硅酸盐生物陶瓷材料由硅、氧及其他生物活性元素组成, 在皮肤修复领域展现出广阔前景[10]。硅酸盐生物陶瓷材料由于生物降解而缓慢释放的硅离子及其他活性离子可促进皮肤等软组织修复[11⇓-13]。例如, 硅酸钙(Calcium Silicate, CS)是最常见的二元硅酸盐生物陶瓷, 其降解释放的钙、硅离子对血管内皮细胞(Human Umbilicle Vein Endothelial Cells, HUVECs)和成纤维细胞(Human Dermal Fibroblasts, HDFs)的增殖、迁移以及蛋白质和生长因子的表达有促进作用[14-15]。Tian等[16]用CS粉体的浸提液培养HUVECs, 发现当CS的浓度为25 mg/mL时, 其浸提液能明显促进HUVECs增殖; 当CS的浓度为0.195~ 0.78 mg/mL时, 其浸提液能明显促进HUVECs的血管内皮生长因子表达。
本研究以具有高比表面积的水合硅酸钙纳米线(Ca6Si6O17(OH)2, CSH)为基体材料、NaCl和KCl为熔盐介质、CuSO4·5H2O为铜金属前驱体, 采用熔盐法制备Cu-CS纳米棒, 再将其复合SA得到Cu-CS纳米棒复合水凝胶(Cu-CS/SA)。详细研究了铜盐添加量和熔盐处理温度对Cu-CS纳米棒催化性能的影响, 探究了Cu-CS/SA水凝胶的生物相容性以及化学动力学性能, 进一步评估了Cu-CS/SA水凝胶分别与HUVECs和HDFs培养时对细胞增殖和迁移的影响。
1 实验方法
1.1 实验药品
四水合硝酸钙(Ca(NO3)2·4H2O, ≥98.5%)、九水合硅酸钠(Na2SiO3·9H2O, AR)、氯化钙(CaCl2, ≥96.0%)、氯化钠(NaCl, ≥99.5%)、氯化钾(KCl, ≥99.8%)、五水合硫酸铜(CuSO4·5H2O, ≥99.0%)均购于中国医药集团上海化学试剂有限公司, 海藻酸钠(SA)购于阿法埃莎化学(中国)有限公司, 2ʹ,7ʹ-二氯二氢荧光素二乙酸酯(DCFH-DA)购于上海碧云天生物科技有限公司。所有试剂在使用前未经过进一步提纯处理。
1.2 水合硅酸钙纳米线的合成
研究采用水热法合成水合硅酸钙纳米线(Ca6Si6O17(OH)2, CSH)[29]。首先, 将11.368 g的Na2SiO3·9H2O和9.446 g的Ca(NO3)2·4H2O分别溶于100 mL去离子水, 常温下磁力搅拌1 h; 接着, 将上述两种溶液混合均匀后转移至水热釜, 200 ℃下反应24 h; 待水热釜自然冷却后, 将白色沉淀物用去离子水和乙醇分别洗涤三次, 在60 ℃烘箱中烘干, 得到CSH纳米线。
1.3 Cu-CS纳米棒的制备
熔盐法制备Cu-CS纳米棒的具体实验步骤如下: 首先, 将0.585 g NaCl、0.746 g KCl、1 g CSH分别与0、5、10、30、50 mg的CuSO4·5H2O混合, 在玛瑙研钵中研磨40 min, 使粉体混合均匀; 接着, 将研磨均匀的粉体转移至坩埚, 再置于管式炉中, 在氩气气氛中以5 ℃/min的升温速度加热至700 ℃, 保温1 h, 冷却至室温后将坩埚取出; 然后, 用去离子水洗涤混合物五次, 除去可溶性盐; 最后, 将洗涤后的粉体在60 ℃的烘箱中干燥24 h, 得到不同铜含量的硅酸钙纳米棒, 并分别命名为CS、0.5Cu-CS、1Cu-CS、3Cu-CS和5Cu-CS。
1.4 Cu-CS纳米棒复合水凝胶的制备
制备Cu-CS纳米棒复合水凝胶的具体实验步骤如下: 首先, 将1.5 g的海藻酸钠(SA)加入100 mL去离子水中, 磁力搅拌24 h, 得到质量分数1.5%的SA溶液; 然后, 分别将0、0.75、1.5、3、6 mg的3Cu-CS和3 mg的CS纳米棒加入到1 g含质量分数1.5% SA溶液中, 搅拌均匀; 最后, 用质量分数3%的CaCl2溶液交联24 h, 得到成型水凝胶, 并根据纳米棒与SA的质量比, 将不同水凝胶分别命名为SA、5%-Cu-CS/SA、10%-Cu-CS/SA、20%-Cu-CS/SA、40%-Cu-CS/ SA和20%-CS/SA。
1.5 结构与理化性能表征
用18 kW靶向X射线衍射仪(X-ray Diffraction, XRD, D8Advance, Bruker, 德国)分析粉体的物相组成; 使用场发射扫描电子显微镜(Scanning Electron Microscope, SEM, S-4800, Hitachi, 日本)观察粉体和水凝胶的微观形貌; 利用透射电子显微镜(Transmission Electron Microscope, TEM, JEM-2100F, 日本)观察粉体的形貌和元素分布。采用电感耦合等离子体原子发射光谱(Inductively Coupled Plasma Atomic Emission Spectrometry, ICP-AES, Varian 715- ES, 美国)法测定纳米棒中Cu含量和水凝胶释放的Ca、Si、Cu离子; 采用X射线光电子能谱仪(X-ray Photoelectron Spectrometer, XPS, ESCAlab250, Thermo Fisher Scientific, 美国)测定纳米棒中Cu元素的价态。采用多功能酶标仪(SpectraFluor Plus, Tecan, Crailsheim, 德国)测试紫外-可见光吸收光谱, 用于分析纳米棒和水凝胶的化学动力学性能。
1.6 水凝胶离子释放测试
研究采用ICP-AES方法测定水凝胶释放的Ca、Si、Cu离子。将 SA、20%-CS/SA、20%-Cu-CS/SA水凝胶浸泡在37 ℃的Tris-HCl缓冲液(pH 7.4)中, 控制水凝胶质量与Tris-HCl缓冲液体积的比例为0.1 g/mL。浸泡1、3、5 d离心后分别吸取上层溶液, 并过孔径为0.22 μm的滤膜收集滤液, 最后检测滤液中Ca、Si、Cu离子浓度。
1.7 纳米棒的化学动力学性能检测
以3,3ʹ,5,5ʹ-四甲基联苯胺(TMB)作为·OH探针, 根据TMB的显色反应分析Cu-CS纳米棒的化学动力学性能。为测试不同制备条件对Cu-CS纳米棒的化学动力学性能的影响, 将不同铜盐添加量和不同熔盐处理温度下制备的纳米棒分散于含有H2O2 (100 mmol/L)的pH 6.0的乙酸钠缓冲溶液中(其终浓度为1 mg/mL), 然后立即加入30 μL的TMB乙醇溶液(10 mg/mL), 反应30 min后, 利用多功能酶标仪检测溶液的紫外-可见光吸收光谱。
为揭示不同pH对Cu-CS纳米棒的化学动力学性能的影响, 本实验将3Cu-CS和CS纳米棒分散于pH 6.0或pH 7.4的含H2O2 (100 mmol/L)的乙酸钠缓冲溶液中, 保持3Cu-CS和CS纳米棒在反应体系中的终浓度为1 mg/mL, 然后立即加入30 μL的TMB乙醇溶液(10 mg/mL), 反应20 min后, 检测溶液的紫外-可见光吸收光谱。
1.8 水凝胶的化学动力学性能检测
按上述1.7节的方法分析Cu-CS纳米棒复合水凝胶的化学动力学性能。将不同Cu-CS添加量的水凝胶置于含有H2O2 (100 mmol/L)的pH 6.0的乙酸钠缓冲溶液中, 其最终浓度为0.1 g/mL, 然后立即加入30 μL的TMB乙醇溶液(10 mg/mL), 反应60 min后, 检测溶液的紫外-可见光吸收光谱。
为探究Cu-CS/SA水凝胶随时间变化的化学动力学性能, 将20%-Cu-CS/SA水凝胶置于含有H2O2 (100 mmol/L)的pH 6.0的乙酸钠缓冲溶液中, 其最终浓度为0.1 g/mL, 然后立即加入30 μL的TMB乙醇溶液(10 mg/mL), 每隔5 min利用多功能酶标仪检测一次溶液的紫外-可见光吸收光谱。
为探究Cu-CS/SA水凝胶在没有H2O2情况下随时间变化的化学动力学性能, 将20%-Cu-CS/SA水凝胶置于不含H2O2的pH 6.0的乙酸钠缓冲溶液中, 其最终浓度为0.1 g/mL, 然后立即加入30 μL的TMB乙醇溶液(10 mg/mL), 每隔5 min利用多功能酶标仪检测一次溶液的紫外-可见光吸收光谱。
为探究TMB随时间变化的化学动力学性能, 将30 μL的TMB乙醇溶液(10 mg/mL)加入到含有H2O2 (100 mmol/L)的pH 6.0的乙酸钠缓冲溶液中, 每隔5 min利用多功能酶标仪检测一次溶液的紫外-可见光吸收光谱。
为探究H2O2 浓度对Cu-CS/SA水凝胶的化学动力学性能的影响, 将20%-Cu-CS/SA水凝胶置于含不同浓度H2O2 (50、75、100 mmol/L)的乙酸钠缓冲溶液(pH 6.0)中, 保持20%-Cu-CS/SA水凝胶在反应体系中的浓度为0.1 g/mL, 然后立即加入30 μL的TMB乙醇溶液(10 mg/mL), 每隔5 min检测一次溶液在652 nm处的吸光值。
为探究溶液的pH对Cu-CS/SA水凝胶的化学动力学性能的影响, 将20%-Cu-CS/SA水凝胶置于pH 6.0或pH 7.4的含H2O2 (100 mmol/L) 的乙酸钠缓冲溶液中, 保持20%-Cu-CS/SA水凝胶在反应体系中的浓度为0.1 g/mL, 然后立即加入30 μL的TMB乙醇溶液(10 mg/mL), 每隔5 min检测一次溶液在652 nm处的吸光值。
1.9 细胞毒性检测
采用CCK-8方法测试计算细胞的存活率, 具体步骤如下: 首先, 将HUVECs以3×104 cells/well或HDFs以2×104 cells/well的密度接种到24孔板内培养24 h, 使其贴壁生长; 接着, 移去孔板里的培养液, 并将装有SA、20%-CS/SA、5%-Cu-CS/SA、10%- Cu-CS/SA、20%-Cu-CS/SA和40%-Cu-CS/SA水凝胶的Transwell细胞培养板小室转移到多孔板的每个孔中, 重新注入培养液, 得到的水凝胶含量为0.1 g/mL, 空白组(Blank组)中换上等量的培养液, 共同培养24 h; 然后, 移去上述的Transwell小室和培养液, 在每孔中加入300 μL的10%CCK-8溶液, 继续培养2 h; 最后, 每孔吸取100 μL的溶液至96孔板中, 利用酶标仪检测450 nm波长处各孔的吸光值(OD), 根据不同组别与空白对照的OD值的比值计算细胞存活率, 从而判断水凝胶的细胞毒性。
1.10 细胞增殖实验
利用CCK-8方法测试细胞增殖, 具体步骤如下: 首先, 将HUVECs (每孔5×103)或HDFs (5×103cells// well)接种到24孔板内培养24 h, 使其贴壁生长; 接着, 移去孔板里的培养液, 并将装有SA、20%-CS/ SA、20%-Cu-CS/SA水凝胶的Transwell小室转移到每孔中, 重新注入培养液, 最终的水凝胶含量为0.1 g/mL, Blank组中换上等量的培养液, 隔天换液; 然后, 在到达预设的时间点(1、3和5 d), 按1.9节的方法检测450 nm波长处各孔的OD值。
1.11 黑色素瘤细胞内活性氧及其体外抗肿瘤检测
采用荧光倒置显微镜观察黑色素瘤细胞(B16F10)内活性氧(ROS)情况, 利用CCK-8方法检测B16F10细胞的活性。实验组别为: (a) Blank (pH 7.4或pH 6.0); (b) SA (pH 7.4或pH 6.0); (c) 20%-CS/SA (pH 7.4或pH 6.0); (d) 20%-Cu-CS/SA (pH 7.4或pH 6.0); (e) Blank+H2O2 (100 μmol/L, pH 7.4或pH 6.0); (f) SA+H2O2 (100 μmol/L, pH 7.4或pH 6.0); (g) 20%-CS/SA+H2O2 (100 μmol/L, pH 7.4或pH 6.0); (h) 20%-Cu-CS/SA+H2O2 (100 μmol/L, pH 7.4或pH 6.0), 具体实验步骤如下述。
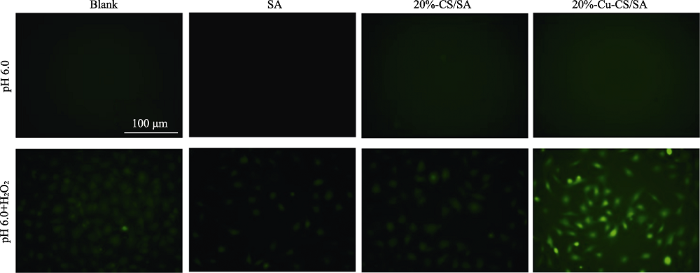
为探究不同处理条件对B16F10细胞内ROS的影响, 利用荧光倒置显微镜观察其胞内荧光变化。首先, 将B16F10细胞(2×104 cells/well)接种到24孔板内培养24 h, 使其贴壁生长; 接着, 移去培养液, 将装有SA、20%-CS/SA、20%-Cu-CS/SA水凝胶的Transwell小室轻轻放入孔板中, Blank组为空白对照, 在这四组中分别加入pH 6.0的不含或者含有100 μmol/L H2O2 的细胞培养液, 水凝胶的最终浓度为0.1 g/mL, 共同培养24 h; 然后, 移去上述Transwell小室和培养液, 各组加入DCFH-DA检测试剂, 继续培养20 min, 移去检测液并用磷酸缓冲液(Phosphate Buffered Saline, PBS)清洗3次后用荧光倒置显微镜观察B16F10细胞内ROS变化, 其中绿色荧光代表产生的ROS。
为探究Cu-CS/SA水凝胶的化学动力学治疗效果, 采用CCK-8法测试不同条件处理的B16F10细胞的活性。首先, 将B16F10细胞(2×104 cells/well)接种到24孔板内培养24 h, 使其贴壁生长; 接着移去培养液, 将装有SA、20%-CS/SA、20%-Cu-CS/SA水凝胶的Transwell小室轻轻放入孔板中, Blank组为对照组, 然后按照实验分组加入不同pH和H2O2的细胞培养液, 使水凝胶的最终浓度为0.1 g/mL, 共同培养24 h; 然后移去上述的Transwell小室和培养基, 在每孔中加入300 μL10% CCK-8溶液, 继续培养2 h; 最后, 每孔吸取100 μL溶液至96孔板中, 利用酶标仪测试450 nm波长处每孔的OD值。
1.12 细胞迁移实验
为测定Cu-CS纳米棒复合水凝胶对正常细胞迁移的影响, 本研究做了HUVECs和HDFs的划痕实验。首先, 将HUVECs (1×105 cells/well)或HDFs (1.5×105 cells/well)接种到24孔板内培养, 培养一段时间使细胞长成融合单层状态; 接着, 使用200 μL的微量移液器枪头垂直向下划出一条均匀的划痕, 用无血清的培养基洗去多余的细胞碎片, 使划痕边缘平整; 然后, 将装有SA、20%-CS/SA、20%-Cu-CS/SA水凝胶的Transwell小室轻轻放入孔板中, Blank组为空白对照, 加入无血清的培养液培养24 h, 水凝胶的最终浓度为0.1 g/mL; 最后, 各组细胞样品中加入300 μL的4%多聚甲醛溶液固定细胞30 min, 用PBS溶液洗涤3次, 再加入200 μL的结晶紫溶液(0.1%)染色2 min, PBS溶液洗去多余的染料, 并用ImageJ软件标记创口边缘, 测定未愈合的创面面积, 计算细胞迁移率。细胞迁移率=(初始创面面积-特定时间点的创面面积)/初始创面面积×100%。
2 结果与讨论
2.1 Cu-CS纳米棒的制备与表征
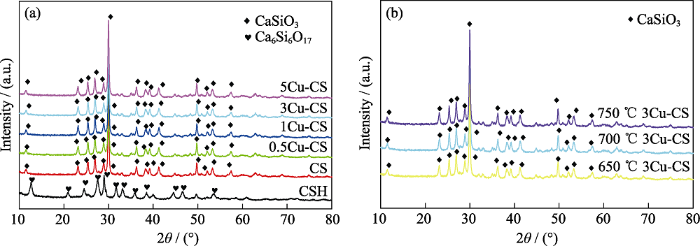
据文献报道[24], 熔盐法制备负载型催化剂时, 前驱体添加量和熔盐处理温度是影响催化剂催化性能的两个重要因素。因此, 本研究在不同铜盐添加量和不同熔盐处理温度下制备了Cu-CS粉体, 并进行表征与分析。图1(a)是不同铜盐添加量的CSH纳米线在700 ℃熔盐处理制备的Cu-CS粉体的XRD图谱。由图谱可知, 铜盐添加量为0、0.5%、1%、3%、5%的CSH纳米线都转变成了硅酸钙(CaSiO3)物相。图1(b)是3%铜盐添加量的CSH纳米线经不同温度熔盐处理制备的Cu-CS粉体的XRD图谱, 可以看出经过650、700和750 ℃熔盐处理的CSH纳米线也都转变成了CaSiO3物相。Shen等[30]研究表明, CSH在高温下会脱去结合水形成CaSiO3。因此, CSH纳米线经过熔盐处理可得到更加稳定的CaSiO3物相。另一方面, 在不同铜盐添加量和不同熔盐温度条件下制备的Cu-CS粉体都没有在XRD图谱上出现铜相关的物相, 可能是由于熔盐处理时熔融离子的强极化力将Cu元素负载在基体表面[24], 未形成铜单质或者铜的化合物, 并且Cu元素负载量极低, 难以形成特征峰。
图1
图1
(a)不同铜盐添加量和(b)不同温度熔盐处理制备的Cu-CS粉体的XRD图谱
Fig. 1
XRD patterns of Cu-CS powders prepared by molten salt method with (a) different amounts of copper salt and (b) different temperatures
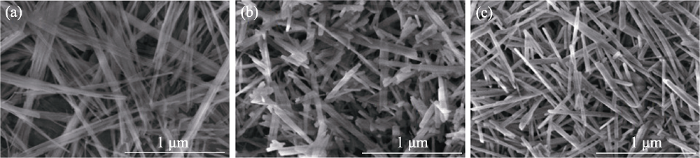
图2
图2
(a) CSH、(b) CS和(c) 3Cu-CS粉体的SEM照片
Fig. 2
SEM images of (a) CSH, (b) CS and (c) 3Cu-CS powders
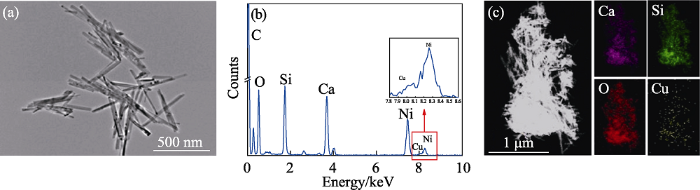
图3
图3
3Cu-CS纳米棒的(a) TEM照片-(b) EDS谱图和(c)元素分布图
Fig. 3
(a) TEM image, (b) EDS spectrum and (c) elemental mapping of 3Cu-CS nanorods
The color figure can be obtained from online edition
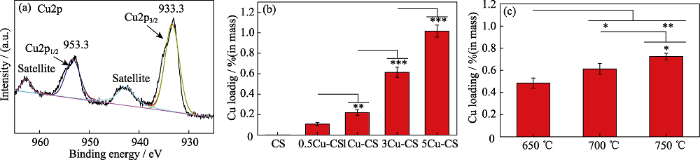
本研究利用XPS图谱进一步分析了3Cu-CS纳米棒表面Cu元素的价态。从3Cu-CS纳米棒的Cu2p图谱可知(图4(a)), Cu 2p1/2和Cu2p3/2主峰结合能为953.3和933.3 eV, 且Cu2p1/2和Cu2p3/2卫星峰结合能为962.6和942.6 eV, 表明3Cu-CS纳米棒中的Cu元素的价态为+2价[19,32]。Lin等[33]研究表明, Cu2+能在酸性条件下与H2O2反应生成•OH。因此, Cu-CS纳米棒表面的Cu元素可以作为活性位点在肿瘤酸性微环境中与H2O2反应生成·OH, 发挥化学动力学效应。进一步地, 本研究采用ICP-AES方法测定了不同工艺条件下制备的Cu-CS纳米棒的Cu负载量。一方面, 在700 oC的熔盐处理温度下, 当铜盐添加量分别为0、0.5%、1%、3%、5%时制备的CS、0.5Cu-CS、1Cu-CS、3Cu-CS、5Cu-CS纳米棒的Cu含量分别是0、0.11%、0.22%、0.61%、1.02% (图4(b))。另一方面, 在铜盐添加量为3%的情况下, 当熔盐处理温度分别为650、700和750 ℃时制备的Cu-CS纳米棒的Cu含量分别是0.48%、0.61%、0.72% (图4(c))。这表明铜盐添加量和熔盐处理温度都会影响Cu-CS纳米棒表面Cu元素的负载量[24], 并且随着铜盐添加量和熔盐处理温度升高, Cu元素在纳米棒表面的负载量也随之显著增加, 这将会对Cu-CS纳米棒的催化性能产生影响。
图4
图4
3Cu-CS纳米棒的(a)Cu2p XPS图谱, ICP-AES方法测定的(b)不同铜盐添加量和(c)不同熔盐处理温度制备的Cu-CS纳米棒的Cu负载量
Fig. 4
(a) Cu2p XPS spectrum of 3Cu-CS nanorods, Cu amounts of Cu-CS nanorods prepared with (b) different copper salt additions and (c) different temperatures by ICP-AES method, respectively. *p< 0.05, **p< 0.01, ***p< 0.001
The color figures can be obtained from online edition
2.2 Cu-CS纳米棒的化学动力学性能
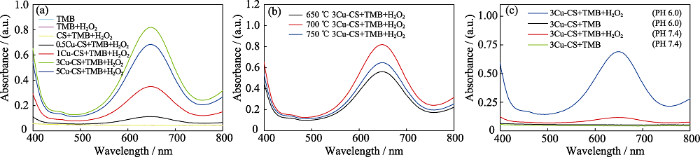
本研究采用TMB分子作为•OH探针测试Cu-CS纳米棒的化学动力学性能, 其原理为Cu-CS纳米棒与H2O2反应的产物•OH能使无色的TMB氧化成蓝色的氧化态TMB(ox-TMB), 因此可以利用紫外-可见光吸收光谱来测定•OH的产生[34]。由图5(a)可以看出, 在酸性条件下, 含有100 mmol/L H2O2的TMB测试液中同时加入0.5Cu-CS、1Cu-CS、3Cu-CS、5Cu-CS纳米棒均在652 nm处出现明显的特征吸收峰, 而加入CS纳米棒的TMB测试液、纯TMB测试液和只加入H2O2的TMB测试液都没有任何特征吸收峰, 这表明只有Cu-CS纳米棒才可以在酸性条件下催化H2O2产生•OH。从图5(a)还可以看出, 随Cu含量增加, Cu-CS的化学动力学性能呈现先增强后减弱的趋势, 即当铜盐添加量为3%时, 化学动力学性能最高。由图5(b)可以看出, 在酸性条件下, 加入不同熔盐处理温度制备的3Cu-CS纳米棒测试液均在652 nm处有明显的特征峰, 即都具有化学动力学性能, 并且随着熔盐处理温度升高, 化学动力学性能也呈现先增强后减弱的趋势。综合以上结果可知700 ℃熔盐处理3%铜盐添加量的CSH纳米线制备的3Cu-CS纳米棒具有最佳的化学动力学性能。Kathleen等[35]研究证明, 催化剂表面暴露的有效催化位点的比例将直接影响其催化活性。本研究中, 随着铜盐添加量增大和熔盐处理温度升高, Cu-CS纳米棒的Cu元素负载量逐渐上升(图4(b, c)), 其表面的Cu催化位点也逐渐增加, 催化性能提高; 但过量的Cu元素负载则容易导致Cu元素团聚, 减少表面有效的催化位点, 造成催化性能下降。
图5
图5
Cu-CS纳米棒的化学动力学性能
Fig. 5
Chemodynamic effects of Cu-CS nanorods
(a, b) UV-Vis absorption spectra of TMB solutions with pH 6.0 and H2O2 (100 mmol/L) after adding Cu-CS nanorod (1 mg/mL) prepared by a molten salt method with (a) different copper salt additions and (b) different treatment temperatures for 30 min; (c) UV-Vis absorption spectra of TMB solutions after adding 3Cu-CS nanorods (1 mg/mL) into TMB solutions under pH 6.0 or pH 7.4 conditions and with or without H2O2 for 20 min The color figures can be obtained from online edition
图5(c)是在pH 6.0或者pH 7.4, 有或没有H2O2条件下, 加入3Cu-CS纳米棒的TMB测试液在400~ 800 nm范围内的紫外-可见光吸收光谱。从图中可以看出, 在没有H2O2的情况下, 不同pH环境下加入3Cu-CS纳米棒都没有使TMB测试液在652 nm处出现特征峰; 在含有H2O2的情况下, pH 7.4的TMB测试液中加入3Cu-CS纳米棒, 其在652 nm处的吸光值略有增加, 出现很弱的特征吸收峰, 而pH 6.0的TMB测试液中加入3Cu-CS纳米棒, 其在652 nm处的吸光值显著增加, 出现了明显的特征吸收峰。以上结果表明, 3Cu-CS纳米棒只有在酸性条件下才能催化H2O2产生大量·OH, 这使得靶向肿瘤组织的化学动力学治疗成为可能。
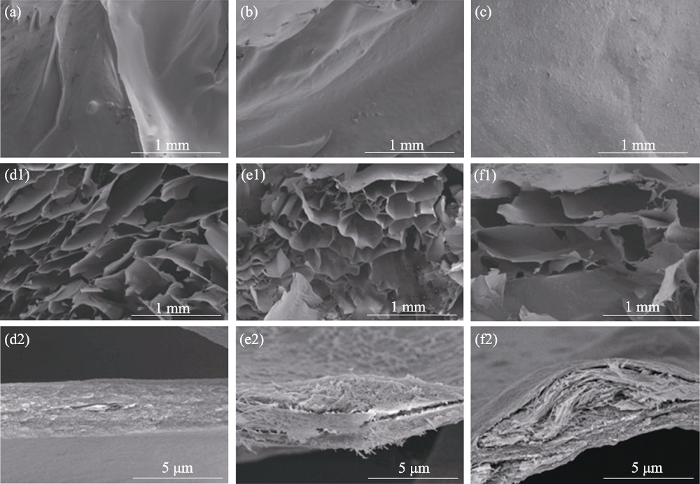
2.3 水凝胶的微观形貌和结构
图6
图6
(a, d1, d2) SA、(b, e1, e2) 20%-CS/SA和(c, f1, f2) 20%-Cu-CS/SA水凝胶的表面以及断面SEM照片
Fig. 6
SEM images of the surfaces and cross sections for (a, d1, d2) SA, (b, e1, e2) 20%-CS/SA and (c, f1, f2) 20%-Cu-CS/SA hydrogels
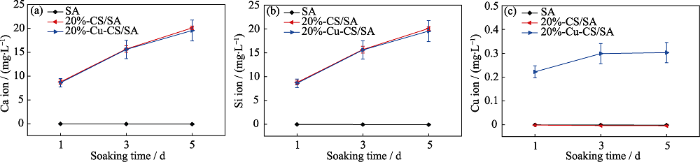
本研究进一步采用ICP-AES方法检测了SA、20%-CS/SA和20%-Cu-CS/SA水凝胶在Tris-HCl缓冲液中的离子释放情况。如图 7 所示, 20%-CS/SA和20%-Cu-CS/SA水凝胶浸泡5 d后的Ca离子累计释放量分别为 20.1和19.6 mg/mL, 浸泡5 d后的Si离子累计释放量分别为7.9和8.0 mg/mL, 两种水凝胶的Ca、Si离子释放量没有显著性差异。20%-Cu- CS/SA水凝胶浸泡1, 3, 5 d后的Cu离子累计释放量分别为 0.22、0.30和0.30 mg/mL, 表明20%-Cu-CS/SA水凝胶对于Cu离子的释放具有初始快速释放的特点, 浸泡3 d后基本没有Cu离子释放,并且Cu离子的累计释放趋缓, 这可能是因为Cu-CS纳米棒的Cu元素含量低且分布在纳米棒表面造成的。由于SA水凝胶中并没有CS纳米棒或者Cu-CS纳米棒, 因此都未测得释放的Ca、Si、Cu离子。已有研究表明, 释放微量的Ca离子和Si离子有利于提高细胞活性[14⇓-16], 且释放微量的Cu离子不仅没有细胞毒性而且能促进血管的生成[16], 同时释放Cu离子还有利于在肿瘤酸性微环境下与H2O2反应产生•OH用于杀死肿瘤细胞[19], 由此推断20%-Cu- CS/SA水凝胶具有良好的生物学效应。
图7
图7
SA、20%-CS/SA和20%-Cu-CS/SA水凝胶在Tris-HCl缓冲液中的(a) Ca、(b) Si、(c) Cu离子释放
Fig. 7
Release behaviors of (a) Ca, (b) Si and (c) Cu ions from SA, 20%-CS/SA and 20%-Cu-CS/SA hydrogels in Tris-HCl buffer
The color figures can be obtained from online edition
2.4 水凝胶的化学动力学性能
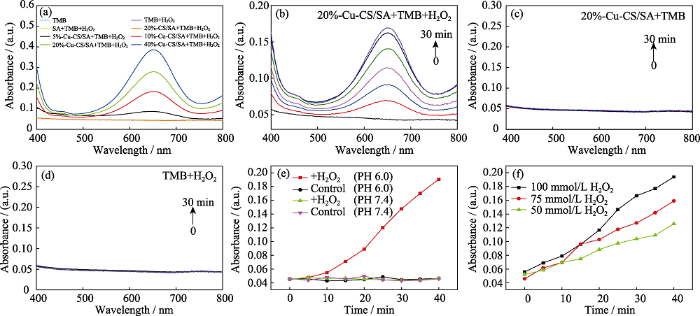
Cu-CS/SA水凝胶的化学动力学性能仍然以TMB分子为探针进行测试分析。由图8(a)可以看出, 在pH 6.0和H2O2 (100 mmol/L)条件下的TMB测试液中分别加入5%-Cu-CS/SA、10%-Cu-CS/SA、20%- Cu-CS/SA、40%-Cu-CS/SA水凝胶(0.1 g/mL), 反应60 min后TMB测试液在652 nm处均出现了对应于ox-TMB的特征吸收峰, 并且强度逐渐增加, 而纯TMB测试液、TMB+H2O2测试液、TMB+H2O2+SA测试液和TMB+ H2O2+20%-CS/SA测试液均没有出现吸收峰, 表明只有Cu-CS/SA水凝胶才能在酸性条件下催化H2O2产生•OH, 并且复合水凝胶的化学动力学性能随Cu- CS纳米棒含量的增加而增强。
图8
图8
Cu-CS/SA水凝胶的化学动力学性能
Fig. 8
Chemodynamic effects of Cu-CS/SA hydrogels
(a) UV-Vis absorption spectra of TMB solutions with pH 6.0 and H2O2 (100 mmmol/L) after adding Cu-CS/SA hydrogel (0.1 g/mL) with different contents of Cu-CS nanorods for 60 min; (b) UV-Vis absorption spectra changes of TMB solutions with time after adding 20%-Cu-CS/SA hydrogel (0.1 g/mL) under a condition of pH 6.0 and H2O2 (100 mmmol/L); (c) UV-Vis absorption spectra changes of TMB solutions with time after adding 20%-Cu-CS/SA hydrogel (0.1 g/mL) under a condition of pH 6.0; (d) UV-Vis absorption spectra changes of TMB solutions with time under a condition of pH 6.0 and H2O2 (100 mmmol/L); (e) Absorbance changes at 652 nm versus time for TMB solutions with 20%-Cu-CS/SA hydrogel under pH 6.0 or pH 7.4 conditions and with or without H2O2; (f) Absorbance changes at 652 nm versus time for TMB solutions with different concentrations of H2O2 at pH 6.0 after adding 20%-Cu-CS/SA hydrogel (0.1 g/mL).
The color figures can be obtained from online edition
图8(b-d)分别是pH 6.0和H2O2 (100 mmol/L)条件下加入20%-Cu-CS/SA水凝胶(0.1 g/mL)、pH 6.0条件下只加入20%-Cu-CS/SA水凝胶(0.1 g/mL)、 pH 6.0和H2O2 (100 mmol/L)条件下不加入20%-Cu-CS/SA水凝胶的TMB测试液在400~800 nm范围内吸光值随时间变化的曲线。从图中可以看出, 只有在pH 6.0和H2O2 (100 mmol/L)条件下加入20%-Cu-CS/SA水凝胶的TMB测试液时, 其在652 nm处对应的特征吸光值随时间延长而逐渐增加, 即生成的·OH量逐渐增加, 而不加入H2O2或者20%-Cu-CS/SA水凝胶的TMB测试液都基本没有出现特征吸收峰, 表明20%-Cu-CS/SA水凝胶是持续催化H2O2产生•OH的关键。
图8(e)是在pH 6.0或者pH 7.4, 有或没有H2O2条件下, 加入20%-Cu-CS/SA水凝胶的TMB测试液在652 nm处的吸光值随时间变化的曲线。从图中可以看出, 在没有H2O2的条件下, 无论是在pH 6.0还是pH 7.4条件下, 20%-Cu-CS/SA水凝胶都不会引起TMB测试液在652 nm处吸光值的变化; 在含有H2O2的情况下, pH 7.4的TMB测试液中加入20%-Cu-CS/SA水凝胶, 其在652 nm处的吸光值基本没有变化, 但是pH 6.0的TMB测试液中加入20%-Cu-CS/SA水凝胶, 其在652 nm处的吸光值随着时间显著增大。由此表明, 20%-Cu-CS/SA水凝胶只有在酸性条件下才能催化H2O2产生•OH。
2.5 水凝胶的细胞毒性
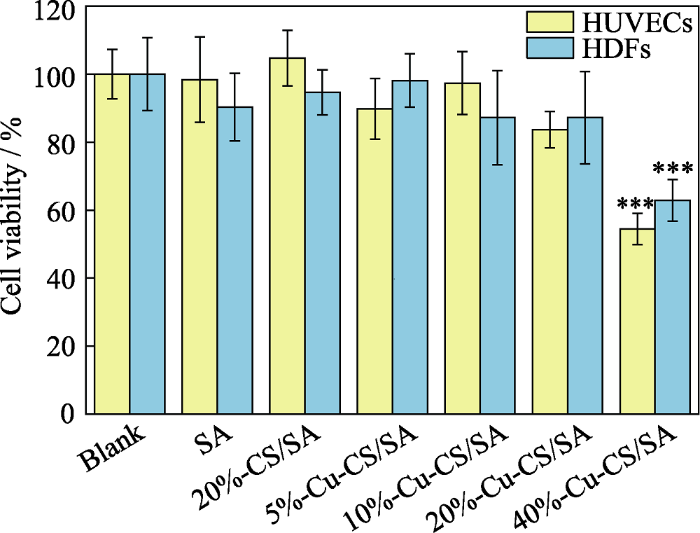
HUVECs和HDFs分别与SA水凝胶、20%-CS/SA水凝胶和不同Cu-CS纳米棒含量的Cu-CS/SA水凝胶培养24 h后的细胞存活率结果显示(图9), 随着水凝胶中Cu-CS纳米棒含量从5%增加到20%, HUVECs和HDFs的细胞存活率均保持80%以上, 与SA水凝胶和20%-CS/SA水凝胶没有显著性差异, 但水凝胶中Cu-CS纳米棒的含量达到40%时, HUVECs和HDFs的细胞存活率分别仅为60.9%和63.1%, 表明其细胞活性较差。一般认为细胞存活率大于80%的材料具有良好的生物相容性。因此, Cu-CS纳米棒含量20%时, Cu-CS/SA水凝胶具有良好的生物相容性。Kong等[20]研究表明, 铜离子浓度高于3.2 μg/mL时, HUVECs的细胞存活率会低于60%。因此, 40%-Cu-CS/SA水凝胶组的HUVECs和HDFs细胞存活率低可能是由水凝胶释放的铜离子浓度过高所致。因此, 本研究选用既有较高催化活性又有良好细胞相容性的20%-Cu-CS/ SA水凝胶进行后续细胞实验研究。
图9
图9
内皮细胞和成纤维细胞与不同Cu-CS纳米棒含量的Cu-CS/SA复合水凝胶培养24 h后的细胞存活率
Fig. 9
Cell viabilities of HUVECs and HDFs after 24 h incubation with hydrogels incorporated with different contents of Cu-CS nanorods
The color figure can be obtained from online edition
***: p< 0.001
2.6 B16F10细胞内ROS分析与治疗效果评价
图10
图10
不同条件处理后B16F10细胞内ROS荧光显微照片
Fig. 10
Fluorescence images of B16F10 cells after different treatments for observing intracellular ROS
The color figures can be obtained from online edition
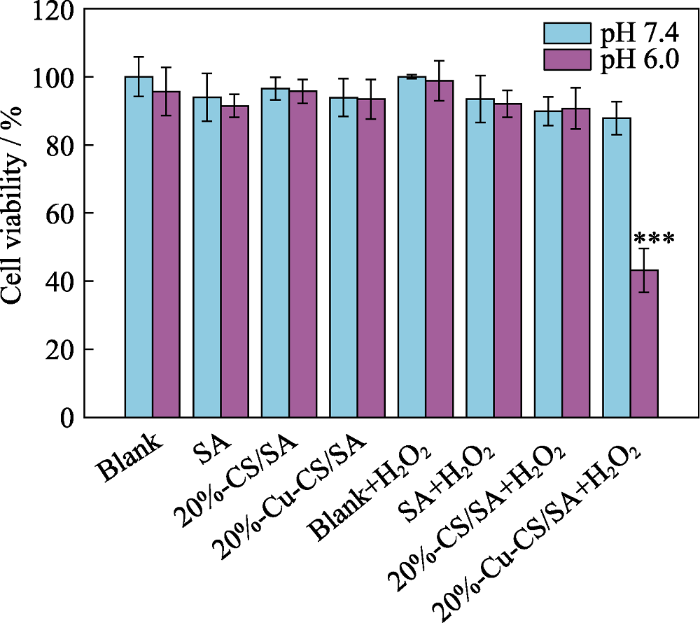
进一步地, 本研究采用CCK-8方法测试不同条件处理的B16F10细胞活性, 以此评估20%-Cu-CS/SA水凝胶对肿瘤细胞的治疗效果。
如图11所示, 当细胞环境调至pH 7.4时, 无论是否升高H2O2的浓度, Blank组、SA组、20%-CS/SA组和20%-Cu-CS/SA组的细胞活性都可达85%以上; 当细胞环境调至pH 6.0时, 无论是否升高H2O2的浓度, Blank组、SA组和20%-CS/SA组的细胞活性均可达90%以上, 不加H2O2的20%-Cu-CS/SA组的细胞活性也可高达93%, 但是加H2O2的20%-Cu- CS/SA组的细胞活性却下降至43%, 表明在弱酸性(pH 6.0)和高浓度H2O2 (100 μmol/L)环境下, 20%- Cu-CS/SA水凝胶对肿瘤细胞的增值有显著抑制作用。20%-Cu-CS/SA水凝胶体外抗肿瘤的机制可能与其在酸性条件下能与H2O2反应催化生成·OH有关。高毒性•OH可引起细胞结构的不可逆损伤, 如线粒体和蛋白质的破坏, 进而引起肿瘤细胞凋亡[37-38]。
图11
图11
不同条件处理下的B16F10细胞的存活率
Fig. 11
Cell viability of B16F10 cells after different treatments
***: p< 0.001
2.7 血管内皮细胞和成纤维细胞的增殖和迁移
HUVECs和HDFs在皮肤伤口愈合中起到重要的作用[28]。本研究以HUVECs和HDFs为细胞模型, 探究了20%-Cu-CS/SA水凝胶对HUVECs和HDFs的细胞增殖和迁移的影响。
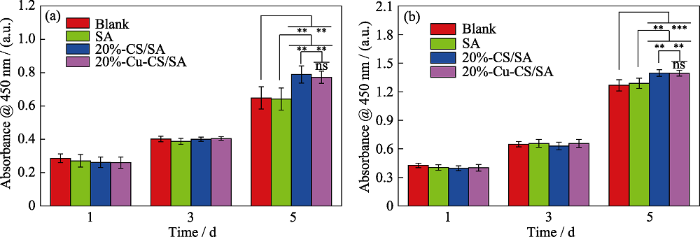
图12(a~b)分别为HUVECs和HDFs培养1、3、5 d的增殖状况。从图中可以看出, SA、20%-CS/SA和20%-Cu-CS/SA水凝胶组均具有良好的生物相容性。随着培养时间延长, 细胞出现增殖。当培养至第5 天时, 相比较于Blank组和SA组, 20%-CS/SA组和20%-Cu-CS/SA组都能显著促进HUVECs和HDFs增殖, 而20%-CS/SA组和20%-Cu-CS/SA组并没有显著性差异。已有研究表明, 微量的Ca离子和Si离子对细胞有刺激作用, 能明显提高细胞活性[14⇓-16]。因此, 本研究可能是20%-CS/SA组和20%-Cu- CS/SA组释放的生物活性Ca离子和Si离子促进了HUVECs和HDFs的增殖, 而20%-Cu-CS/SA组释放的Cu离子对HUVECs和HDFs的增殖影响不大。
图12
图12
与不同水凝胶共培养的(a)血管内皮细胞和(b)成纤维细胞的增殖状况
Fig. 12
Cell proliferations of (a) HUVECs and (b) HDFs cultured with different hydrogels
ns: p>0.05, **: p< 0.01, ***: p< 0.001; The color figures can be obtained from online edition
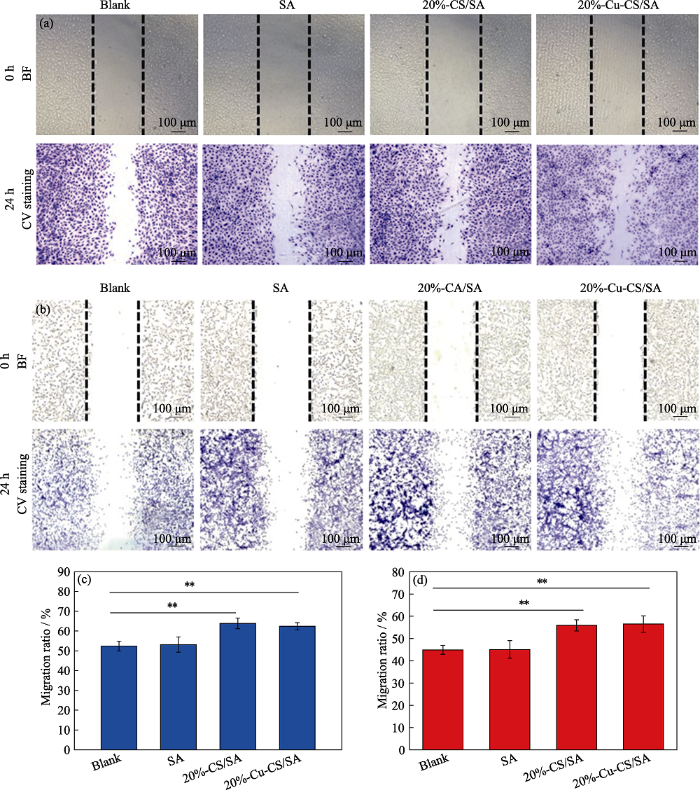
进一步, 本研究通过划痕法研究了20%-Cu-CS/ SA水凝胶对HUVECs和HDFs的迁移影响。由图13可以看出, 相较于Blank组和SA组, 20%-CS/SA组和20%-Cu-CS/SA组中有更多的HUVECs和HDFs迁移到划痕区。对HUVECs和HDFs的迁移作定量统计结果表明: 对于HUVECs而言, Blank组和SA组的细胞迁移率分别为50%和52%, 而20%- CS/SA组和20%-Cu-CS/SA组的细胞迁移率为66%和64%; 对于HDFs而言, Blank组和SA组的细胞迁移率为47%和46%, 而20%-CS/SA组和20%-Cu- CS/SA组的细胞迁移率为56%和59%。因此, 相较于Blank组和SA组, 20%-CS/SA组和20%-Cu-CS/ SA组都能促进HUVECs和HDFs 迁移, 这有利于皮肤伤口的愈合。已有研究表明, 微量的Ca离子和Si离子对HUVECs和HDFs的迁移起促进作用[39-40]。20%- CS/SA组和20%-Cu-CS/SA组对HUVECs和HDFs的细胞迁移效果相当, 可能是由于20%-CS/SA组和20%-Cu-CS/SA组释放的生物活性Ca离子和Si离子起到了促进HUVECs和HDFs迁移的作用。
图13
图13
与SA、20%-CS/SA或20%-Cu-CS/SA水凝胶共培养24 h后(a, c)内皮细胞和(b, d)成纤维细胞的迁移显微照片以及(c, d)相应定量统计的细胞迁移率(**p< 0.01)
Fig. 13
(a, b) Cell migration images and (c, d) corresponding migration rates of (a, c) HUVECs and (b, d) HDFs after cultured with SA, 20%-CS/SA and 20%-Cu-CS/SA hydrogels for 24 h, respectively ( **: p< 0.01)
The color figures can be obtained from online edition
3 结论
本研究采用熔盐法制备了Cu-CS纳米棒, 并将其与海藻酸钠复合得到Cu-CS/SA复合水凝胶。Cu-CS纳米棒表面的Cu元素负载量随铜盐添加量和熔盐处理温度升高而增加, 但Cu-CS纳米棒催化H2O2生成•OH的催化性能则随Cu含量增加呈现先升高后下降的趋势, 其中3%铜盐添加量和熔盐处理温度700 ℃条件下制备的3Cu-CS纳米棒的催化活性最高, 而且其Cu元素含量仅为0.61%。Cu-CS纳米棒含量不高于20%的Cu-CS/SA复合水凝胶具有良好的生物相容性。在模拟肿瘤弱酸性和高H2O2含量的微环境中, Cu-CS/SA复合水凝胶能催化H2O2生成高细胞毒性的·OH而具有化学动力学治疗功能, 导致黑色素瘤细胞的活性降至43%。另一方面, Cu-CS/SA复合水凝胶能促进内皮细胞和成纤维细胞增殖和迁移, 有利于皮肤伤口愈合。因此, Cu-CS/SA复合水凝胶对于肿瘤治疗和皮肤伤口愈合具有较大的应用前景。
参考文献
Topical treatments for skin cancer
Global cancer statistics
Nanoscale theranostics for physical stimulus-responsive cancer therapies
MoS2 nanoclusters-based biomaterials for disease-impaired wound therapy
Grape seed-inspired smart hydrogel scaffolds for melanoma therapy and wound healing
Grape-seed extracts contain rich flavonoids with oligomeric proanthocyanidins (OPC). In this study, OPC containing hydrogel scaffolds can function as a natural photothermal agent for melanoma therapy and bioactive biomaterial for wound healing. Inspired by grape-seed extracts, OPC were explored as a photothermal agent and endowed the hydrogel scaffolds with excellent and controlled photothermal ability. The rheological property of the hydrogel scaffolds responded to irradiation time of near infrared (NIR) laser, and OPC contents. The compressive mechanical property of the hydrogel scaffolds was well modulated by NIR laser irradiation with different impact durations. The controlled high temperature induced by OPC-containing hydrogel scaffolds under NIR laser irradiation could effectively kill melanoma cells and suppress tumor growth. In addition, OPC-containing hydrogel scaffolds supported the proliferation and migration of human dermal fibroblasts and human umbilical vein endothelial cells, as well as obviously promoted angiogenesis and skin regeneration in both tumor-caused and chronic wounds. Therefore, OPC-containing hydrogel scaffolds possessed controlled photothermal, rheological, and compressive mechanical properties under NIR laser stimuli, as well as excellent biocompatibility and bioactivity for melanoma therapy and wound healing.
Nanozymes-engineered mental-organic frameworks for catalytic cascades-enhanced syner- gistic cancer therapy
Chemodynamic therapy: tumor microenvironment-mediated Fenton and Fenton-like reaction
Engineering nanoparticles for optimized photodynamic therapy
A multifunctional cascade bioreactor based on hollow structured Cu2MoS4for synergetic cancer chemodynamic therapy/starvation therapy/phototherapy/ immunotherapy with remarkably enhanced efficacy
Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect
Angiogenesis is critical in tissue engineering, and bioceramic-induced angiogenesis has been reported. However, the role of other types of cells such as fibroblasts in this bioceramic-induced angiogenesis process has not been reported, and is closer to the in vivo situation of tissue regeneration. In this study, the paracrine effect of silicate bioceramic-induced angiogenesis in the presence of fibroblasts was confirmed by investigating the effects of calcium silicate (CS), one of the simplest silicate bioactive ceramics, on angiogenesis in co-cultures of human dermal fibroblasts (HDF) and human umbilical vein endothelial cells (HUVEC). Results showed that CS extracts stimulated the expression of vascular endothelial growth factor (VEGF) from co-cultured HDF and subsequently enhanced the expression of VEGF receptor 2 on co-cultured HUVEC (co-HUVEC). The endothelial nitric oxide synthase and nitric oxide production in co-HUVEC was then increased to finally initiate the proangiogenesis. During this process, the expression of vascular endothelial cadherin from co-HUVEC was up-regulated, and cadherin proteins were concentrated at the cell junctions to facilitate tube formation. Silicon ions are confirmed to play an important role during silicate bioceramic-inducing angiogenesis, and effective silicon ion concentrations (0.7-1.8μgml(-1)) are proposed.Copyright © 2013 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Effects of wollastonite on proliferation and differentiation of human bone marrow-derived stromal cells in PHBV/wollastonite composite scaffolds
In this study, the effects of wollastonite on proliferation and differentiation of human bone marrow-derived stromal cells (hBMSCs) have been investigated based on a polyhydroxybutyrate-co-hydroxyvalerate (PHBV)/ wollastonite (W) composite scaffolds system. Cell morphology, proliferation, and differentiation were measured. The results showed that the incorporation of wollastonite benefited hBMSCs adhesion, proliferation, and differentiation rate. In addition, an increase of proliferation and differentiation rate was observed when the wollastonite content in the PHBV/W composite scaffolds increased from 10 to 20 wt%. Based on our previous studies on PHBV/W composite discs, the differentiation measurements in this paper further proved that the wollastonite itself can stimulate the hBMSCs to differentiate toward osteoblasts without any osteogenic medium, and the ionic products (Ca and Si) released from wollastonite might contribute to this advantage. It is also suggested that the osteogenic differentiation of the hBMSCs can be affected by adjusting the wollastonite content in the composite scaffolds.
Osteogenic differentiation and immune response of human bone-marrow-derived mesenchymal stem cells on injectable calcium-silicate-based bone grafts
Silicate bioceramics induce angiogenesis during bone regeneration
The capacity to induce rapid vascular ingrowth during new bone formation is an important feature of biomaterials that are to be used for bone regeneration. Akermanite, a Ca-, Mg- and Si-containing bioceramic, has been demonstrated to be osteoinductive and to promote bone repair. This study further demonstrates the ability of akermanite to promote angiogenesis and investigates the mechanism of this behavior. The akermanite ion extract predominantly caused Si-ion-stimulated proliferation of human aortic endothelial cells. The Si ion in the extract was the most important component for the effect and the most effective concentration was found to be 0.6-2 μg ml(-1). In this range of Si ion concentration, the stimulating effect of the ceramic ion extract was demonstrated by the morphology of cells at the primary, interim and late stages during in vitro angiogenesis using ECMatrix™. The akermanite ion extract up-regulated the expression of genes encoding the receptors of proangiogenic cytokines and also increased the expression level of genes encoding the proangiogenic downstream cytokines, such as nitric oxide synthase and nitric oxide synthesis. Akermanite implanted in rabbit femoral condyle model promoted neovascularization after 8 and 16 weeks of implantation, which further confirmed its stimulation effect on angiogenesis in vivo. These results indicate that akermanite ceramic, an appropriate Si ion concentration source, could induce angiogenesis through increasing gene expression of proangiogenic cytokine receptors and up-regulated downstream signaling. To our knowledge, akermanite ceramic is the first Si-containing ceramic demonstrated to be capable of inducing angiogenesis during bone regeneration.Copyright © 2011 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Bioglass promotes wound healing by affecting gap junction connexin 43 mediated endothelial cell behavior
Bioglass activated skin tissue engineering constructs for wound healing
Preparation and in vitro osteogenic, angiogenic and antibacterial properties of cuprorivaite (CaCuSi4O10, Cup) bioceramics
Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity
It is of great importance to develop multifunctional bioactive scaffolds, which combine angiogenesis capacity, osteostimulation, and antibacterial properties for regenerating lost bone tissues. In order to achieve this aim, we prepared copper (Cu)-containing mesoporous bioactive glass (Cu-MBG) scaffolds with interconnective large pores (several hundred micrometer) and well-ordered mesopore channels (around 5 nm). Both Cu-MBG scaffolds and their ionic extracts could stimulate hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF) expression in human bone marrow stromal cells (hBMSCs). In addition, both Cu-MBG scaffolds and their ionic extracts significantly promoted the osteogenic differentiation of hBMSCs by improving their bone-related gene expression (alkaline phosphatase (ALP), osteopontin (OPN) and osteocalcin (OCN)). Furthermore, Cu-MBG scaffolds could maintain a sustained release of ibuprofen and significantly inhibited the viability of bacteria. This study indicates that the incorporation of Cu(2+) ions into MBG scaffolds significantly enhances hypoxia-like tissue reaction leading to the coupling of angiogenesis and osteogenesis. Cu(2+) ions play an important role to offer the multifunctional properties of MBG scaffold system. This study has demonstrated that it is possible to develop multifunctional scaffolds by combining enhanced angiogenesis potential, osteostimulation, and antibacterial properties for the treatment of large bone defects.Copyright © 2012 Elsevier Ltd. All rights reserved.
Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy
A responsive microneedle system for efficient anti-melanoma by combining self-enhanced chemodynamic therapy with photothermal therapy
Synergy effects of copper and silicon ions on stimulation of vascularization by copper-doped calcium silicate
Copper (Cu) has been reported to be able to stimulate vascularization/angiogenesis, which is critical for regeneration of vascularized tissue in tissue engineering. Silicate bioceramics have also been reported to have stimulatory effects on vascularization due to the silicon (Si) ions released from silicate biomaterials. Therefore, we hypothesize that a combination of Cu and Si ions may show synergy effects on vascularization. Therefore, a copper-doped calcium silicate bioceramic (Cu-CaSiO, Cu-CS) was designed and synthesized with the purpose to enhance the stimulatory effects of copper salts or pure silicate bioceramics on vascularization by combining the effects of Cu and Si ions. The cytocompatibility of Cu-CS was firstly assessed by testing the influence of Cu-CS ion extracts on proliferation of human umbilical vein endothelial cells (HUVECs). Thereafter, vascularization of HUVECs on ECMatrix™ gel or co-cultured with human dermal fibroblasts (HDFs) in Cu-CS extracts was evaluated and expression of angiogenic growth factors was analyzed. Results revealed that, as compared to CS extracts and media containing soluble CuSO, Cu-CS extracts possessed stronger stimulatory effects on upregulation of angiogenic growth factors, which finally resulted in better stimulatory effects on vascularization. During the vascularization process, paracrine effects dominated in the co-culture system. In addition, lower concentrations of Cu and Si ions released from Cu-CS than those released from pure CS or CuSO were enough to stimulate vascularization, which indicated that there were synergy effects between Cu and Si ions during stimulation of vascularization by Cu-CS. Taken together, the designed Cu-CS may be suitable as a new biomaterial for regenerating blood vessels in tissue engineering.
Magnesium calcium silicate bioactive glass doped with copper ions: synthesis and in-vitro bioactivity characterization
Ionic liquid (molten salt) phase organometallic catalysis
Molten salt synthesis, formation mechanism, and oxidation behavior of nanocrystalline HfB2 powders
Moten-salt-mediated synthesis of an atomic nickel Co-catalyst on TiO2 for improved photocatalytic H2 evolution
Wound dressings
Alginate-based nano materials: fabrication techniques, properties, and applications
Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: a review
Sprayable β-FeSi2 composite hydrogel for portable skin tumor treatment and wound healing
A simple method to synthesize single-crystalline β-wollastonite nanowires
Dehydration/recrystallization mechanisms, energetics, and kinetics of hydrated calcium silicate minerals: an in situ TGA/DSC and synchrotron radiation SAXS/WAXS study
A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte
Two-dimensional carbides and nitrides of transition metals, known as MXenes, are a fast-growing family of materials that have attracted attention as energy storage materials. MXenes are mainly prepared from Al-containing MAX phases (where A = Al) by Al dissolution in F-containing solution; most other MAX phases have not been explored. Here a redox-controlled A-site etching of MAX phases in Lewis acidic melts is proposed and validated by the synthesis of various MXenes from unconventional MAX-phase precursors with A elements Si, Zn and Ga. A negative electrode of TiC MXene material obtained through this molten salt synthesis method delivers a Li storage capacity of up to 738 C g (205 mAh g) with high charge-discharge rate and a pseudocapacitive-like electrochemical signature in 1 M LiPF carbonate-based electrolyte. MXenes prepared via this molten salt synthesis route may prove suitable for use as high-rate negative-electrode materials for electrochemical energy storage applications.
Effect of Mn doping on the microstructure and magnetic properties of CuFeO2 ceramics
Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy
Determination of Hg2+ based on the selective enhancement of peroxidase mimetic activity of hollow porous gold nanoparticles
Synthesis-structure-activity relationships in Co3O4 catalyzed CO oxidation
Methods of detection of vascular reactive species nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite
Overcoming the heat endurance of tumor cells by interfering with the anaerobic glycolysis metabolism for improved photothermal therapy
Perfluorooctyl bromide & indo- cyanine green co-loaded nanoliposomes for enhanced multimodal imaging-guided phototherapy
A novel "hot spring"- mimetic hydrogel with excellent angiogenic properties for chronic wound healing