Langmuir吸附平衡常数 (L/mg)。
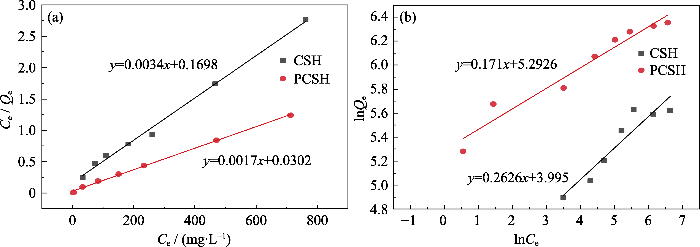
拟合曲线和拟合参数如图S3和表S1所示。样品在Langmuir等温模型中的相关系数R2(CSH, R2=0.9945; PCSH, R2=0.9982)均高于Freundlich模型(CSH, R2=0.867; PCSH, R2= 0.9450)。因此, 可以认为Cu(II)离子通过单层吸附均匀分布在材料的粗糙表面上。根据Langmuir模型可知, CSH和PCSH的理论饱和吸附容量分别为294和588 mg/g, 接近实验值276和575 mg/g。并且CSH与PCSH在Langmuir等温模型中吸附常数KL分别为0.0200、0.0563(介于0和1之间), 这表明吸附过程是平稳且自发的。将本工作中样品的理论饱和吸附量与已报道的吸附剂的性能进行比较(表S2), 其中PCSH表现出最高的吸附能力。
为探讨水合硅酸钙在多元金属水溶液中对Cu(II)的吸附特性, 测试了其在不同干扰离子初始浓度(CZn=CPb=10, 20, 30 mg/L)的三元金属溶液中对Cu(II)(CCu=20 mg/L)的去除能力[21]。初始pH调整为5.0±0.3, 吸附剂用量为0.5 g/L, 吸附30 min后取样。如图3(d)所示, 在无干扰离子存在的情况下, CSH与PCSH对溶液中Cu(II)的去除率分别为99.1%和99.3%。在Cu-Zn-Pb溶液中, 当Zn(II)-Pb(II)初始浓度由10 mg/L增大至30 mg/L时, CSH与PCSH的Cu(II)去除率分别由98.6%、98.9%降低至94.1%、98.6%, 整体呈现下降趋势, 这是由于溶液中Zn(II)与Pb(II)的存在导致了竞争吸附。与CSH相比, PCSH去除率的下降幅度十分微弱, 这可能是氨基对Cu(II)的强选择性吸附发挥了作用。上述结果表明, 在Zn(II)与Pb(II)存在的情况下, CSH与PCSH对Cu(II)吸附能力仍然保持在较高的状态, 并且PCSH具有更强的抗干扰能力。
2.3 吸附机理
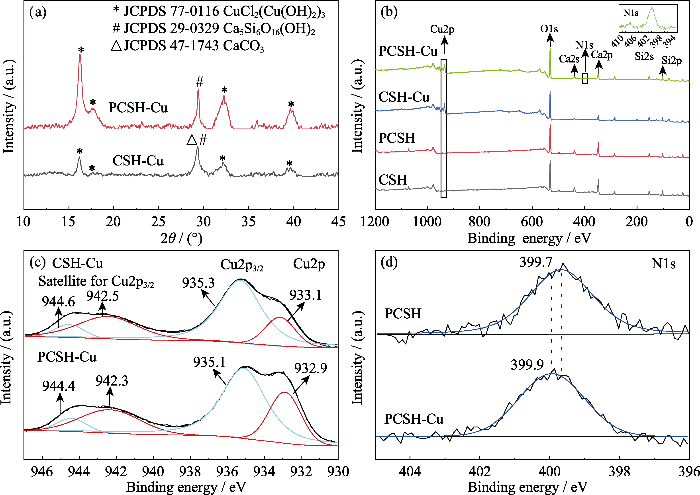
为探究吸附机理, 对吸附后的样品进行了XRD测试, 如图4(a)所示。CSH-Cu和PCSH-Cu在2θ=16.2°、17.6°、32.2°和39.4°处均出现了一组对应于碱式氯化铜(CuCl2 (Cu(OH)2)3, JCPDS 77-0116)的特征峰, 这表明PEI改性对所吸附Cu的物相组成无影响。但PCSH-Cu的XRD峰强明显高于CSH-Cu, 这与PCSH对Cu(II)吸附能力更强的结果一致。
图4
图4
吸附Cu后样品的表征
Fig. 4
Characteristics of Cu-absorbed samples
(a) XRD patterns; (b) Survey scans and high-resolution scans of (c) Cu2p, (d) N1s XPS spectra
图4(b~d)为吸附后样品的XPS谱图。吸附后两个样品的全谱(图4(b))中均出现了Cu2p特征峰。同时, Ca2p特征峰强度与吸附前相比有所减弱, 表明在吸附过程中均发生了离子交换。图4(c)为样品的Cu2p3/2精细谱。在CSH-Cu中, Cu2p3/2和相应的卫星峰分别出现在933.1~935.3和942.5~944.6 eV范围内。相比于CSH-Cu, PCSH-Cu发生了红移现象(Cu2p的峰向低结合能处移动了约0.2 eV), 这表明Cu(II)离子在结合过程中与相应的活性官能团相互作用(Cu-Cl、Cu-OH从-NH2获得电子以补偿电荷)[22]。为了进一步确认PCSH固定金属离子的吸附机制, 对吸附前后PCSH的N1s精细光谱进行分析。如图4(d)所示, 在PCSH的399.7 eV结合能处检测到属于-NH2/-NH-基团的N1s峰[23]。吸附Cu(II)离子后, PCSH-Cu的N1s结合能移动到更高的位置(从399.7 eV到399.9 eV), 这表明PCSH对Cu(II)的化学吸附与N元素相关。正如前面所述, PEI对Cu(II)的选择性高于其他金属, 当PCSH接触Cu(II)后, 可能会有部分与Ca2+离子结合的氨基与其解离, 转而与Cu(II)离子发生结合, 进一步使N1s结合能受到影响。
以上结果说明, 离子交换对不同水合硅酸钙样品吸附过程均有所贡献。而对于PCSH, 除优异的微观结构可以提供大量活性位点外, PEI的氨基还显著增强了其化学吸附强度。上述因素共同作用, 使PCSH展现出较强的Cu(II)吸附能力。
2.4 催化性能
图5
图5
(a) CSH-Cu, (b) PCSH-Cu, (c) CSH-Cu-c, (d) PCSH- Cu-c的SEM照片
Fig. 5
SEM images of (a) CSH-Cu, (b) PCSH-Cu, (c) CSH- Cu-c, and (d) PCSH-Cu-c
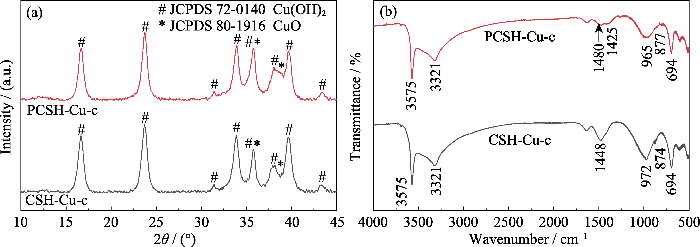
通过分析碱热处理后样品的XRD图谱(图6(a))可以得知, CSH-Cu与PCSH-Cu中的碱式氯化铜在处理后几乎全部转化为Cu(OH)2 (JCPDS 72-0140) 与少量CuO (JCPDS 80-1916)的混合物。此外, 与碱处理前样品相比, CSH-Cu-c与PCSH-Cu-c在2θ=29°附近归属于水合硅酸钙的主峰消失。这可能是由于吸附过程中样品内部的大量Ca(II)被溶液中Cu(II)的离子交换所替换, 随后样品中这些具有结构支撑作用的Cu(II)在碱热处理过程中参与反应, 进而破坏样品的基础晶体结构框架。
图6
图6
CSH-Cu-c与PCSH-Cu-c的(a) XRD和(b) FT-IR图谱
Fig. 6
(a) XRD patterns and (b) FT-IR spectra of CSH-Cu-c and PCSH-Cu-c
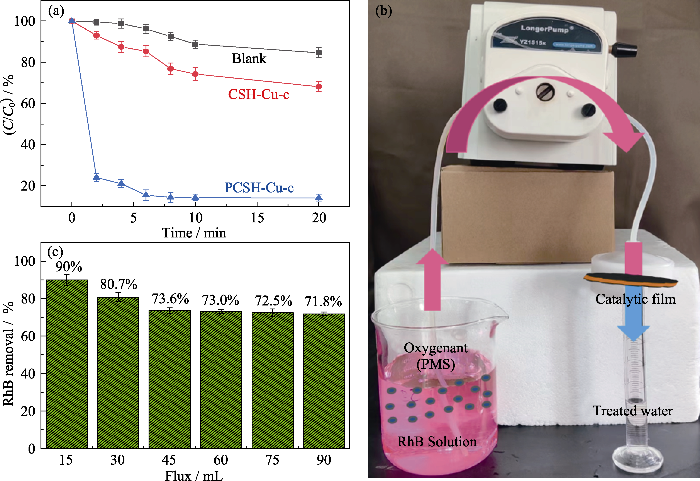
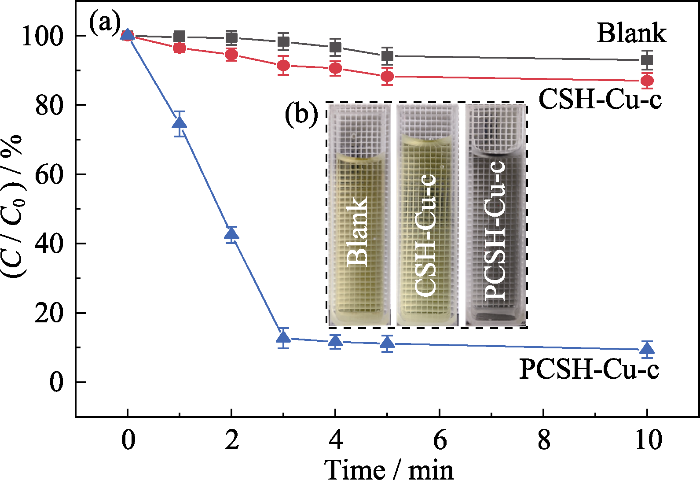
将CSH-Cu-c和PCSH-Cu-c用于催化PMS降解RhB, 具体实验条件为RhB, 20 mg/L; PMS, 0.12 g/L; 催化剂, 0.8 g/L; pH (7.0±0.3)。为比较降解反应动力学, 使用准一级反应动力学模型(公式(5))来描述RhB和随后的4-NP的降解。
其中, A0和At分别为初始时刻和t时刻降解产物的吸光度, k是准一级速率常数。
图7
图7
CSH-Cu-c和PCSH-Cu-c催化PMS降解RhB的性能
Fig. 7
Degradation of RhB by PMS with CSH-Cu-c and PCSH-Cu-c
(a) RhB residue percentage; (b) Photo of catalytic device; (c) Catalytic performance of PCSH-Cu-c-M
表1 不同材料催化PMS降解RhB的速率常数(k)
Table 1
| Catalyst | C1/(g·L-1) | CPMS/(g·L-1) | CRhB/(mg·L-1) | k/min-1 | Ref. |
|---|---|---|---|---|---|
| MPC | 1 | 0.616 | 20 | 0.013 | [27] |
| α-MnO2/Pal | 0.1 | 0.1 | 20 | 0.0204 | [28] |
| Vis/BiVO4 | 0.5 | 0.616 | 10 | 0.04 | [29] |
| rGO-CoPc | 0.3 | 0.616 | 10 | 0.288 | [30] |
| CSH-Cu-c | 0.8 | 0.12 | 20 | 0.036 | This work |
| PCSH-Cu-c | 0.8 | 0.12 | 20 | 0.7135 | This work |
C1, CPMS, and CRhB are the concentrations of catalyst, PMS and RhB, respectively
为充分发挥PCSH-Cu-c快速降解染料的优势, 将样品分散在水中并使用聚酯膜过滤悬浮液中的样品粉末, 制备得到一种简易催化膜(PCSH-Cu-c-M)。以1 mg/L的RhB溶液作为降解对象, 在液体流速为3 mL/min的膜催化装置中测试其催化效率。如图7(b)所示, RhB溶液的颜色在经过简易催化膜后基本消失。处理后溶液的RhB浓度测试结果显示, RhB的降解率在溶液通量为15~45 mL范围区间内由90%迅速下降至73.6%(图7(c))。这可能是由于PCSH- Cu-c颗粒在聚酯膜上的附着力较差, 从而造成简易催化膜的结构被水溶液破坏(本实验中没有使用成膜剂)。随后, 由于RhB对催化剂的污染以及其他因素的影响, 其降解效率随着溶液通量的增加而缓慢下降。以上测试结果表明PCSH-Cu-c对于PMS具有显著的激活作用, 并且PCSH-Cu-c-M在实际应用领域中表现出极大的潜力。
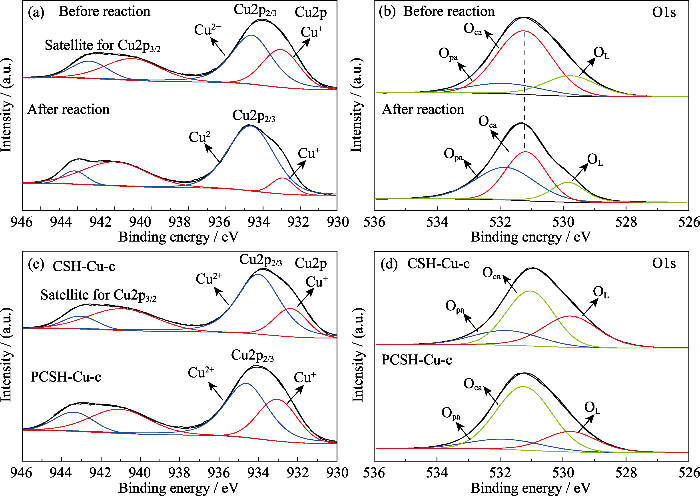
图8
图8
样品表面元素和化学状态表征
Fig. 8
Suface elements and chemical status of samples
(a) Cu2p and (b) O1s XPS spectra of PCSH-Cu-c before and after reaction; (c) Cu2p and (d) O1s XPS spectra of CSH-Cu-c and PCSH-Cu-c before reaction
图9
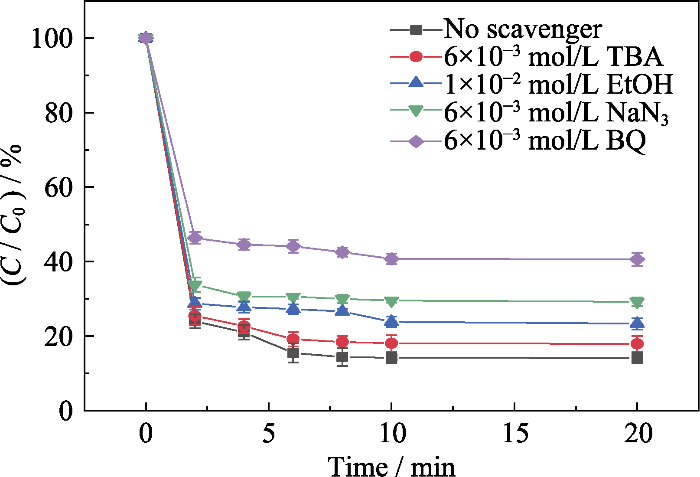
此外, PMS的活化能力还会受到催化剂中金属成分的负载量以及形态分布影响。本工作所制催化剂中含有大量的金属活性成分, 并且未经高温处理, 具有良好的晶型结构与小尺寸的晶粒分布, 因此表现出优异的催化能力。
进一步将CSH-Cu-c和PCSH-Cu-c用于催化NaBH4还原降解4-NP实验, 具体条件: [4-NP]= 10-4 mol/L; [NaBH4]= 5×10-3 mol/L; [催化剂]= 0.167 g/L; pH (11.0±0.3)。NaBH4将4-NP降解为4-氨基苯酚的机制较为复杂, 在金属催化剂体系中, 可能是通过金属离子完成氢化物转移(从BH4-到4-NP), 从而促使还原反应高效进行[37]。图10(a)显示使用PCSH- Cu-c作为催化剂, 3 min内4-NP的降解率达到87%, 反应混合物的亮黄色几乎变成无色(图10插图)。值得注意的是, 本实验在较低浓度的还原剂(n(NaBH4)∶ n(4-NP)=50∶1)和催化剂条件下取得了理想的实验结果(表2)。
图10
图10
4-NP剩余浓度随时间的变化曲线
Fig. 10
Change of 4-NP residue percentage
Inset: photograph of the 4-NP solution after 3 min degradation
表2 不同材料催化NaBH4降解4-NP的速率常数(k)
Table 2
| Catalyst | C1/ (g·L-1) | CNaBH4/ (mmol·L-1) | C4-NP/ (mmol·L-1) | k/(×10-3, s-1) | Ref. |
|---|---|---|---|---|---|
| CuO NLs | 0.307 | 10 | 0.12 | 0.36 | [38] |
| Ag-NP/C | 0.333 | 6.667 | 4.7×10-2 | 1.69 | [39] |
| Cu2−xSe/ rGO/PVP | 2.5 | 62.5 | 0.125 | 2.3 | [40] |
| Pd-FG | 0.5 | 10 | 0.1 | 2.35 | [41] |
| CSH-Cu-c | 0.167 | 5 | 0.1 | 0.61 | This work |
| PCSH-Cu-c | 0.167 | 5 | 0.1 | 11.47 | This work |
C1, CNaBH4, and C4-NP are the concentrations of catalyst, NaBH4, and 4-NP, respectively
上述结果表明, 由PCSH吸附剂制备的催化剂在不同领域都表现出显著的催化性能, 这是因为样品表面含有大量的有效催化活性成分, 并且PCSH- Cu-c的形态较为均匀, 分散性较高, 呈现出有利于传质的微观结构。上述工作既证实了“金属污染物转化为催化材料”的实际应用潜力, 又揭示了桥接材料水合硅酸钙的相关性质对催化能力的重要影响。
3 结论
本工作利用粉煤灰为硅源制备的改性水合硅酸钙作为中间桥接材料, 探索了一条将水溶液中的铜元素转化为催化剂活性物质的途径。结果证明PEI对CaCO3的形成有显著限制作用。与CSH相比, PEI的空间位阻作用使PCSH呈现出大比表面积 (371 m2/g)、细微孔径、高度多孔的优异显微结构, 为样品与吸附质的接触提供了更多的活性位点。结合氨基官能团对Cu(II)的高选择性络合, 使PCSH对Cu(II)的吸附量高达588 mg/g。因此, 相应催化剂的有效成分含量与形态也得到了改善。以PCSH为桥接材料制得的催化剂在PMS氧化反应(6 min时RhB降解率达到85%)和NaBH4还原反应(3 min时4-NP降解率达到87%)中表现出优异的催化性能。本工作提供了一种适用于多领域的铜基高效催化剂, 实现了“金属污染物”转化为“催化材料”的可持续发展策略, 同时具有原料成本低、制备工艺简单、应用范围广等多种优点, 将有助于环境领域的治理改善。
补充材料
粉煤灰衍生水合硅酸钙PEI改性及吸附去除Cu(II)与催化降解有机污染物
汤亚1,2, 孙盛睿2, 樊佳1,2, 杨庆峰3, 董满江2, 寇佳慧1, 刘阳桥2
(1. 南京工业大学 材料科学与工程学院, 材料化学工程国家重点实验室, 南京210009; 2. 中国科学院 上海硅酸盐研究所, 上海200050; 3. 中国科学院 上海高等研究院, 上海201210)
图S1
图S2
图S3
图S3
CSH与PCSH在(a) Langmuir模型和(b) Freundlich模型中的吸附等温线拟合
Fig. S3
Linear fitting curves of (a) Langmuir model and (b) Freundlich model for isotherms of CSH and PCSH
图S4
表S1 Langmuir和Freundlich等温线拟合参数
Table S1
| Sample | Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | n | KF | R2 | |
| CSH | 294.10 | 0.0200 | 0.9945 | 3.810 | 54.00 | 0.8670 |
| PCSH | 588.23 | 0.0563 | 0.9982 | 5.848 | 198.86 | 0.9450 |
表S2 文献中报道的各种吸附剂对Cu(II)的最大吸附能力比较
Table S2
| Sample | q / (mg·g-1) | SBET/ (m2·g-1) | Ref. |
|---|---|---|---|
| Activated carbon | 10 | 921 | [S1] |
| Modified SBA-15 mesoporous silica | 46 | 317 | [S2] |
| MCM-48 | 126 | 511 | [S3] |
| Citrate-LDH | 137 | 8 | [S4] |
| Mesoporous silica | 153 | 462 | [S5] |
| Humulus scandens-derived biochars | 221 | 450 | [S6] |
| Steel slag-derived CSH | 244 | 77 | [S7] |
| Amorphous molybdenum sulphide | 259 | 28 | [S8] |
| NPCS-PEI | 276 | 491 | [S9] |
| CSH | 294 | 240 | This work |
| PCSH | 588 | 371 | This work |
参考文献:
[S1] MACIAS-GARCIA A, GOMEZ CORZO M, ALFARO DOMINGUEZ M, et al. Study of the adsorption and electroadsorption process of Cu(II) ions within thermally and chemically modified activated carbon. Journal of Hazardous Materials, 2017, 328: 46.
[S2] MURESEANU M, REISS A, STEFANESCU I, et al. Modified SBA-15 mesoporous silica for heavy metal ions remediation. Chemosphere, 2008, 73(9): 1499.
[S3] ANBIA M, KARGOSHA K, KHOSHBOOEI S. Heavy metal ions removal from aqueous media by modified magnetic mesoporous silica MCM-48. Chemical Engineering Research & Design, 2015, 93: 779.
[S4] HAI NGUYEN T, LIN C-C, WOO S H, et al. Efficient removal of copper and lead by Mg/Al layered double hydroxides intercalated with organic acid anions: Adsorption kinetics, isotherms, and thermodynamics. Applied Clay Science, 2018, 154: 17.
[S5] SANTHAMOORTHY M, THIRUPATHI K, PERIYASAMY T, et al. Synthesis of bifunctional groups-integrated mesoporous silica hybrid adsorbent for simultaneous removal of Hg2+ and Cu2+ ions from aqueous solution. Surfaces and Interfaces, 2022, 29: 101808.
[S6] BAI X, XING L, LIU N, et al. Humulus scandens-derived biochars for the effective removal of heavy metal ions: Isotherm/Kinetic study, column adsorption and mechanism investigation. Nanomaterials, 2021, 11(12): 3255.
[S7] WANG S, PENG X, TANG L, et al. Influence of hydrothermal synthesis conditions on the formation of calcium silicate hydrates: from amorphous to crystalline phases. Journal of Wuhan University of Technology-Materials Science Edition, 2018, 33(5): 1150.
[S8] FU W, JI G, CHEN H, et al. Engineering anion resin based amorphous molybdenum sulphide composite for treatment of authentic acid mine drainage. Journal of Environmental Chemical Engineering, 2020, 8(5): 104072.
[S9] LIU T, GOU S, HE Y, et al. N-methylene phosphonic chitosan aerogels for efficient capture of Cu2+ and Pb2+ from aqueous environment. Carbohydrate Polymers, 2021, 269: 118355.
参考文献
Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions
Cu and Cu-based nanoparticles: synthesis and applications in review catalysis
A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants
Remarkable facet selective reduction of 4-nitrophenol by morphologically tailored (111) faceted Cu2O nanocatalyst
Relating silica scaling in reverse osmosis to membrane surface properties
Anti-organic fouling and anti-biofouling poly(piperazineamide) thin film nanocomposite membranes for low pressure removal of heavy metal ions
Propensity towards anti-organic fouling, anti-biofouling property and low rejection of multivalent cation (monovalent counter ion) restricts the application of the state-of-art poly(piperazineamide) [poly(PIP)] thin film composite (TFC) nanofiltration (NF) membrane for the treatment of water containing toxic heavy metal ions, organic fouling agents and microbes. Herein, we report the preparation of thin film nanocomposite (TFNC) NF membranes with improved heavy metal ions rejection efficacy, anti-biofouling property, and anti-organic fouling properties compared to that of poly(PIP) TFC NF membrane. The TFNC NF membranes were prepared by the interfacial polymerization (IP) between PIP and trimesoyl chloride followed by post-treatment with polyethyleneimine (PEI) or PEI-polyethylene glycol conjugate and then immobilization of Ag NP. The IP was conducted on a polyethersulfone/poly(methyl methacrylate)-co-poly(vinyl pyrollidone)/silver nanoparticle (Ag NP) blend ultrafiltration membrane support. The TFNC membranes exhibited >99% rejection of Pb, 91-97% rejection of Cd, 90-96% rejection of Co and 95-99% rejection of Cu with permeate flux ∼40Lmh at applied pressure 0.5MPa. The improved heavy metal ions rejection efficacy of the modified NF membranes is attributed to the development of positive surface charge as well as lowering of surface pore size compared to that of unmodified poly(PIP) TFC NF membrane.Copyright © 2017 Elsevier B.V. All rights reserved.
Removal of heavy metal ions from wastewaters: a review
Heavy metal pollution has become one of the most serious environmental problems today. The treatment of heavy metals is of special concern due to their recalcitrance and persistence in the environment. In recent years, various methods for heavy metal removal from wastewater have been extensively studied. This paper reviews the current methods that have been used to treat heavy metal wastewater and evaluates these techniques. These technologies include chemical precipitation, ion-exchange, adsorption, membrane filtration, coagulation-flocculation, flotation and electrochemical methods. About 185 published studies (1988-2010) are reviewed in this paper. It is evident from the literature survey articles that ion-exchange, adsorption and membrane filtration are the most frequently studied for the treatment of heavy metal wastewater.Copyright © 2010 Elsevier Ltd. All rights reserved.
CO2-assisted ‘Weathering’ of steel slag-derived calcium silicate hydrate: A generalized strategy for recycling noble metals and constructing SiO2-based nanocomposites
Hierarchically structured calcium silicate hydrate-based nanocomposites derived from steel slag for highly efficient heavy metal removal from wastewater
Recycling heavy metals from wastewater for photocatalytic CO2 reduction
Application of covalent organic frameworks and metal-organic frameworks nanomaterials in organic/inorganic pollutants removal from solutions through sorption-catalysis strategies
With the fast development of agriculture, industrialization and urbanization, large amounts of different (in)organic pollutants are inevitably discharged into the ecosystems. The efficient decontamination of the (in)organic contaminants is crucial to human health and ecosystem pollution remediation. Covalent organic frameworks (COFs) and metal–organic frameworks (MOFs) have attracted multidisciplinary research interests because of their outstanding physicochemical properties like high stability, large surface areas, high sorption capacity or catalytic activity. In this review, we summarized the recent works about the elimination/extraction of organic pollutants, heavy metal ions, and radionuclides by MOFs and COFs nanomaterials through the sorption-catalytic degradation for organic chemicals and sorption-catalytic reduction-precipitation-extraction for metals or radionuclides. The interactions between the (in)organic pollutants and COFs/MOFs nanomaterials at the molecular level were discussed from the density functional theory calculation and spectroscopy analysis. The sorption of organic chemicals was mainly dominated by electrostatic attraction, π-π interaction, surface complexation and H-bonding interaction, whereas the sorption of radionuclides and metal ions was mainly attributed to surface complexation, ion exchange, reduction and incorporation reactions. The porous structures, surface functional groups, and active sites were important for the sorption ability and selectivity. The doping or co-doping of metal/nonmetal, or the incorporation with other materials could change the visible light harvest and the generation/separation of electrons/holes (e−/h+) pairs, thereby enhanced the photocatalytic activity. The challenges for the possible application of COFs/MOFs nanomaterials in the elimination of pollutants from water were described in the end.
Strong metal-support interactions- occurrence among binary oxides of groups IIA-VB
COF-based composites: extraordinary removal performance for heavy metals and radionuclides from aqueous solutions
Recent advances in carbon nitride-based nanomaterials for the removal of heavy metal ions from aqueous solution
Graphitic-like carbon nitride (g-C3N4), one of the most significant two-dimensional layered materials, has attracted worldwide attention in multidisciplinary areas such as photocatalysis, energy conversion and environmental pollution management. Its derivative compounds have also attracted multifarious attention owing to the intrinsic characters of their stable physicochemical properties, low cost and environmentally friendly features. This review focus on the design of high-performance g-C3N4-based nanomaterials and their potential for pollutant elimination in environmental pollution cleanup. Over the past few years, signi?cant advances have been achieved to synthesize g-C3N4 and g-C3N4-based nanomaterials, and their properties have been enhanced and characterized in detail. In this review, recent developments in the synthesis and modification of g-C3N4-based nanomaterials are summarized. The applications in heavy metal ions adsorption from wastewaters are gathered and their underlying reaction mechanisms are discussed. Finally, a summary and outlook are also briefly illustrated.
In-situ fabrication of polyelectrolyte-CSH superhydrophilic coatings via layer-by-layer assembly
Structural characterization of gel-derived calcium silicate systems
The main aim of this study is to synthesize calcium silicate ceramics that exhibit suitable properties to be used for biomedical applications. In the present work, attention was paid to the understanding of processing-structure relationships. A particular effort was made to clarify the identification of Ca-O-Si bonds by means of spectroscopy. The calcium silicate systems were prepared via a sol-gel route, varying the chemical compositions, the catalyst concentration, and the temperature and time of aging and heat treatment. The processes and the phases evolved during the sol-gel procedure were determined. The bond systems were investigated by Fourier transform infrared (FTIR) and (29)Si magic angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy and the aggregate structures by scanning electron microscopy (SEM), small-angle neutron scattering (SANS), small-angle X-ray scattering (SAXS), wide-angle X-ray scattering (WAXS), and X-ray diffraction (XRD) measurements.
The coordination state of copper(II) complexes anchored and grafted onto the surface of organically modified silicates
Amine- bilayer-functionalized cellulose-chitosan composite hydrogel for the efficient uptake of hazardous metal cations and catalysis in polluted water
Polyethylenimine in medicinal chemistry
Polyethylenimine (PEI), an organic branched or linear polyamine polymer, has been successfully used in the past for DNA complexation and transfection in vitro and in vivo into several cell lines and tissues. PEI was also applied in different fields from gene therapy and several studies have emphasized the importance of this polymer in medicinal chemistry. In this brief critical review the uses and applications of this versatile polymeric molecule will be discussed.
Polyethyleneimine functionalized mesoporous diatomite particles for selective copper recovery from aqueous media
Enhanced copper adsorption by DTPA-chitosan/alginate composite beads: Mechanism and application in simulated electroplating wastewater
Calibration of XPS core-level binding-energies-influence of the surface chemical-shift
Equilibrium, kinetic and mechanism studies of Cu(II) and Cd(II) ions adsorption by modified chitosan beads
In this study, the Cu(II) and Cd(II) ions removal behavior of crosslinked chitosan beads grafted poly(methacrylamide) (abbreviated as crosslinked chitosan-g-PMAm) from single metal ion solutions was investigated. The modified chitosan beads presented a remarkable improvement in acid resistance. The batch experiments demonstrated that pH of solution played a significant role in adsorption. It was found that the adsorption of Cu(II) and Cd(II) were optimum at pH 4 and pH 5, respectively. The maximum adsorption capacities for Cu(II) and Cd(II) based on Langmuir equation were 140.9 mg g and 178.6 mg g, respectively. Pseudo-second order gave a better fit for adsorption data with respect to linearity coefficients than pseudo-first order suggesting that chemisorption or electron transfer is the dominant mechanism of the metal ions onto crosslinked chitosan-g-PMAm. In addition, X-ray photoelectron spectroscopy (XPS) investigations revealed that adsorption of both metal ions took place on the surfaces of crosslinked chitosan-g-PMAm by chelation through CNH, CO and CO groups. Overall, the modified chitosan has proved a promising adsorbent for removal of metal ions.Copyright © 2018 Elsevier B.V. All rights reserved.
Facile synthesis of Fe3O4@Cu(OH)2 composites and their arsenic adsorption application
Reaction pathway led by silicate structure transformation on decomposition of CaSiO3 in alkali fusion process using NaOH
Effect of TiO2 content on the structure of CaO-SiO2-TiO2 system by molecular dynamics simulation
Non-radical pathway dominated degradation of organic pollutants by nitrogen-doped microtube porous graphitic carbon derived from biomass for activating peroxymonosulfate: performance, mechanism and environmental application
The agricultural waste-derived biochar can be used as an effective green catalyst for peroxymonosulfate (PMS) activation to utilize the biomass resource. Herein, nitrogen-doped microtubes porous graphitic carbon (N-MPGC) derived from loofah sponge was facilely prepared via the impregnation-calcination method. The amount of N doping was positively correlated with the catalytic performance of N-MPGC. The obtained N-MPGC-2 as a metal-free carbon catalyst showed excellent ability for rhodamine B (RhB) degradation via PMS activation with the pseudo-first-order reaction rate constant (k) of 0.293 min, which was 22.5-fold as high as that over microtube porous carbon (MPC). Besides, N-MPGC-2 showed still outstanding stability and reusability for RhB degradation after ten successive cycles. Excitingly, the N-MPGC-2 membrane exhibited good catalytic activity after the N-MPGC-2 had been immobilized in the polytetrafluoroethylene (PTFE) membrane. Non-radical pathways including singlet oxygen and electron transfer played a major role in RhB degradation for the N-MPGC-2/PMS/RhB system. The carbonyl (CO) group and graphitic N of N-MPGC-2 were the main active sites for PMS activation. This work opened a new idea for synthesizing N-doped biochar as a low-cost and high-efficiency catalyst and provided theoretical support for the mechanism of biochar-based carbonaceous materials activation of PMS for practical applications.Copyright © 2022 Elsevier Inc. All rights reserved.
α-MnO2/palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate PMS for the degradation of Rhodamine B
Activation of peroxymonosulfate by BiVO4 under visible light for degradation of Rhodamine B
Cobalt phthalocyanine-supported reduced graphene oxide: a highly efficient catalyst for heterogeneous activation of peroxymonosulfate for Rhodamine B and pentachlorophenol degradation
Impact of crystal types of AgFeO2 nanoparticles on the peroxymonosulfate activation in the water
Spongelike porous CuO as an efficient peroxymonosulfate activator for degradation of Acid Orange 7
Co-doped CuO/visible light synergistic activation of PMS for degradation of Rhodamine B and its mechanism
Elemental doping is an important method for improving the efficiency of catalysts. In this study, cobalt-doped CuO catalysts were prepared using a rapid precipitation method for the effective degradation of organic pollutants by photo-assisted activation of potassium peroxymonosulfate (PMS). The Co-doped CuO catalyst demonstrated degradation efficiency as high as 96% within 20 min reaction under visible light conditions, which is much higher than that of the undoped CuO. Cobalt doping transformed copper oxide nanoparticles from a three-dimensional needle-shape structure to a near-two-dimensional thin ribbon-like structure. Cobalt doping increases the flat-band potential of CuO and thus enhances the charge transfer efficiency. Furthermore, the effects of solution pH, initial dye concentration and catalyst dosage on the degradation efficiency were also investigated. XPS and EPR results show that cobalt doping can increase the oxygen vacancy content in CuO and thus improves the activation properties. The result of the trapping agent experiments indicates that main active species in the reaction are holes, while hydroxyl radicals, singlet oxygen, superoxide radicals and sulfate radicals are also involved. Finally, the reaction mechanism for the photo-assisted Co-doped CuO activated PMS degradation of RhB are generally proposed.
Activation of peroxymonosulfate/ persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies
Insights into perovskite-catalyzed peroxymonosulfate activation: maneuverable cobalt sites for promoted evolution of sulfate radicals
Recovery of CuO/C catalyst from spent anode material in battery to activate peroxymonosulfate for refractory organic contaminants degradation
Nickel-doped cobalt ferrite nanoparticles: efficient catalysts for the reduction of nitroaromatic compounds and photo-oxidative degradation of toxic dyes
This study deals with the exploration of NixCo₁-xFe₂O₄ (x = 0.0, 0.2, 0.4, 0.6, 0.8, 1.0) ferrite nanoparticles as catalysts for reduction of 4-nitrophenol and photo-oxidative degradation of Rhodamine B. The ferrite samples with uniform size distribution were synthesized using the reverse micelle technique. The structural investigation was performed using powder X-ray diffraction, high-resolution transmission electron microscopy, energy dispersive X-ray and scanning tunneling microscopy. The spherical particles with ordered cubic spinel structure were found to have the crystallite size of 4-6 nm. Diffused UV-visible reflectance spectroscopy was employed to investigate the optical properties of the synthesized ferrite nanoparticles. The surface area calculated using BET method was found to be highest for Co₀.₄Ni₀.₆Fe₂O₄ (154.02 m(2) g(-1)). Co₀.₄Ni₀.₆Fe₂O₄ showed the best catalytic activity for reduction of 4-nitrophenol to 4-aminophenol in the presence of NaBH4 as reducing agent, whereas CoFe₂O₄ was found to be catalytically inactive. The reduction reaction followed pseudo-first order kinetics. The effect of varying the concentration of catalyst and NaBH₄ on the reaction rates was also scrutinized. The photo-oxidative degradation of Rhodamine B, enhanced oxidation efficacy was observed with the introduction of Ni(2+) in to the cobalt ferrite lattice due to octahedral site preference of Ni(2+). Almost 99% degradation was achieved in 20 min using NiFe₂O₄ nanoparticles as catalyst.
Green synthesis of 2D CuO nanoleaves (NLs) and its application for the reduction of p-nitrophenol
Carbon spheres with controllable silver nanoparticle doping
Synergetic catalytic effect of Cu2-xSe nanoparticles and reduced graphene oxide coembedded in electrospu n nanofibers for the reduction of a typical refractory organic compound