目前已有多种方法用于去除废气中的放射性碘污染物, 常用的方法大致分为两类: 湿法洗涤和固体吸附法[5-6]。湿法洗涤通过使用溶剂从气相中洗涤碘[7], 但工艺复杂、腐蚀性强、设备成本高, 且会产生大量的二次废物。固体吸附法操作简单、维护和运行成本低, 对放射性碘的吸附分离具有良好的应用前景。常用的吸附剂有沸石[8]、活性炭[9]、活性氧化铝[10]、气凝胶[11]、层状双氢氧化物(LDH)[12]、多孔有机聚合物(POPs)[13]和金属-有机框架(MOFs)[14-15]等。活性炭因其多孔结构和比表面积高而具有较高的碘吸附能力, 但由于自身着火点低, 限制了在后处理厂的高温尾气处理中的应用。载银沸石(AgX、AgZ等)是最常用的碘吸附材料, 通过形成难溶性的AgI, 实现对碘的高吸附容量[16], 但银存在毒性、高成本等缺陷。为了最大限度地克服上述弊端, 有必要开发环境友好且具有成本效益的碘吸附材料。
1 实验方法
1.1 试剂
聚甲基倍半硅氧烷(MK树脂)购自德国瓦克化学有限公司; 五水合硝酸铋(Bi(NO3)3∙5H2O, AR)、四氢呋喃(THF, AR)、硝酸(HNO3, AR)、碘(I2, AR)均购自阿拉丁试剂有限公司。
1.2 材料制备
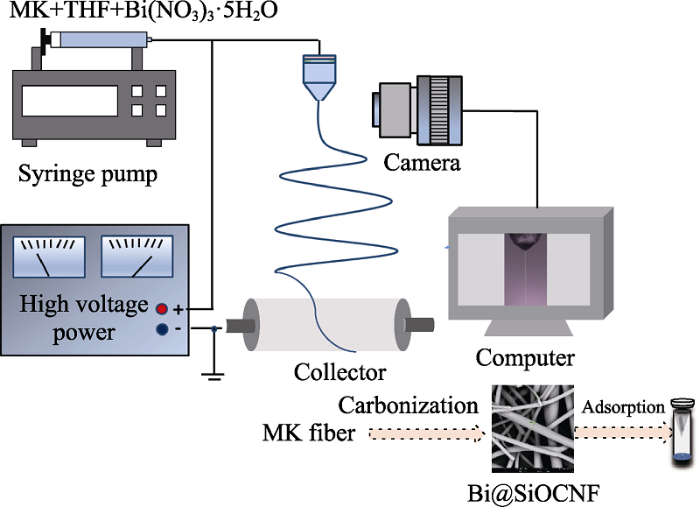
配置纺丝溶液: Bi@SiOCNF的制备流程如图1所示, 首先将6 g MK树脂加入到4 g四氢呋喃中搅拌1 h至完全溶解, 得到质量分数为60%的MK溶液, 然后在溶液中加入30 μL HNO3搅拌10 min, 最后加入不同质量(0.5、0.8、1 g)的Bi(NO3)3∙5H2O, 超声30 min后继续搅拌3 h, 得到三种不同负载量的纺丝溶液。
图1
静电纺丝过程: 将上述得到的纺丝溶液吸入5 mL塑料注射器中, 然后放入静电纺丝设备进行纺丝成型(如图1)(纺丝参数为: 针头: 25号不锈钢针头; 纺丝电压: 12 kV; 电极距: 15 cm; 注射泵推进速率: 0.08 mL/min; 环境温度: 18 ℃; 环境湿度: 40%), 得到MK纤维膜。
热还原过程: 在氩氢混合气氛中将上述静电纺丝得到的3种纤维膜以3 ℃/min升温至800 ℃, 煅烧2 h, 得到Bi@SiOCNF复合纤维膜, 按不同负载量分别命名为: Bi@SiOCNF-5、Bi@SiOCNF-8、Bi@SiOCNF-10。
1.3 表征
利用X射线衍射仪(XRD, D8 ADVANCE, 德国Bruker公司)对吸附碘前后的Bi@SiOCNF和I-Bi@SiOCNF样品进行结构表征, 分析样品的物相组成; 采用X射线光电子能谱(XPS, K-Alpha, 美国赛默飞世尔公司)分析吸附前后样品表面的元素组成和价态变化。利用扫描电子显微镜(SEM, Prox, 荷兰Phenom公司)和超高分辨场发射透射电子显微镜(TEM, JEM-2100F, 日本JEOL公司)分析吸附前后材料的微观形貌。利用能谱分析仪(EDS, Prox, 荷兰Phenom公司)对元素分布图谱进行分析。利用同步热重分析仪(TGA, STA 8000, 美国PerkinElmer公司)检测材料在升温过程中的热稳定性(气氛: 氩气, 升温速率: 10 ℃/min, 温度范围: 室温~800 ℃)。
1.4 吸附实验
由于放射性碘具有毒性和放射性, 本实验均选用非放射性的碘单质(I2)进行模拟吸附实验。将0.3 g碘和0.03 g的Bi@SiOCNF吸附剂分别放置在30 mL玻璃瓶的底部和锥形滤纸中并密封(如图1所示), 在静态条件(75 ℃和环境压力)下进行吸附实验。经过不同的时间间隔进行称重测量。根据公式(1)计算材料对碘的吸附容量。
其中, qe表示总吸附容量, 单位为mg/g; m0和m1分别表示吸附剂的初始质量和吸附后的质量, 单位为mg。
同时, 为了确定实验结果中吸附剂质量的增加是由吸附作用而不是与空气反应形成氧化物引起的, 进行了空白对照组实验。类似地, 将Bi@SiOCNF吸附剂放置在不含碘的装置中, 其他条件相同, 测定最终质量。另外热重分析表明, Bi@SiOCNF在75 ℃下几乎不与空气反应。
为了研究材料的碘吸附性能与气态碘浓度之间的关系, 通过添加不同质量的碘来控制装置中碘蒸气的浓度(0~300 mg/L)。为确保达到饱和吸附量, 吸附实验的吸附时间均为12 h。
为了验证该材料对碘气体吸附的循环性, 进行了循环吸附测试实验。将上述吸附后的吸附剂放入150 ℃的烘箱中进行碘气体脱附, 脱附3 h进行称重测量, 再用公式(2)计算出物理吸附容量, 用公式(3)算出化学吸附容量, 将上述步骤重复3次, 取平均值得到不同负载量材料的物理及化学吸附容量。
其中, qp表示物理吸附容量, qc表示化学吸附容量, 单位为mg/g; m0和m1分别表示吸附剂的初始质量和吸附后的质量, m2表示脱吸附后吸附剂的质量, 单位为mg。
2 结果与讨论
2.1 材料表征
图2
图2
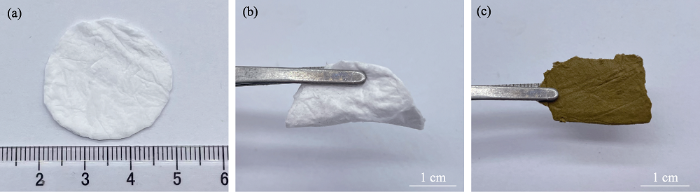
(a,b)MK纤维膜和(c)Bi@SiOCNF的光学照片
Fig. 2
Photos of (a,b) MK fiber membrane and (c) Bi@SiOCNF
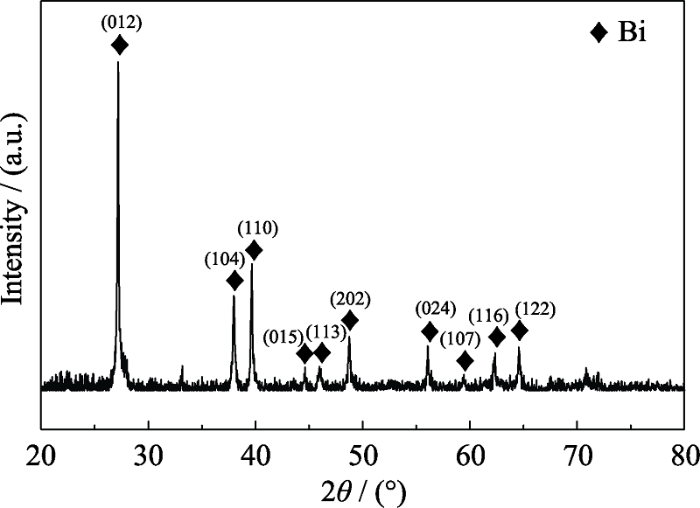
图3为Bi@SiOCNF材料的XRD图谱, 2θ=27.1°、38.0°、39.6°、44.6°、46.0°、48.7°、55.6、59.3、62.1°和64.5°处的特征峰分别对应金属Bi的(012)、(104)、(110)、(015)、(113)、(202)、(024)、(107)、(116)和(122)晶面(ICDD PDF 85-1330), 证明Bi@SiOCNF材料中存在铋单质。
图3
图4
图4
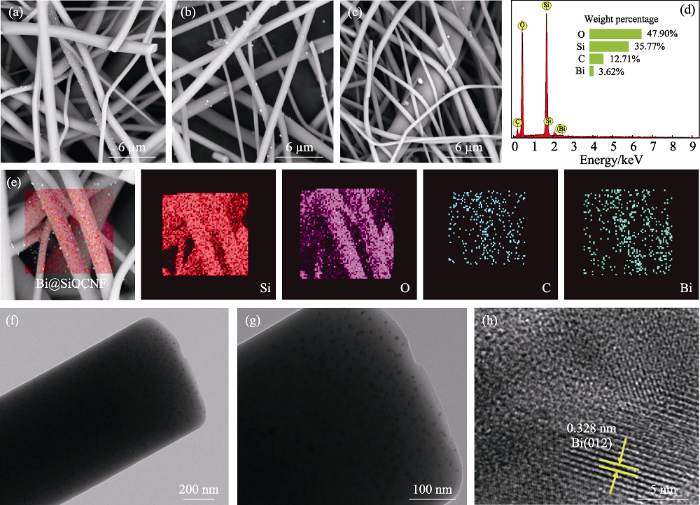
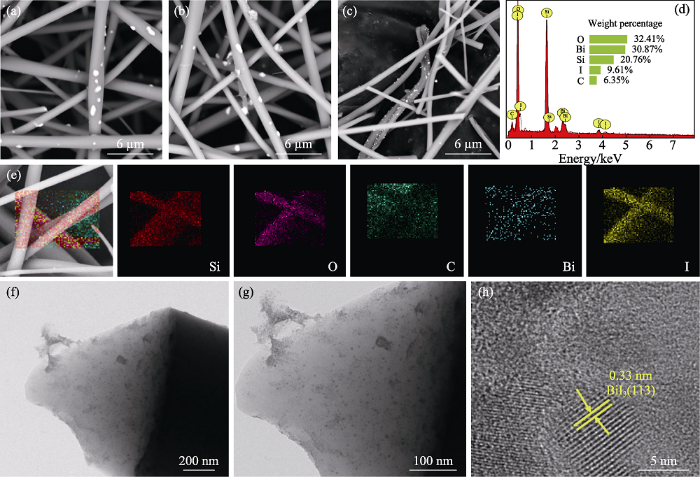
(a~c)Bi@SiOCNF-5, Bi@SiOCNF-8, Bi@SiOCNF-10的SEM照片, (d~e)Bi@SiOCNF-8的EDS能谱和元素分布图, (f~h)Bi@SiOCNF-8的TEM照片
Fig. 4
(a-c) SEM images of Bi@SiOCNF-5, Bi@SiOCNF-8, Bi@SiOCNF-10, (d-e) EDS spectrum and mappings of Bi@SiOCNF-8, and (f-h) TEM images of Bi@SiOCNF-8
2.2 碘吸附性能研究
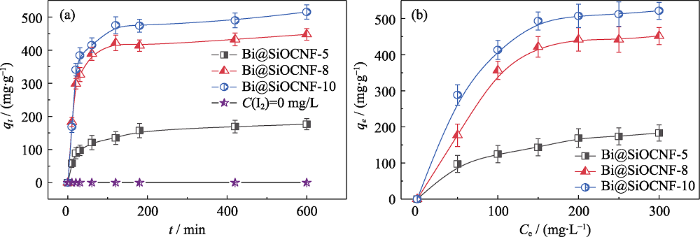
为了评估Bi@SiOCNF材料对碘蒸气的吸附能力, 测试了材料的吸附动力学曲线和等温吸附曲线。如图5(a)所示, Bi@SiOCNF对碘蒸气的吸附速率较快, 约120 min即可达到饱和吸附容量。不同负载量的Bi@SiOCNF-5, Bi@SiOCNF-8, Bi@SiOCNF-10的最大吸附量分别为176、448、515.2 mg/g。在空白对照组中, Bi@SiOCNF-10在无碘蒸气环境中10 h仍保持稳定, 表明它与空气未发生反应。图5(b)为Bi@SiOCNF-5, Bi@SiOCNF-8, Bi@SiOCNF-10对不同浓度碘的吸附容量。结果表明, Bi@SiOCNF-10吸附性能最佳, 且在SiOC含量一定的情况下, 铋负载量越大, 吸附容量越高, 说明该材料对碘气体的吸附性能关键是单质金属铋的作用, Bi@SiOCNF-10的最大吸附容量可达515.2 mg/g, 高于大多数无机材料(表1), 如Ag基沸石, Ag-ETS-2, Ag@Mon-POF等, 吸附容量均低于300 mg/g; 但小于多孔有机聚合物。相较于其他粉体铋基材料(如Bi6O7、Bi-BP2-O等)[21-22], Bi@SiOCNF的吸附能力可与之相媲美, 且宏观的膜材料特性更易于回收利用和固化处理, 从而更具有竞争优势。
图5
图5
Bi@SiOCNF吸附碘蒸气的特性曲线
Fig. 5
Bi@SiOCNF adsorption characteristic curves of iodine vapor
(a) Adsorption kinetic curves; (b) Adsorption isotherm curves
表1 不同吸附材料对碘的吸附性能
Table 1
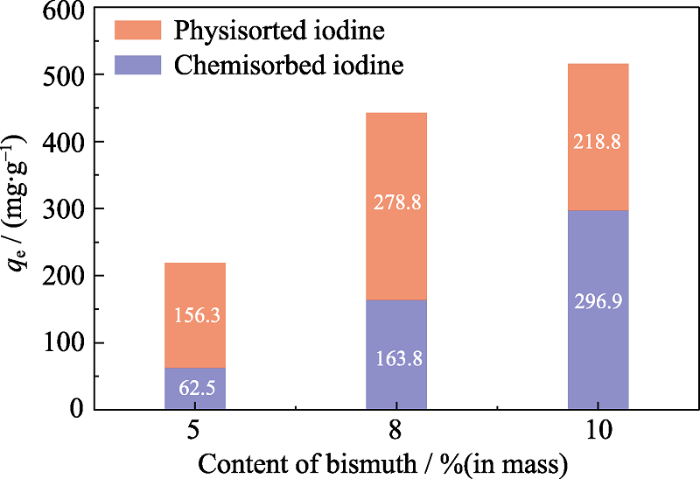
图6为不同负载量Bi@SiOCNF的物理吸附和化学吸附容量柱状图, Bi@SiOCNF-5, Bi@SiOCNF-8中的铋含量较少, 吸附形成的BiI3的含量较少, 所以主要以物理吸附为主, 物理吸附容量比化学吸附容量高; 而随着铋含量的增加, 化学吸附所占比例增加, Bi@SiOCNF-10主要以化学吸附为主。通过碘气体循环实验发现, 物理吸附部分可以通过150 ℃脱吸附后再进行物理吸附; 而化学吸附部分由于形成了BiI3, 不能再循环吸附。但该材料通过负载金属单质铋可用于对碘气体的固化作用。
图6
图6
不同负载量Bi@SiOCNF的物理吸附和化学吸附容量柱状图
Fig. 6
Histograms of physisorption and chemisorption capacities of Bi@SiOCNF with different loadings
2.3 机理分析
图7
图7
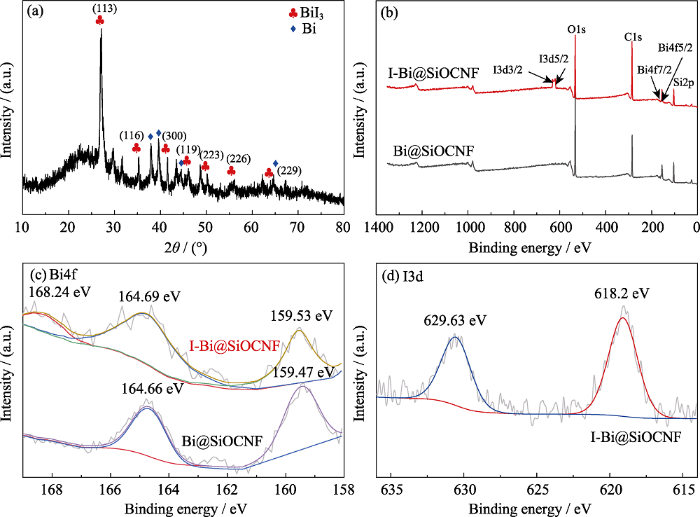
I-Bi@SiOCNF的(a)XRD图谱, I-Bi@SiOCNF和Bi@SiOCNF的(b) XPS全谱及其(c) Bi4f图谱, (d) I-Bi@SiOCNF的I3d XPS图谱
Fig. 7
(a) XRD pattern of I-Bi@SiOCNF, (b) XPS survey spectra, corresponding (c) Bi4f Spectra of I-Bi@SiOCNF and Bi@SiOCNF and (d) I3d spectra of I-Bi@SiOCNF
为了进一步验证, 对I-Bi@SiOCNF进行形貌分析。如图8(a~e)所示, 吸附后的I-Bi@SiOCNF表面分布Bi和I元素, 与EDS结果一致。TEM照片 (图8(f~h))显示, 晶格间距为0.33 nm, 对应于BiI3的(113)晶面, 表明白色颗粒是BiI3。通过XPS光谱分析得到碘吸附前后的价态变化, 如图7(b)所示, Bi@SiOCNF吸附碘蒸气后, 在630 eV结合能附近出现了I3d光谱, 说明存在碘元素。在图7(c)的Bi4f的光谱中, 159.47和164.66 eV分别归属于Bi@SiOCNF中Bi4f7/2和Bi4f5/2处的金属Bi峰[31]。而在吸附碘气体后, I-Bi@SiOCNF中Bi的峰值位置Bi4f7/2和Bi4f5/2分别变化为164.69 和168.24 eV。Bi元素的氧化态从0变化到+3, 但在159.53 eV处仍有铋单质的峰, 说明Bi@SiOCNF中的金属铋单质还未完全与碘气体反应。从图7(a)的XRD图谱也可看出吸附后的I-Bi@SiOCNF中还存在金属铋的峰,这可能是因为有少量铋被包覆在纤维内部而未与碘反应。在图7(d)的I-Bi@SiOCNF的I3d光谱中, 618.2和629.63 eV处的峰属于I-的特征峰[32]。结合上述结果可以得出, Bi@SiOCNF不仅能以BiI3形式化学吸附碘气体, 还能以SiOCNF孔结构物理吸附I2分子, 这与吸附实验结果一致。
图8
图8
(a~c)I-Bi@SiOCNF-5, I-Bi@SiOCNF-8, I-Bi@SiOCNF-10的SEM照片, (d~e)I-Bi@SiOCNF-8的EDS能谱和元素分布图, (f~h)I-Bi@SiOCNF-8的TEM照片
Fig. 8
(a-c) SEM images of I-Bi@SiOCNF-5, I-Bi@SiOCNF-8, I-Bi@SiOCNF-10, (d, e) EDS spectrum and mappings of I-Bi@SiOCNF-8, and (f-h) TEM images of I-Bi@SiOCNF-8
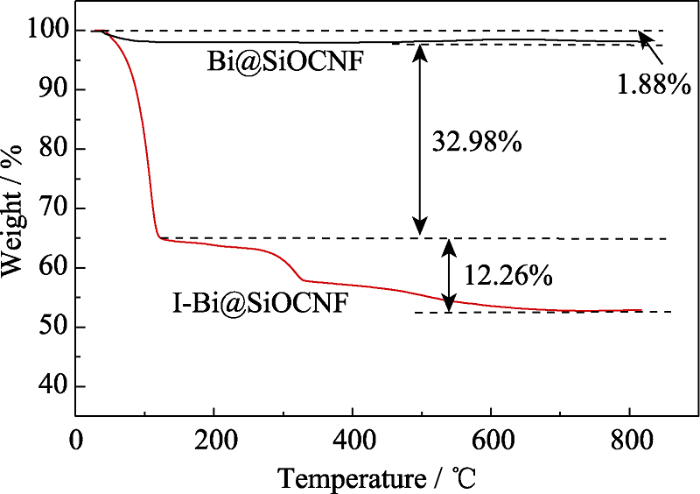
为了探究Bi@SiOCNF的热稳定性, 对碘吸附前后样品进行了TGA表征, 如图9所示, 当温度升高至800 ℃时, Bi@SiOCNF-10的质量略有下降, 但仅下降了1.88%, 而I-Bi@SiOCNF-10的质量损失了47.12%。通过对比分析, I-Bi@SiOCNF-10的重量损失可归结为以下三个部分: 1.88%是由于吸附空气中的水或吸附剂本身的少量分解; 32.98%为物理吸附的碘分子挥发, 曲线从75 ℃左右开始下降; 其余12.26%是由于化学吸附产生的BiI3在127~ 800 ℃的分解。室温升高到800 ℃的过程中, I-Bi@SiOCNF-10总共失重47.12%, 与前面吸附实验获得的碘吸附性能515.2 mg/g大致吻合。
图9
图9
Bi@SiOCNF-10和I-Bi@SiOCNF-10的热重曲线
Fig. 9
TGA curves of Bi@SiOCNF-10 and I-Bi@SiOCNF-10
3 结论
本研究通过静电纺丝工艺和热还原方法将前驱体Bi(NO3)3/MK中的铋离子还原为分散良好的金属铋单质, 成功制备出一种新型铋基复合材料(Bi@SiOCNF)。该材料以硅氧碳纤维膜为骨架, 金属铋单质负载在SiOC纤维的表面和内部, 提供了大量与碘蒸气的反应位点, 同时该结构避免了金属铋的团聚, 从而大大提高了碘气体吸附性能。在75 ℃, Bi@SiOCNF-10对碘气体的吸附容量可达515.2 mg/g, 约为商业银基沸石(Ag0Z)的两倍左右。研究表明, 高吸附容量主要是由于Bi和I2的化学反应和纤维膜的物理吸附。更重要的是, 与大多数粉体吸附剂相比, 宏观形态的纤维膜材料更适用于处理和固定放射性气态碘。因此, Bi@SiOCNF是一种非常有潜力的放射性气态碘吸附剂。
参考文献
Radioactive iodine and krypton control for nuclear fuel reprocessing. facilities
Fluorescent aminal linked porous organic polymer for reversible iodine capture and sensing
A novel triazene-anthracene-based fluorescent aminal linked porous organic polymer (TALPOP) was prepared via metal free-Schiff base polycondensation reaction of 9,10-bis-(4,6-diamino-S-triazin-2-yl)anthracene and 2-furaldehyde. The polymer has exceptional chemical and thermal stabilities and exhibit good porosity with Brunauer-Emmett-Teller surface area of 401 mg. The combination of such porosity along with the highly conjugated heteroatom-rich framework enabled the polymer to exhibit exceptional iodine vapor uptake of up to 314 wt % and reversible iodine adsorption in solution. Because of the inclusion of the anthracene moieties, the TALPOP exhibited excellent detection sensitivity towards iodine via florescence quenching with K value of 2.9 × 10 L mol. The cost effective TALPOP along with its high uptake and sensing of iodine, make it an ideal material for environmental remediation.
Comparative analysis of the countermeasures taken to mitigate exposure of the public to radioiodine following the Chernobyl and Fukushima accidents: lessons from both accidents
Radioactive iodine: an unappreciated threat to salivary gland function
Crystallization behavior of boron in low-temperature immobilization of iodine waste
Materials and processes for the effective capture and immobilization of radioiodine: a review
Experimental studies on retention of iodine in a wet scrubber
Porous sorbents for the capture of radioactive iodine compounds: a review
Study on adsorption performance of coal based activated carbon to radioactive iodine and stable iodine
Adsorption of iodine from aqueous solutions by aminosilane-grafted mesoporous alumina
Iodine capture with mechanically robust heat-treated Ag-Al-Si-O xerogel sorbents
Various radionuclides are released as gases during reprocessing of used nuclear fuel or during nuclear accidents including iodine-129 (I) and iodine-131 (I). These isotopes are of particular concern to the environment and human health as they are environmentally mobile and can cause thyroid cancer. In this work, silver-loaded heat-treated aluminosilicate xerogels (Ag-HTX) were evaluated as sorbents for iodine [I] capture. After synthesis of the base NaAlSiO xerogel, a heat-treatment step was performed to help increase the mechanical integrity of the NaAlSiO gels (Na-HTX) prior to Ag-exchanging to create Ag-HTX xerogels. Samples were characterized by powder X-ray diffraction, scanning electron microscopy, energy-dispersive X-ray spectroscopy, transmission electron microscopy, Brunauer-Emmett-Teller analysis, gravimetric iodine loading, nanoindentation, and dynamic mechanical analysis. The structural and chemical analyses of Ag-HTX showed uniform distribution of Ag throughout the gel network after Ag-exchange. After I capture, the AgI crystallites were observed in the sorbent, verifying chemisorption as the primary iodine capture mechanism. Iodine loading of this xerogel was 0.43 g g at 150 °C over 1 day and 0.52 g g at 22 °C over 33 days. The specific surface area of Ag-HTX was 202 m g and decreased to 87 m g after iodine loading. The hardness of the Na-HTX was >145 times higher than that of the heat-treated aerogel of the same starting composition. The heat-treatment process increased Young's modulus (compressive) value to 40.8 MPa from 7.0 MPa of as-made xerogel, demonstrating the need for this added step in the sample preparation process. These results show that Ag-HTX is a promising sorbent for I capture with good iodine loading capacity and mechanical stability.© 2021 The Authors. Published by American Chemical Society.
Efficient capture of iodine by a polysulfide-inserted inorganic NiTi-layered double hydroxides
Functional porous organic polymer with high S and N for reversible iodine capture
Metal organic framework MIL-101 for radioiodine capture and storage
IL-induced formation of dynamic complex iodide anions in IL@MOF composites for efficient iodine capture
Sorption and desorption of radioactive organic iodine by silver doped zeolite and zeolite X.
Bismuth-impregnated aluminum/copper oxide-pillared montmorillonite for efficient vapor iodine sorption
Bismuth-based materials for iodine capture and storage: a review
Development of the functionalized nanocomposite materials for adsorption/decontamination of radioactive pollutants
Superparamagnetic polyvinylpyrrolidone/chitosan/Fe3O4 electrospun nanofibers as effective U(VI) adsorbents
Nanosheets-built flowerlike micro/nanostructured Bi2O2.33 and its highly efficient iodine removal performances
Novel synthesis of bismuth-based adsorbents for the removal of 129I in off-gas
Viologen-based conjugated covalent organic networks via Zincke reaction
Sorption of iodine by polyurethane and melamine-formaldehyde foams using iodine sublimation and iodine solutions
Controllable synthesis of porous Cu-BTC@polymer composite beads for iodine capture
Al-O-F materials as novel adsorbents for gaseous radioiodine capture
Re-processing used nuclear fuel requires a method to effectively capture and dispose of gaseous radioiodine. Previous work has shown that nanoporous Al-O materials are effective at capturing gaseous iodine; molecular dynamics simulations have shown that the addition of fluoride to the Al-O surface should increase the amount of iodine capture. Twelve different materials with different ratios of F:Al were created. These materials were chemically characterized and functionally characterized with respect to gaseous iodine uptake. The addition of fluoride does in fact lead to a substantial (10-100×) increase in iodine uptake per unit surface area. However, the amount of uptake does not appear to be directly related to the total fluoride content of the solid phase material. Copyright © 2013 Elsevier Ltd. All rights reserved.
Adsorption of iodine on hydrogen-reduced silver-exchanged mordenite: experiments and modeling
Iodine adsorption on silver-exchanged titania-derived adsorbents
Functional monolithic polymeric organic framework aerogel as reducing and hosting media for Ag nanoparticles and application in capturing of iodine vapors
Gaseous iodine sorbents: a comparison between Ag-loaded aerogel and xerogel scaffolds
Novel synthesis of Bi-Bi2O3-TiO2-C composite for capturing iodine-129 in off-gas