燃料电池可以将化学能转化为电能, 具有节能环保的特点, 发展前景广阔。直接乙醇燃料电池阳极Pt基催化剂相比于Pd、Au基催化剂, 在酸性条件下表现出更加优异的性能。然而, 纯金属Pt催化剂的成本较高、催化活性和原子利用率较低, 阻碍了实际推广应用。与其他金属合金化, 并设计合成具有高指数晶面结构的催化剂是降低成本和提高Pt催化活性与原子利用率的有效方法[1⇓⇓⇓⇓⇓⇓-8]。一方面, 引入非贵金属合金化可以调节原子比例, 减少Pt消耗并改善其电催化性能[9-10];另一方面, 具有高指数晶面结构的Pt基纳米晶粒可以暴露大量缺陷原子(如台阶、扭结原子等), 提供更多的活性位点, 进而提高Pt原子的利用率[11-12]。
为进一步提高Pt基催化剂的电催化性能, 可以将Pt基纳米晶粒负载到导电性良好的碳材料载体上[13]。Vulcan XC-72R(简称XC-72R)炭黑的比表面积(~250 m2/g)和电导率(~2.77 S/cm)之间的平衡良好, 被广泛用于直接醇类燃料电池[14]。制备Pt基/碳材料纳米催化剂, 通常先合成Pt基纳米晶粒, 然后与碳载体物理混合, 完成负载。但是这种方式的碳载体与Pt基纳米晶粒之间的结合力较弱, 且操作繁琐。合成负载型Pt基高指数晶面催化剂最理想的方式是在碳载体表面直接原位生长Pt基金属纳米晶粒。然而, 早期原位生长法制备的XC-72R炭黑负载Pt基纳米催化剂, 晶粒多呈现出常规的基础晶面, 且目前在XC-72R炭黑上原位生长具有高指数晶面的Pt基纳米材料鲜有报道。因此, 探索在XC-72R炭黑上原位生长具有高指数晶面的Pt基合金纳米材料, 对直接乙醇燃料电池的发展具有重要的意义。
本研究以XC-72R炭黑为碳载体, 以聚乙烯吡咯烷酮(PVP k-25)为分散剂和还原剂、甘氨酸为表面控制剂和共还原剂, 通过简单绿色的液相水热合成法代替复杂的电化学方法, 一步制备得到XC-72R炭黑负载的高指数晶面取向的Pt1Cox/C合金催化剂, 实现具有高指数晶面取向催化剂纳米晶粒在碳载体上的原位生长。添加非贵金属Co形成合金催化剂, 利用Pt、Co之间的相互作用, 在降低催化剂成本的同时, 保证了催化剂的催化活性。
1 实验方法
1.1 试剂
氯铂酸(H2PtCl6·6H2O, ≥37%(Pt))购自天津科密欧化学试剂有限公司;氯化钴(CoCl2·6H2O, 分析纯)购自上海麦克林生化科技有限公司;聚乙烯吡咯烷酮k-25 (分子量~30000, ≥97%)购自广东粤美化工有限公司;甘氨酸(C2H5NO2, 分析纯)购自上海麦克林生化科技有限公司;Vulcan XC-72R炭黑购自上海凯茵化工有限公司;Pt/C(JM) (质量分数30%)为商业催化剂。
1.2 催化剂的制备
PVP功能化处理XC-72R炭黑 将0.0069 g炭黑粉末添加到6.9 mL去离子水中, 超声分散30 min。然后添加3.71 g PVP-k25(分子量~30000), 室温下匀速搅拌12 h。
Pt1Cox/C高指数晶面纳米催化剂的制备 向上述功能化处理后的炭黑悬浊液中加入1.62 g甘氨酸、30.60 mL去离子水, 按照不同的铂钴摩尔比, 添加CoCl2·6H2O和H2PtCl6·6H2O (38.62 mmol/L, 金属总质量为61.824 mg, 占催化剂总质量的90%), 室温下搅拌30 min, 超声10 min。将溶液转移至100 mL不锈钢高压反应釜, 在200 ℃鼓风干燥箱中反应9 h。反应结束后, 取出反应釜并自然冷却至室温, 在104 r/min下高速离心收集样品, 分别用去离子水与无水乙醇洗涤, 除去表面有机物。然后将离心管放入45 ℃真空干燥箱中干燥, 研磨后收集粉末, 得到Pt1Cox/C (x=0、1/4、1/3、1/2、1, 摩尔比)高指数晶面纳米催化剂。
1.3 表征方法
采用日本理学公司的Miniflex600 X射线衍射仪对催化剂的物相组成和结晶情况进行分析, 工作电压40 kV, 工作电流15 mA, 光源CuKα, 波长0.15406 nm, 扫描速度2 (°)/min, 扫描范围为2θ=10º~90°。采用日本电子公司的JEM-2500SE透射电镜分析所制备催化剂的形貌及粒径分布。采用英国赛默飞世尔科技公司的ESCALAB250 XI光电子能谱仪分析催化剂的元素组成和化学价态变化, 测试前先对制备的催化剂粉末样品进行磁处理。以AlKα 射线为激发源, C1s (284.6 eV)为基准, 仪器功率300 W, 测试压力10-8 Pa。
采用英国苏立强公司的电化学综合测试系统(1260A+1287A)进行电化学测试, 使用三电极体系, 铂丝为对电极, 饱和甘汞电极为参比电极。按照体积分数配制1 mL含有20.00%乙醇、73.75%水、6.25% Nafion (5.00%)的混合溶液, 取10 mg催化剂添加到上述混合溶液中, 超声后取7 μL混合溶液涂在工作电极表面, 干燥后进行测试。在电化学测试之前向测试溶液中通入氮气, 去除O2的影响。在0.5 mol/L的硫酸溶液中测定电化学活性表面积, 扫描电位区间-0.3~0.6 V, 扫描速度50 mV/s;循环伏安电化学测试使用0.5 mol/L硫酸与1 mol/L无水乙醇的混合溶液, 扫描电位区间0~1.2 V, 扫描速度50 mV/s;计时电流测试使用0.5 mol/L硫酸与1 mol/L无水乙醇的混合溶液, 极化电位0.6 V, 测试时间1100 s;CO溶出测试使用0.5 mol/L H2SO4为测试溶液, 扫描电位区间0~1.0 V, 扫描速度50 mV/s。测试前预先通CO气体30 min, 气体流速为10 mL/min。
2 结果与讨论
2.1 Pt1Cox/C高指数晶面催化剂的物相组成和晶体结构
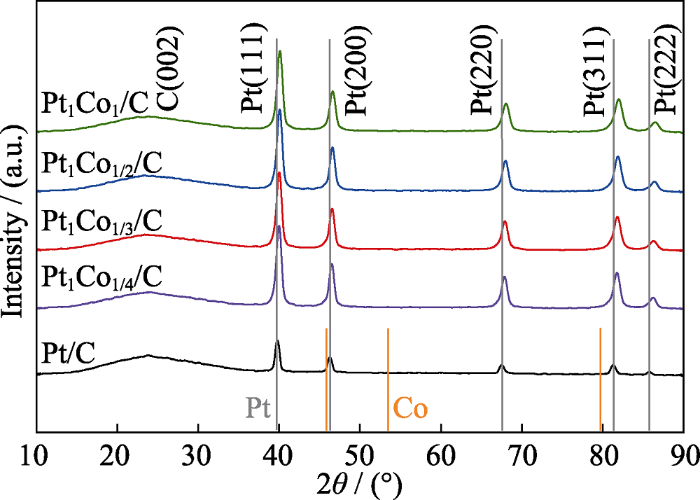
为了研究Co掺杂对催化剂晶体结构的影响, 对Pt1Cox/C高指数晶面催化剂进行XRD表征, 如图1所示。各催化剂在2θ=23.80°附近均出现一个宽峰, 对应于载体XC-72R炭黑的C(002)晶面。未掺杂Co的Pt/C催化剂在2θ=39.78°、46.22°、67.53°、81.31°和85.79°出现了5个特征衍射峰, 分别对应于Pt的(111)、(200)、(220)、(311)和(222)晶面。掺杂Co后, 各催化剂均没有出现Co及其氧化物的特征峰, 说明Co并不以单晶的形式存在。与Pt/C的衍射峰对比, 掺杂Co后催化剂的Pt特征衍射峰均向大角度方向发生了偏移。这是因为Co的原子半径小于Pt, 掺杂Co引起Pt晶格收缩[15-16], 表明Pt与Co形成了合金。
图1
2.2 Pt1Cox/C高指数晶面催化剂的晶面形貌和粒径分布
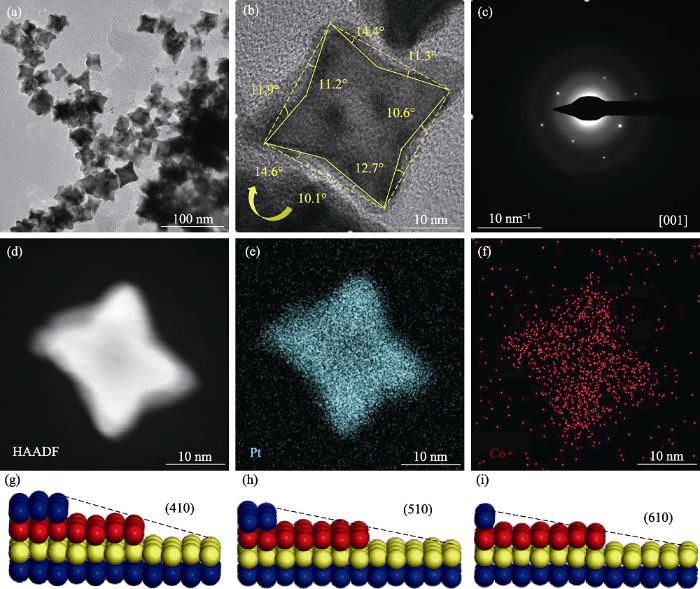
水热法是控制合成Pt基催化剂某种特定形貌的常用方法。, 水热法制备的Pt1Co1/3/C高指数晶面纳米催化剂晶粒呈现内凹的形貌, 且形貌选择性在90%以上(图2(a))。内凹是一种典型的高指数晶面{hk0}形貌, 根据经典的研究方法, 通过选区电子衍射(Selected Area Electron Diffraction, SAED)确定[001]的入射方向, 观察单个晶体并量取凹陷角度, 如图2(b)所示。其凹陷角度依次为14.4°、11.3°、10.6°、12.7°、10.1°、14.6°、11.9°、11.2°(按顺时针方向)。通过比对高指数晶面界面角与晶面的理论关系[15,17⇓-19], 可以解析Pt1Co1/3/C催化剂晶粒主要暴露的高指数晶面有(410)、(510)和(610)晶面。图2(c)点阵状电子衍射图显示所制高指数晶面纳米粒子为 单晶。由图2(d~f)能量色散谱(Energy Dispersive Spectrometer, EDS)面扫描分布可知, 催化剂纳米晶粒中Pt元素与Co元素分布均匀, 形成了Pt-Co合金, 这与XRD表征结果一致。对纳米晶粒所暴露的高指数晶面进行原子建模, 如图2(g~i)所示, 从图中可以看出这三种高指数晶面上的原子呈台阶状排列, 与典型的基础晶面原子排列相比, 台阶状排列的原子可以暴露更多的Pt原子参与催化反应, 提高Pt原子的利用率, 增大晶粒的活性表面积, 并作为活性位点催化氧化反应[20-21]。
图2
图2
Pt1Co1/3/C高指数晶面纳米催化剂的表面形貌
Fig. 2
Surface morphologies of Pt1Co1/3/C high-index crystalline nanocatalyst
(a) TEM and (b) HRTEM images; (c) SAED image; (d-f) EDS surface sweep mapping images; (g-i) High-index crystalline atomic model for Pt1Co1/3/C high-index crystalline nanocatalysts; Colorful spheres in (g-i) represent different layers of atoms
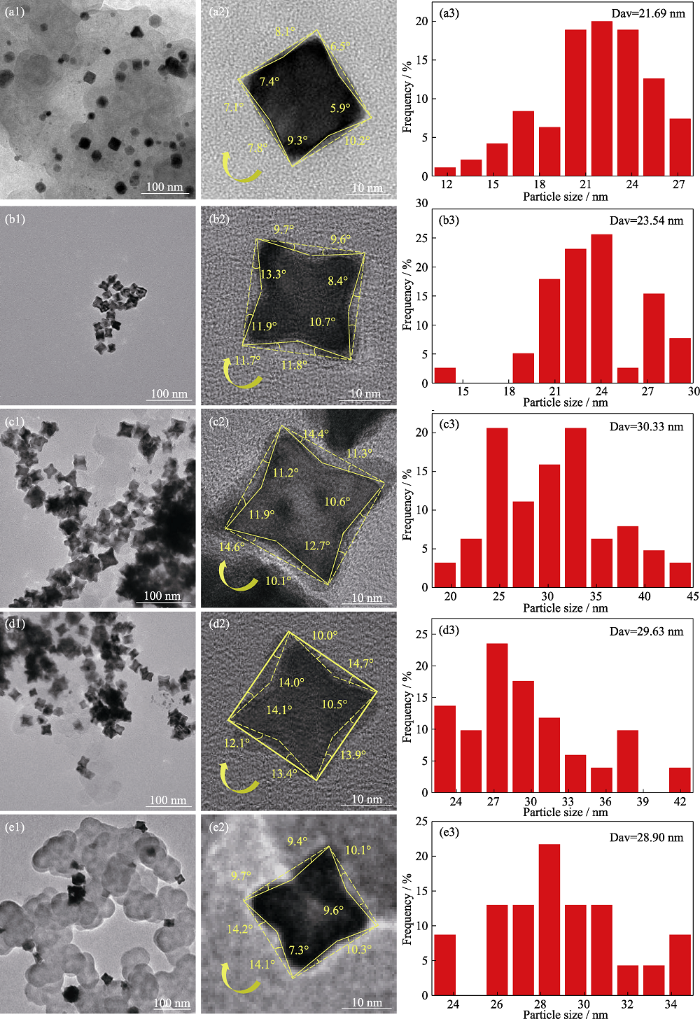
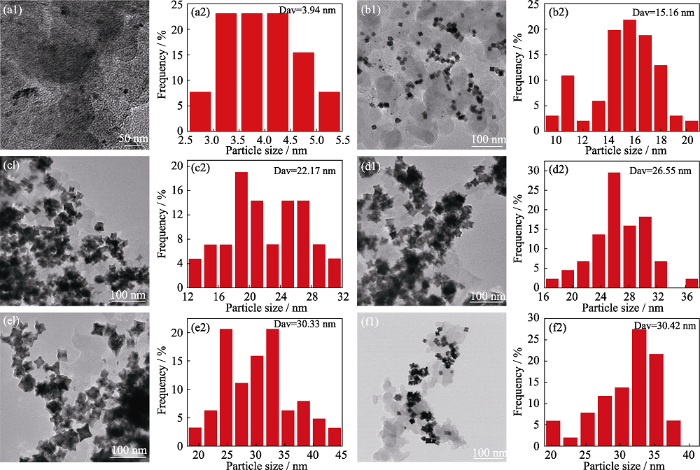
调控Pt与Co摩尔比制备Pt1Cox/C高指数晶面催化剂, 分别随机选取100个纳米晶粒进行粒径统计(图S1)。Pt1Cox/C催化剂晶粒均出现了内凹形貌的催化剂晶粒, 如表S1所示。结合图S1与表S1可以发现, 未掺杂Co的催化剂晶粒中具有高指数晶面内凹形貌的选择性较低, 平均粒径为21.69 nm, 主要暴露(610)、(710)和(810)晶面。掺杂Co元素后, 晶体中内凹形貌的选择性逐渐增大, 凹陷角度略有增加, 当n(Pt) : n(Co)=1 : 1/4时, 主要暴露晶面(410)、(510)、(610)和(710), 粒径略有增大, 平均粒径为23.54 nm。当n(Pt) : n(Co)=1 : 1/3时, 主要暴露的晶面有(410)、(510)、(610), 粒径继续增大, 平均粒径为30.33 nm。当n(Pt) : n(Co)=1 : 1/2时, 形貌选择性略有降低, 主要暴露的晶面基本与Pt1Co1/3/C一致, 平均粒径为29.63 nm。当Co元素掺杂量增大到n(Pt) : n(Co)=1 : 1时, 催化剂晶粒内凹形貌的选择性大幅度下降, 只有少部分保持内凹形貌, 主要暴露的晶面为(410)、(610)、(810), 且晶粒大小不均, 平均粒径为28.90 nm。这是由于原子尺寸较小的Co取代了Pt晶格上的原子, 晶格发生压缩畸变, 产生压缩应变效应, 周围Pt-Pt原子间距离缩短, 导致Pt表面电子结构发生变化, 进而影响合金晶粒的形貌结构[22]。这说明在该合成体系中, 内凹形貌的Pt1Cox/C二元合金高指数晶面纳米催化剂的合成机理与金属前驱体的摩尔比具有一定关系。此外, 与文献[23]相比, 本研究合成的原位负载的高指数晶面纳米合金催化剂的晶粒进一步减小, 有利于提高催化剂的电催化性能。
图S1
图S1
Pt1Cox/C高指数晶面纳米催化剂TEM、HRTEM照片及粒径分布直方图
Fig. S1
TEM, HRTEM images and histograms of particle size distributions for Pt1Cox/C high index crystalline nanocatalysts
(a1-a3) Pt/C; (b1-b3) Pt1Co1/4/C; (c1-c3) Pt1Co1/3/C; (d1-d3) Pt1Co1/2/C; (e1-e3) Pt1Co1/C
表S1 Pt1Cox/C高指数晶面催化剂的凹陷角度与暴露晶面
Table 1
| Catalyst | Angle of depression/(°) | Exposure of crystalline surfaces |
|---|---|---|
| Pt/C | 8.1, 6.5, 5.9, 10.2, 9.3, 7.8, 7.1, 7.4 | (610), (710), (810) |
| Pt1Co1/4/C | 9.7, 9.6, 8.4, 10.7, 11.8, 11.7, 11.9, 13.3 | (410), (510), (610), (710) |
| Pt1Co1/3/C | 14.4, 11.3, 10.6, 12.7, 10.1, 14.6, 11.9, 11.2 | (410), (510), (610) |
| Pt1Co1/2/C | 10.0, 14.7, 10.5, 13.9, 13.4, 12.1, 14.1, 14.0 | (410), (510), (610) |
| Pt1Co1/C | 9.4, 10.1, 9.6, 10.3, 7.3, 14.1, 14.2, 9.7 | (410), (610), (810) |
2.3 Pt1Cox/C高指数晶面催化剂的元素组成和化学价态变化
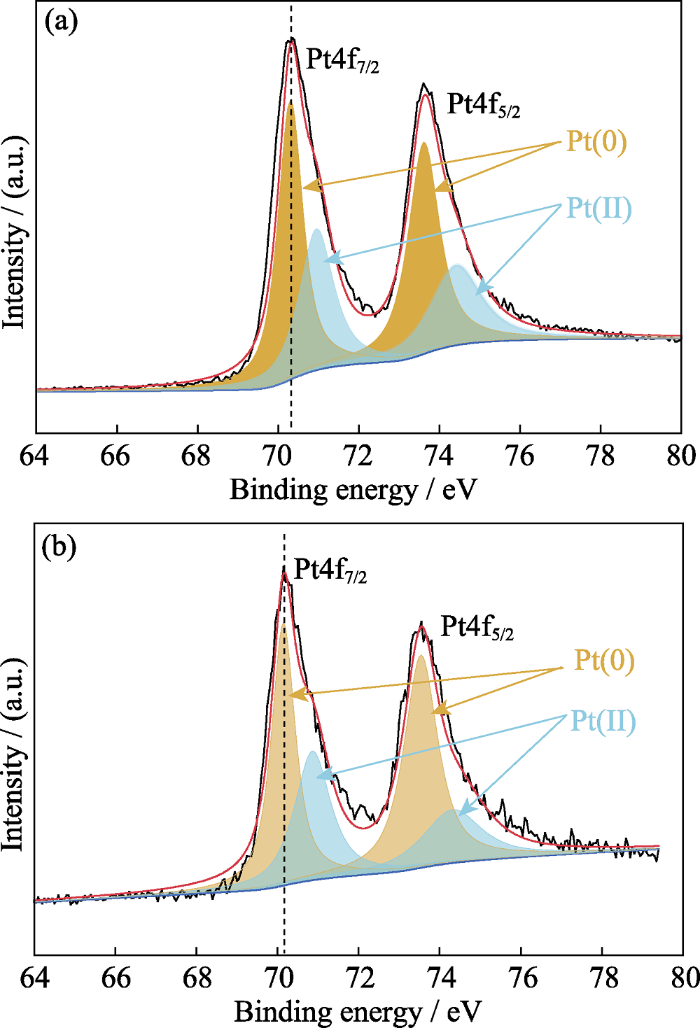
合金化可以改善直接乙醇燃料电池Pt基催化剂的性能。金属Pt与过渡金属形成的合金会导致d带中心发生偏移, d带中心相对于费米能级的位置与催化剂催化氧化反应过程有直接关系[24]。为了进一步探究掺杂Co催化剂的表面电子结构的变化, 对Pt/C和Pt1Co1/3/C高指数晶面纳米催化剂进行XPS测试, 如图3所示, 拟合数据如表1所示。由表1可知, 在所制备的催化剂中Pt主要以金属态的形式存在。Pt/C和Pt1Co1/3/C催化剂的Pt4f XPS图谱中, Pt存在Pt(0)和Pt(Ⅱ)两种状态。在Pt/C高指数晶面纳米催化剂的Pt4f XPS光谱图(图 3(a))分别在70.30 (Pt4f7/2)和73.60 eV (Pt4f5/2)出现了归属于Pt金属态的2个结合能峰。催化剂掺杂Co后(图3(b)), 其金属态的2个结合能峰分别为70.15 (Pt4f7/2)和73.55 eV (Pt4f5/2), Pt4f峰向低结合能方向发生偏移, 表明Pt表层的电子结构受到下层中Co的影响。
图3
图3
(a)Pt/C和(b)Pt1Co1/3/C催化剂的Pt4f XPS图谱
Fig. 3
Pt4f XPS spectra of (a) Pt/C and (b) Pt1Co1/3/C
表1 Pt/C与Pt1Co1/3/C催化剂的XPS拟合结果
Table 1
| Catalyst | Pt(0)/eV | Relative ratio/% | Pt(II)/eV | Relative ratio/% |
|---|---|---|---|---|
| Pt/C | 70.30,73.60 | 52.67 | 70.95,74.45 | 47.33 |
| Pt1Co1/3/C | 70.15,73.55 | 53.04 | 70.85,74.35 | 46.96 |
2.4 Pt1Cox/C高指数晶面催化剂形貌生长规律
为了研究铂钴高指数晶面纳米催化剂的生长和形貌的变化过程, 对不同保温时间节点的Pt1Co1/3/C纳米晶粒进行TEM形貌观察及粒径统计, 如图S2所示。在该水热体系中, 保温1 h的Pt1Co1/3/C纳米晶粒形核生长优先呈现热力学稳定的“类球体”形貌, 这些小晶粒的平均粒径在3.94 nm左右。随着生长过程进行, 保温3 h的Pt1Co1/3/C的大部分纳米晶粒由“类球体”生长为立方块形貌, 晶粒明显增大, 平均粒径为15.16 nm。反应体系的保温时间继续延长到5 h, 由于立方块的楞和顶点的生长速度超过面的生长速度, Pt1Co1/3/C立方块开始出现凹陷, 并且粒径进一步增大, 平均粒径为22.17 nm。随着保温时间延长, 催化剂晶粒继续生长, 凹陷程度略有增大, 保温9 h的Pt1Co1/3/C纳米晶粒的平均尺寸为30.33 nm左右, 且催化剂晶粒整体形貌选择性较高。保温时间继续延长至10 h, Pt1Co1/3/C纳米晶粒形貌结构与粒径变化不大, 说明保温9 h的Pt1Co1/3/C高指数晶面纳米催化剂就已经完成生长。甘氨酸对晶体表面控制的机理可能是由于甘氨酸分子的两个可电离的氨基和羧基功能团与Pt前驱体发生配位作用, 形成的Gly-Pt复合物附着于载体表面形核生长。随着Pt-Co合金晶粒生长时间延长, 这种配位作用使金属原子在晶体楞和角上的沉积速率超过面上的沉积速率, 影响原子排列, 进而形成内凹的高指数晶面[23,26-27]。
图S2
图S2
Pt1Co1/3/C高指数晶面催化剂在不同保温时间的TEM照片及粒径分布直方图
Fig. S2
TEM images and histograms of particle size distributions of Pt1Co1/3/C high index crystalline catalysts at different holding time (a1, a2) 1 h; (b1, b2) 3 h; (c1, c2) 5h; (d1, d2) 7 h; (e1, e2) 9 h; (f1, f2) 10 h
2.5 Pt1Cox/C高指数晶面催化剂的电催化性能
图4
图4
催化剂的电催化性能
Fig. 4
Electrocatalytic performance of catalysts
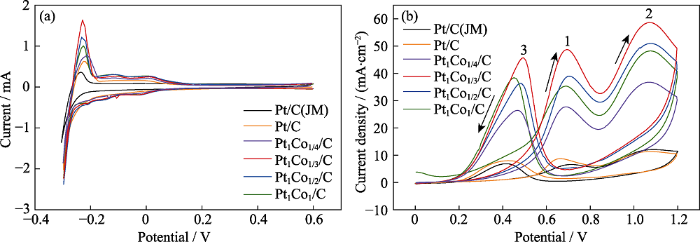
(a) H adsorption-desorption curves of the catalysts in 0.5 mol/L H2SO4 saturated with N2; (b) Cyclic voltammetric curves of the catalysts in 0.5 mol/L H2SO4+1 mol/L CH3CH2OH
Colorful figures are available on website
其中, ECSA为电化学活性表面积, m2/g;Q为H吸附时的电量, C;[Pt]为电极的载Pt量, g;s为吸附峰的面积, m2;v为扫描速度, 0.05 V/s;单位表面积Pt吸附H所需的电量为0.21 mC/cm2。
图4(a)中6组催化剂在Pt电位上均出现H的电化学吸-脱附峰。正向扫描时, 电位在-0.2~-0.25 V之间出现氢的氧化峰;负向扫描时, 在-0.3 V附近出现氢的还原峰。这说明氢的吸-脱附反应是一个可逆的过程。根据式(1)和式(2)计算, Pt/C(JM)(30%)、Pt/C、Pt1Co1/4/C、Pt1Co1/3/C、Pt1Co1/2/C和Pt1Co1/C催化剂的ECSA分别为7.67、9.76、13.46、18.46、16.92 和15.74 m2/g。与 Pt/C(JM)相比, 具有高指数晶面取向的Pt1Cox/C催化剂的ECSA较大, 这是因为Pt/C(JM)的微观呈基础晶面取向的球形, 而具有高指数晶面取向的催化剂的晶体表面存在台阶和扭结原子, 为催化反应提供了大量的活性位点, 并且高指数晶面原子的配位数较低。配位数越低的原子越倾向于与反应物质结合, 越有利于化学键断裂(如C-C、C-H键), 从而提高催化剂的催化活性[20-21]。掺杂Co后, 各组Pt1Cox/C催化剂与Pt/C催化剂相比, ECSA均有所提高, 这归因于二元催化剂成分之间的相互作用。此外, 随着Co含量增大, 催化剂的ECSA先增大后减小, 在n(Pt):n(Co)=1:1/3条件下催化剂的ECSA达到最大。
图4(b)为6组催化剂在0.5 mol/L H2SO4+ 1 mol/L CH3CH2OH溶液中的循环伏安曲线, 由图可知, 催化剂的两个氧化峰(峰1、峰2)出现在正向扫描过程中, 并在负向扫描时均重新出现了一个氧化峰(峰3)。在0.6~0.8 V电位区间出现的氧化峰(峰1), 主要对应乙醇完全氧化生成CO2的反应。随着电位增大, 在0.9~1.2 V区间内, 氧化峰再次出现, 对应于部分乙醇被氧化生成乙酸、乙醛。另外在该电位区间内, Pt也会被氧化成“Pt-O”物种[29-30]。电位负向扫描时, 氧化物种“Pt-O”会通过还原反应重新生成Pt, 其表面的活性位点重新暴露, 从而再次出现一个对应乙醇氧化的电流密度峰[31]。由于峰1对应于乙醇完全氧化生成CO2的过程, 因此催化剂的催化性能可以用峰1的电流密度来衡量。图4(b)中, Pt/C(JM)、Pt/C、Pt1Co1/4/C、Pt1Co1/3/C、Pt1Co1/2/C和Pt1Co1/C催化剂对乙醇氧化的峰电流密度分别为6.55、8.69、27.71、48.70、38.92和35.28 mA/cm2, 其中Pt1Co1/3/C催化剂的峰电流密度最大。一方面Pt1Co1/3/C纳米催化剂晶粒为高指数晶面取向, 相对于低指数的基础晶面, 其表面的台阶、扭结等缺陷位原子可作为吸附和反应的活性位点, 促进催化反应进行。另一方面, 乙醇的电氧化过程涉及乙醇脱氢、键断裂、氧化和催化剂表面Pt原子的吸脱附过程, 掺入Co之后, 两种组分之间的“相互作用”有利于提高催化剂的电催化性能[15]。
2.6 Pt1Cox/C高指数晶面催化剂的稳定性及抗中毒性
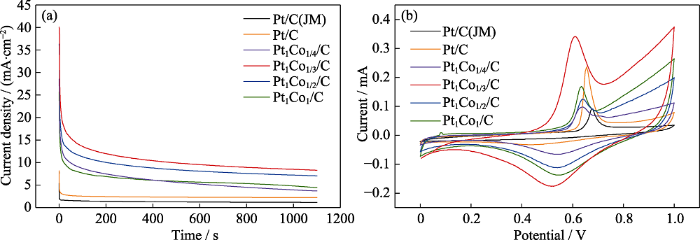
图5(a)为6组催化剂在0.5 mol/L H2SO4+ 1 mol/L CH3CH2OH溶液中的计时电流曲线。各组催化剂在最开始的100 s极化时间内, 电流密度急剧下降, 这是由乙醇电氧化过程中产生的中间产物“COads”使催化剂中毒所致。之后, 随着极化时间延长, 曲线显示出一定电流衰减现象, 当Pt基催化剂表面的活性位点上中间产物的累积和去除达到相对平衡时, 电流曲线则逐渐趋于稳定平缓, 该时间的电流密度可以作为判断催化剂稳定性及抗中毒能力的标准。经过1100 s稳定性测试, Pt/C(JM)、Pt/C、Pt1Co1/4/C、Pt1Co1/3/C、Pt1Co1/2/C和Pt1Co1/C催化剂的稳态电流密度分别为1.17、2.23、3.78、8.29、7.06和4.45 mA/cm2。Pt1Co1/3/C高指数晶面纳米催化剂的稳态电流密度最高, 稳定性最佳, 这与图4的电化学测试结果一致。
图5
图5
催化剂的稳定性和抗中毒性能
Fig. 5
Stability and anti-poisoning performance of catalysts
(a) Timing current curves of the catalysts in 0.5 mol/L H2SO4+1 mol/L CH3CH2OH; (b) CO dissolution curves of catalysts in 0.5 mol/L H2SO4
Colorful figures are available on website
为了研究Pt1Cox/C高指数晶面纳米催化剂的抗中毒性, 对各组催化剂进行CO溶出测试, 测试结果如图5(b)所示。由图可知, Pt/C(JM)、Pt/C、Pt1Co1/4/C、Pt1Co1/3/C、Pt1Co1/2/C和Pt1Co1/C催化剂的CO氧化峰电位分别为0.675、0.655、0.645、0.610、0.635和0.640 V。在催化剂的催化氧化过程中, CO氧化峰的电位越低, 则催化剂的抗CO中毒能力越强[32⇓-34]。经过比较可知, 具有高指数晶面取向的Pt1Cox/C催化剂具有优异的抗CO中毒能力。一方面, 与Pt/C(JM)相比, Pt1Cox/C具有独特的高指数晶面取向的表面结构, 有利于催化剂抗CO中毒;另一方面, 受到电负性的影响, CO通过“C”端以桥式或线性等多种方式吸附于纯铂表面, 而掺杂金属Co可以使CO只能以线性吸附的方式与Pt原子结合[35-36], 大大减小了CO在Pt表面的吸附量, 从而提高了催化剂的抗CO中毒能力。其中, Pt1Co1/3/C催化剂的抗CO中毒能力最优异。
3 结论
1)通过简单的水热法, 在XC-72R炭黑载体上一步制备Pt1Cox/C纳米催化剂, 对晶面进行解析后发现Pt1Cox/C纳米催化剂暴露的晶面主要包括(410)、(510)、(610)、(710)和(810)晶面, 实现了具有高指数晶面取向的纳米晶粒在碳载体上的原位生长。
2)调节金属前驱体的摩尔比, 控制Pt1Cox/C高指数晶面纳米催化剂的形貌选择性。当Pt与Co摩尔比为1 : 1/3时, Pt1Co1/3/C纳米催化剂的形貌选择性最高, 暴露的晶面包括(410)、(510)、(610)晶面。Pt、Co之间的相互作用使Pt1Co1/3/C高指数晶面纳米催化剂对乙醇具有优异的电催化氧化性能。
3)通过对催化剂晶粒生长过程的研究发现, 合成时间是影响催化剂生长规律的关键因素。在反应初期, Pt1Co1/3/C纳米催化剂的纳米晶粒优先呈热力学稳定的“类球体”形貌;然后, 纳米晶粒由“类球体”逐渐生长成立方块形貌;接着立方块开始产生凹陷, 最终形成了具有内凹形貌的Pt1Co1/3/C高指数晶面纳米晶粒。
补充材料
本文相关补充材料可登陆
参考文献
Concave platinum-copper octopod nanoframes bounded with multiple high-index facets for efficient electrooxidation catalysis
Multimetallic nanoframes with three-dimensional (3D) catalytic surfaces represent an emerging class of efficient nanocatalysts. However, it still remains a challenge in engineering nanoframes via simple and economical methods. Herein, we report a facile one-pot synthetic strategy to synthesize Pt-Cu nanoframes bounded with multiple high-index facets as highly active electrooxidation catalysts. Two distinct octopod nanoframes, namely, concave PtCu octopod nanoframes (PtCu CONFs) and ultrathin PtCu octopod nanoframes (PtCu UONFs) were successfully synthesized by simply changing the feeding Pt and Cu precursors. Interestingly, the PtCu CONFs are constructed by eight symmetric feet with sharp tips, which are enclosed by high-index facets of n (111)-(111), such as {553}, {331}, and {221}. Benefiting from their 3D accessible surfaces and multiple high-index facets, the self-supported PtCu CONFs catalysts exhibit excellent electrocatalytic performance and superior CO-tolerant ability. For methanol oxidation reaction, the PtCu CONFs catalysts exhibit more than 7-fold increase in activities, 205 mV lower in the onset potential compared with commercial Pt/C. More importantly, when facing harsh electrochemical reaction conditions, the PtCu CONFs are well-preserved in the catalytic activities, architectural features, and stepped surfaces. The PtCu UONFs with 12 ultrathin edges, however, suffer from breakdown. The present work provides guidelines for the rational design and synthesis of nanoframe catalysts with both high activity and stability.
Hexapod Pt RuCu nanocrystalline alloy for highly efficient and stablemethanol oxidation
Simultaneous formation of trimetallic Pt-Ni-Cu excavated rhombicdodecahedrons with enhanced catalytic performance for the methanol oxidation reaction
One-pot synthesis of concave platinum-cobalt nanocrystals and their superior catalytic performances for methanol electrochemical oxidation and oxygen electrochemical reduction
Surface and near-surface engineering of PtCo nanowires at atomic scale for enhanced electrochemical sensing and catalysis
Laser-irradiation induced synthesis of spongy AuAgPt alloy nanospheres with high-index facets, rich grain boundaries and subtle lattice distortion for enhanced electrocatalytic activity
Solvothermal synthesis of alloyed PtNi colloidal nanocrystal clusters (CNCs) with enhanced catalytic activity for methanol oxidation
Synthesis and property of platinum- cobalt alloy nano catalyst
Crystal phase and architecture engineering of lotus-thalamus-shaped Pt-Ni anisotropic superstructures for highly efficient electrochemical hydrogen evolution
Atomic-scale preparation of octopod nanoframes with high-index facets as highly active and stable catalysts
Stable high-index faceted Pt skin on zigzag-like Pt Fe nanowires enhances oxygen reduction catalysis
Atomic layer-by-layer deposition of Pt on Pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction
An effective strategy for reducing the Pt content while retaining the activity of a Pt-based catalyst is to deposit the Pt atoms as ultrathin skins of only a few atomic layers thick on nanoscale substrates made of another metal. During deposition, however, the Pt atoms often take an island growth mode because of a strong bonding between Pt atoms. Here we report a versatile route to the conformal deposition of Pt as uniform, ultrathin shells on Pd nanocubes in a solution phase. The introduction of the Pt precursor at a relatively slow rate and high temperature allowed the deposited Pt atoms to spread across the entire surface of a Pd nanocube to generate a uniform shell. The thickness of the Pt shell could be controlled from one to six atomic layers by varying the amount of Pt precursor added into the system. Compared to a commercial Pt/C catalyst, the Pd@PtnL (n = 1-6) core-shell nanocubes showed enhancements in specific activity and durability toward the oxygen reduction reaction (ORR). Density functional theory (DFT) calculations on model (100) surfaces suggest that the enhancement in specific activity can be attributed to the weakening of OH binding through ligand and strain effects, which, in turn, increases the rate of OH hydrogenation. A volcano-type relationship between the ORR specific activity and the number of Pt atomic layers was derived, in good agreement with the experimental results. Both theoretical and experimental studies indicate that the ORR specific activity was maximized for the catalysts based on Pd@Pt2-3L nanocubes. Because of the reduction in Pt content used and the enhancement in specific activity, the Pd@Pt1L nanocubes showed a Pt mass activity with almost three-fold enhancement relative to the Pt/C catalyst.
Research progress on advanced carbon materials as Pt support for proton exchange membrane fuel cells
Proton Exchange Membrane Fuel Cell (PEMFC) has the characteristics of high energy conversion efficiency, high power density, fast start-up at room temperature, low noise and zero pollution, which is expected to alleviate the energy crisis and reduce carbon dioxide emissions. It has broad application prospects in rail transit, aerospace and other fields. Catalyst is one of the key materials of PEMFC. Moreover, Pt catalysts are widely used and considered difficult to be replaced because of their good activity and stability in oxygen reduction reaction. Pt is expensive because of its limited storage. However, Pt loading could be significantly lessened by Pt support to improve PEMFC utilization. Carbon materials are widely used as Pt supports because of their low cost, high specific surface area, pore structure, adjustable conductivity and surface properties, but commercial carbon black supports have low utilization efficiency and poor electrochemical corrosion resistance for Pt. For realizing the large-scale application of PEMFC, it is necessary to develop new carbon supports which can uniformly disperse Pt, efficiently utilize Pt, be resistant to electrochemical corrosion, and have good conductivity, thus the performance and sustainability of PEMFC are improved. Carbon aerogels, carbon nanotubes, graphene and other new carbon supports with unique structures and properties, which are expected to improve PEMFC performance and life, have attracted the attention of many researchers. In this paper, the research progress on new carbon material as Pt support for PEMFC in recent years is reviewed systematically, and the development trend is also commented appropriately.
Concave cubic gold nanocrystals with high-index facets
A new class of gold nanostructures, concave nanocubes, enclosed by 24 high-index {720} facets, have been prepared in a monodisperse fashion by a modified seed-mediated synthetic method. The Cl(-) counterion in the surfactant plays an essential role in controlling the concave morphology of the final product. The concave nanocubes exhibit higher chemical activities compared with low-index {111}-faceted octahedra.
Graphene oxide-assisted synthesis of Pt-Co alloy nanocrystals with high-index facets and enhanced electrocatalytic properties
Metal nanocrystals (NCs) are grown directly on the surface of reduced graphene oxide (rGO), which can maximize the rGO-NCs contact/interaction to achieve the enhanced catalytic activity. However, it is difficult to control the size and morphology of metal NCs by in situ method due to the effects of functional groups on the surface of GO, and as a result, the metal NCs/rGO hybrids are conventionally synthesized by two-step method. Herein, one-pot synthesis of Pt-Co alloy NCs is demonstrated with concave-polyhedrons and concave-nanocubes bounded by {hkl} and {hk0} high-index facets (HIFs) distributed on rGO. GO can affect the geometry and electronic structure of Pt-Co NCs. Thanks to the synergy of the HIFs and the electronic effect of the intimate contact/interaction between Pt-Co alloy and rGO, these as-prepared Pt-Co NCs/rGO hybrids presents enhanced catalytic properties for the electrooxidation of formic acid, as well as for the oxygen reduction reaction. © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Pt-group bimetallic nanocrystals with high-index facets as high performance electrocatalysts
Bimetallic nanocrystals bound by high-index facets are promising catalysts, as they have both electronic effects from alloying and surface structure effects from high-index facets. Herein, we mainly focused on electrochemical preparation of two new Pt-Rh nanocrystals with high-index facets: {830}-bound tetrahexahedron and {311}-bound trapezohedron, and their excellent electrocatalytic properties for ethanol oxidation, especially the ability to break C-C bonds. Combining previous results about surface-modified tetrahexahedral Pt nanocrystals, we discuss the correlation of alloy and surface effects of the bimetallic system.
Fe-Ni-Mo nitride porous nanotubes for full water splitting and Zn-air batteries
Tuning Pt-skinned PtAg nanotubes in nanoscales to efficiently modify electronic structure for boosting performance of methanol electrooxidation
The in situ etching assisted synthesis of Pt-Fe-Mn ternary alloys with high-index facets as efficient catalysts for electro-oxidation reactions
Fluctuations and bistabilities on catalyst nanoparticles
We show that coverage fluctuations on catalyst particles can drastically alter their macroscopic catalytic behavior. Scrutinizing the occurrence of kinetic bistabilities, it is demonstrated by molecular beam experiments on model catalysts that macroscopically observable bistabilities vanish completely with decreasing particle size, as previously predicted by theory. The effect is attributed to fluctuation-induced transitions between two kinetic reaction regimes, with a transition rate controlled by both particle size and surface defects. These results suggest that fluctuation-induced effects represent a general phenomenon affecting the reaction kinetics on nanostructured surfaces.
Ammonia synthesis from first-principles calculations
The rate of ammonia synthesis over a nanoparticle ruthenium catalyst can be calculated directly on the basis of a quantum chemical treatment of the problem using density functional theory. We compared the results to measured rates over a ruthenium catalyst supported on magnesium aluminum spinel. When the size distribution of ruthenium particles measured by transmission electron microscopy was used as the link between the catalyst material and the theoretical treatment, the calculated rate was within a factor of 3 to 20 of the experimental rate. This offers hope for computer-based methods in the search for catalysts.
Recent advances in electrocatalysts for oxygen reduction reaction
The recent advances in electrocatalysis for oxygen reduction reaction (ORR) for proton exchange membrane fuel cells (PEMFCs) are thoroughly reviewed. This comprehensive Review focuses on the low- and non-platinum electrocatalysts including advanced platinum alloys, core-shell structures, palladium-based catalysts, metal oxides and chalcogenides, carbon-based non-noble metal catalysts, and metal-free catalysts. The recent development of ORR electrocatalysts with novel structures and compositions is highlighted. The understandings of the correlation between the activity and the shape, size, composition, and synthesis method are summarized. For the carbon-based materials, their performance and stability in fuel cells and comparisons with those of platinum are documented. The research directions as well as perspectives on the further development of more active and less expensive electrocatalysts are provided.
Glycine-mediated syntheses of Pt concave nanocubes with high-index {hk0} facets and their enhanced electrocatalytic activities
Three-dimensional Pt-on-Pd bimetallic nanodendrites supported on graphene nanosheet: facile synthesis and used as an advanced nanoelectrocatalyst for methanol oxidation
Graphene nanosheet, the hottest material in physics and materials science, has been studied extensively because of its unique electronic, thermal, mechanical, and chemical properties arising from its strictly 2D structure and because of its potential technical applications. Particularly, these remarkable characteristics enable it to be a promising candidate as a new 2D support to load metal nanoparticles (NPs) for application in fuel cells. However, constructing high-quality graphene/bimetallic NP hybrids with high electrochemical surface area (ECSA) remains a great challenge to date. In this paper, we demonstrate for the first time a wet-chemical approach for the synthesis of high-quality three-dimensional (3D) Pt-on-Pd bimetallic nanodendrites supported on graphene nanosheets (TP-BNGN), which represents a new type of graphene/metal heterostructure. The resulting hybrids were characterized by atomic force microscopy (AFM), transmission electron microscopy (TEM), high-resolution TEM (HRTEM), energy-dispersive X-ray (EDX) spectroscopy, X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), Raman spectroscopy, and electrochemical technique. It is found that small single-crystal Pt nanobranches supported on Pd NCs with porous structure and good dispersion were directly grown onto the surface of graphene nanosheets, which exhibits high electrochemical active area. Furthermore, the number of nanobranches for Pt-on-Pd bimetallic nanodendrites on the surface of graphene nanosheets could be easily controlled via simply changing the synthetic parameters, thus resulting in the tunable catalytic properties. Most importantly, the electrochemical data indicate that the as-prepared graphene/bimetallic nanodendrite hybrids exhibited much higher electrocatalytic activity toward methanol oxidation reaction than the platinum black (PB) and commercial E-TEK Pt/C catalysts.
Structural, chemical, and electronic properties of Pt/Ni thin film electrodes for methanol electrooxidation
High performance electrocatalyst: Pt-Cu hollow nanocrystals
Synergistic effect between undercoordinated platinum atoms and defective nickel hydroxide on enhanced hydrogen evolution reaction in alkaline solution
Effect of acid treatment on electrocatalytic performance of PtNi catalyst
Roughening of Pt nanoparticles induced by surface-oxide formation
Using density functional theory (DFT) and thermodynamic considerations we studied the equilibrium shape of Pt nanoparticles (NPs) under electrochemical conditions. We found that at very high oxygen coverage, obtained at high electrode potentials, the experimentally-observed tetrahexahedral (THH) NPs consist of high-index (520) faces. Since high-index surfaces often show higher (electro-)chemical activity in comparison to their close-packed counterparts, the THH NPs can be promising candidates for various (electro-)catalytic applications.
Platinum concave nanocubes with high-index facets and their enhanced activity for oxygen reduction reaction
Pt-Cu alloy with high density of surface Pt defects for efficient catalysis of breaking C-C bond in ethanol
Electronic metal-support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction
Tuning metal-support interaction has been considered as an effective approach to modulate the electronic structure and catalytic activity of supported metal catalysts. At the atomic level, the understanding of the structure-activity relationship still remains obscure in heterogeneous catalysis, such as the conversion of water (alkaline) or hydronium ions (acid) to hydrogen (hydrogen evolution reaction, HER). Here, we reveal that the fine control over the oxidation states of single-atom Pt catalysts through electronic metal-support interaction significantly modulates the catalytic activities in either acidic or alkaline HER. Combined with detailed spectroscopic and electrochemical characterizations, the structure-activity relationship is established by correlating the acidic/alkaline HER activity with the average oxidation state of single-atom Pt and the Pt-H/Pt-OH interaction. This study sheds light on the atomic-level mechanistic understanding of acidic and alkaline HER, and further provides guidelines for the rational design of high-performance single-atom catalysts.
Optimizing alkyne hydrogenation performance of Pd on carbon in situ decorated with oxygen-deficient TiO2 by integrating the reaction and diffusion
Neighboring Pt atom sites in ultrathin FePt nanosheet for efficient and highly CO-tolerant oxygen reduction reaction
DFT studies on Pt3M (M=Pt, Ni, Mo, Ru, Pd, Rh) clusters for CO oxidation