The MN+1AXN phases: a new class of solids; thermodynamically stable nanolaminates
3
2000
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
... As previous reported, Sc2InC was listed as one of possible stable MAX phases[1,3], where the structure, properties and potential applications are investigated via theoretical predictions[18,19,20], but not been experimentally identified yet. The Sc2InC is expected to be a promising candidate for optoelectronic devices for the visible light and ultraviolet regions, as well as coating materials to avoid solar heating[20]. In addition, theoretical calculations indicate that the Sc2CT2 (T= F, OH) MXenes can be promising candidate materials for the next generation electronic devices[21]. Kuchida, et al[22] focused on non- transition metal M2AX compounds which embody Sc, Y, and Lu atoms in M site, however, only polycrystalline sample of Lu2SnC was reported. As a result, the study of new MAX phases taking Sc as M site element is an intriguing and challenging work. ...
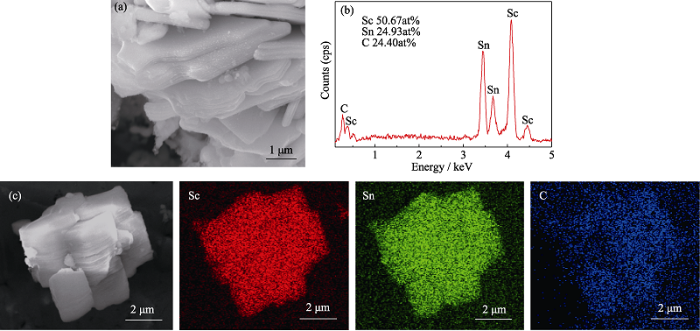
... It is well known that MAX phases crystallize in hexagonal structures and their grains are generally layered hexagonsin morphology[1]. To confirm that Sc2SnC has a similar microstructure, the microstructure of as-prepared powder was observed by SEM. It can be seen from Fig. 3(a) that Sc2SnC exhibits the microstructure of typical thin hexagons. EDS equipped in SEM detected all constitutive elements (Sc, Sn and C) within these particles (as shown in Fig. 3(b)). Although the EDS analysis is semi-quantitative and the accurate determination of light elements like C is difficult, the relative atomic ratio of (Sc : Sn : C) could be revealed by EDS as about (2 : 1 : 1), consistent with the stoichiometry of 211 MAX phases. The elemental mapping of Sc, Sn and C corroborated that all of these three elements have the same distribution. The above results further confirm that the new MAX phase compound Sc2SnC is experimentally synthesized. ...
On the chemical diversity of the MAX phases
1
2009
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
The Mn+1AXn phases: materials science and thin-film processing
2
2010
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
... As previous reported, Sc2InC was listed as one of possible stable MAX phases[1,3], where the structure, properties and potential applications are investigated via theoretical predictions[18,19,20], but not been experimentally identified yet. The Sc2InC is expected to be a promising candidate for optoelectronic devices for the visible light and ultraviolet regions, as well as coating materials to avoid solar heating[20]. In addition, theoretical calculations indicate that the Sc2CT2 (T= F, OH) MXenes can be promising candidate materials for the next generation electronic devices[21]. Kuchida, et al[22] focused on non- transition metal M2AX compounds which embody Sc, Y, and Lu atoms in M site, however, only polycrystalline sample of Lu2SnC was reported. As a result, the study of new MAX phases taking Sc as M site element is an intriguing and challenging work. ...
Radiation tolerance of Mn+1AXn phases, Ti3AlC2 and Ti3SiC2
1
2010
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
Synthesis of Ti3AuC2, Ti3Au2C2 and Ti3IrC2 by noble metal substitution reaction in Ti3SiC2 for high-temperature-stable Ohmic contacts to SiC
1
2017
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
Corrosion performance of Ti3SiC2, Ti3AlC2, Ti2AlC and Cr2AlC MAX phases in simulated primary water conditions
1
2018
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
Tribological properties of Ti3SiC2 coupled with different counterfaces
1
2012
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2
1
2011
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide
1
2013
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
2D metal carbides and nitrides (MXenes) for energy storage
1
2017
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte
1
2020
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
Synthesis and characterization of a new (Ti1-ε,Cuε)3(Al,Cu)C2 MAX phase solid solution
1
2019
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes
2
2019
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
... Density functional theory (DFT) calculations were programmed in the CASTEP code[29,30], using the generalized gradient approximation (GGA) as implemented in the Perdew-Breke-Ernzerhof (PBE) functional[31,32]. Phonon calculations were carried out to evaluate the dynamical stability using the finite displacement approach, as implemented in CASTEP[33,34]. The equation E= (Ebroken-Ebulk)/S[13] was adopted to calculate the cleavage energy E, where Ebulk and Ebroken represent the total energies of bulk MAX and the cleaving structures respectively with a 1 nm vacuum separation in the corresponding M and A atomic layers, and S is the cross- sectional surface area of the MAX phase materials. The Rietveld refinement of powder XRD pattern of Sc2SnC was by Total Pattern Solution (TOPAS-Academic V6) software. ...
Single-atom-thick active layers realized in nanolaminated Ti3(AlxCu1-x)C2 and its artificial enzyme behavior
1
2019
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
Synthesis of MAX phases Nb2CuC and Ti2(Al0.1Cu0.9)N by A-site replacement reaction in molten salts
1
2019
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
Multielemental single-atom-thick A layers in nanolaminated V2(Sn,A)C (A=Fe, Co, Ni, Mn) for tailoring magnetic properties
2
2020
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
... The powders were mixed in a stoichiometric ratio of Sc : Sn : C=2 : 1.1 : 1 (Due to the melting point of Sn is relatively low, we increased the content ratio of tin for compensating the weight loss of tin at a high temperature, as in the preparation of V2(Sn,A)C MAX phases)[16]. The starting powders of Sc, Sn and graphite are mixed with inorganic salt (NaCl + KCl), and the mole ratio of (Sc + Sn + C) : (NaCl + KCl) was 1 : 10, and the mole ratio of (NaCl : KCl) was 1 : 1. After ground for 10 min, the powder mixture was put into an aluminum oxide boat, and then moved to a tube furnace and heated to 1000 ℃ for 3 h at heating rate of 5 ℃/min under argon atmosphere, respectively. After the reaction was finished, the product was washed, filtered and dried at 40 ℃ in vacuum; and the excess Sn element was removed by ferric chloride (Aladdim Industrial Co. Ltd, Shanghai, China; FeCl3, 99.5wt% purity). ...
A genomic approach to the stability, elastic, and electronic properties of the MAX phases
1
2014
... The MAX phases are a family of nanolayered ternary carbides or nitrides with a hexagonal lattice structure (P63/mmc), the chemical formula is Mn+1AXn (where M is an early transition metal; A is an element mainly from 13-16; and X is carbon or/and nitrogen, n=1-3)[1,2,3]. Generally, the heterodesmic feature of MAX phases contributes to a unique combination of both metallic and ceramic properties, which have been investigated as promising candidates for structural applications in many fields[4,5,6,7]. Moreover, MAX phases are used as a precursor to synthesize two-dimensional (2D) MXene with many attractive physical and chemical properties, and show promise in a broad range of applications, notably electrochemical energy storage[8,9,10,11]. Due to the continuing efforts from the scientific community, about 155 MAX phases have been reported so far, including some novel MAX phases that A-site elements are late transition metals[12,13,14,15,16]. The theoretical studies have predicted around 665 ternary MAX phases that could be experimentally synthesized[17], for example, the ones which M site element is rare earth Sc. ...
First-principles study of structural and elastic properties of Sc2AC (A=Al, Ga, In, Tl)
1
2008
... As previous reported, Sc2InC was listed as one of possible stable MAX phases[1,3], where the structure, properties and potential applications are investigated via theoretical predictions[18,19,20], but not been experimentally identified yet. The Sc2InC is expected to be a promising candidate for optoelectronic devices for the visible light and ultraviolet regions, as well as coating materials to avoid solar heating[20]. In addition, theoretical calculations indicate that the Sc2CT2 (T= F, OH) MXenes can be promising candidate materials for the next generation electronic devices[21]. Kuchida, et al[22] focused on non- transition metal M2AX compounds which embody Sc, Y, and Lu atoms in M site, however, only polycrystalline sample of Lu2SnC was reported. As a result, the study of new MAX phases taking Sc as M site element is an intriguing and challenging work. ...
A comprehensive survey of MAX phase elastic properties
1
2009
... As previous reported, Sc2InC was listed as one of possible stable MAX phases[1,3], where the structure, properties and potential applications are investigated via theoretical predictions[18,19,20], but not been experimentally identified yet. The Sc2InC is expected to be a promising candidate for optoelectronic devices for the visible light and ultraviolet regions, as well as coating materials to avoid solar heating[20]. In addition, theoretical calculations indicate that the Sc2CT2 (T= F, OH) MXenes can be promising candidate materials for the next generation electronic devices[21]. Kuchida, et al[22] focused on non- transition metal M2AX compounds which embody Sc, Y, and Lu atoms in M site, however, only polycrystalline sample of Lu2SnC was reported. As a result, the study of new MAX phases taking Sc as M site element is an intriguing and challenging work. ...
Predicted MAX phase Sc2InC: dynamical stability, vibrational and optical properties
3
2018
... As previous reported, Sc2InC was listed as one of possible stable MAX phases[1,3], where the structure, properties and potential applications are investigated via theoretical predictions[18,19,20], but not been experimentally identified yet. The Sc2InC is expected to be a promising candidate for optoelectronic devices for the visible light and ultraviolet regions, as well as coating materials to avoid solar heating[20]. In addition, theoretical calculations indicate that the Sc2CT2 (T= F, OH) MXenes can be promising candidate materials for the next generation electronic devices[21]. Kuchida, et al[22] focused on non- transition metal M2AX compounds which embody Sc, Y, and Lu atoms in M site, however, only polycrystalline sample of Lu2SnC was reported. As a result, the study of new MAX phases taking Sc as M site element is an intriguing and challenging work. ...
... [20]. In addition, theoretical calculations indicate that the Sc2CT2 (T= F, OH) MXenes can be promising candidate materials for the next generation electronic devices[21]. Kuchida, et al[22] focused on non- transition metal M2AX compounds which embody Sc, Y, and Lu atoms in M site, however, only polycrystalline sample of Lu2SnC was reported. As a result, the study of new MAX phases taking Sc as M site element is an intriguing and challenging work. ...
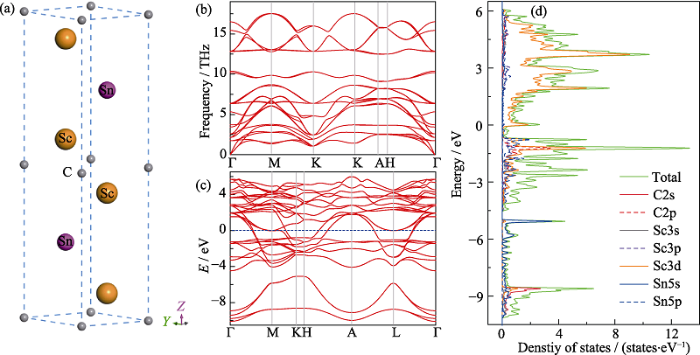
... The relative small value of C33 indicates that the compound is more compressible along the c-axis compared to other studied compounds; while low C44 indicates being subject to shear deformation along $[11\bar{2}0]$ (0001); and small C66 probably means lower resistance to shear in the <110> direction[20,35,37]. The low shear deformation of Sc2SnC is also reflected from shear modulus G, which represents the resistance to shape change of the polycrystalline material[38]. The calculated value of G/B >0.5 indicates that the phase is brittle in nature following Pugh’s criterion. Furthermore, the obtained value of v (0.238) for Sc2SnC shows that it locates at the boundary between covalent and ionic materials. The calculated band structure of Sc2SnC and the projected density of states (DOS) of Sc, Sn, and C atoms with k-points are shown in Fig. 4(c, d), respectively. Similar to other MAX phases and MAX phase-like compounds, Sc2SnC exhibits metallic nature, and the overlapping between valence and conduction bands across the Fermi level also reveals the presence of metallic bonding, which can be treated as the origin of the quasi-ductility of Sc2SnC (Fig. 4(c)). From Fig. 4(d), it can be observed that the Sc-3d electrons are mainly contributing to the DOS at the Fermi level, and should be involved in the conduction properties, while the minor contributions come from Sn5p electrons. ...
Bipolar magnetic semiconductors among intermediate states during the conversion from Sc2C(OH)2 to Sc2CO2 MXene
1
2018
... As previous reported, Sc2InC was listed as one of possible stable MAX phases[1,3], where the structure, properties and potential applications are investigated via theoretical predictions[18,19,20], but not been experimentally identified yet. The Sc2InC is expected to be a promising candidate for optoelectronic devices for the visible light and ultraviolet regions, as well as coating materials to avoid solar heating[20]. In addition, theoretical calculations indicate that the Sc2CT2 (T= F, OH) MXenes can be promising candidate materials for the next generation electronic devices[21]. Kuchida, et al[22] focused on non- transition metal M2AX compounds which embody Sc, Y, and Lu atoms in M site, however, only polycrystalline sample of Lu2SnC was reported. As a result, the study of new MAX phases taking Sc as M site element is an intriguing and challenging work. ...
Superconductivity in Lu2SnC
1
2013
... As previous reported, Sc2InC was listed as one of possible stable MAX phases[1,3], where the structure, properties and potential applications are investigated via theoretical predictions[18,19,20], but not been experimentally identified yet. The Sc2InC is expected to be a promising candidate for optoelectronic devices for the visible light and ultraviolet regions, as well as coating materials to avoid solar heating[20]. In addition, theoretical calculations indicate that the Sc2CT2 (T= F, OH) MXenes can be promising candidate materials for the next generation electronic devices[21]. Kuchida, et al[22] focused on non- transition metal M2AX compounds which embody Sc, Y, and Lu atoms in M site, however, only polycrystalline sample of Lu2SnC was reported. As a result, the study of new MAX phases taking Sc as M site element is an intriguing and challenging work. ...
Salt melt synthesis of ceramics, semiconductors and carbon nanostructures
1
2013
... Now, the common methods to synthesize MAX phases are hot pressing (HP) and spark plasma sintering (SPS). Compared to HP and SPS, the molten salt method is a simple and cost-effective route for preparing MAX phase powders. As a high-temperature ionic solvent, the molten salt bath offers high solvation power and liquid environment for reactants that will greatly facilitate the mass transport and nucleation processes, thus need lower synthesis temperature and bold time[23]. Some MAX phases (e.g. Ti3SiC2, Ti3AlC2, V2AlC, Cr2AlC) have been synthesized by molten salt method[24,25,26,27,28]. In the present work, we synthesized a MAX phase of Sc2SnC in molten salts environment where the Sc element belongs to rare earth. The crystal structure and chemical composition were confirmed by XRD and SEM-EDS, respectively. Furthermore, the structure stability, electronic structure and mechanical properties of Sc2SnC are also be investigated via density functional theory (DFT). ...
Synthesis and oxidation resistance of V2AlC powders by molten salt method
1
2017
... Now, the common methods to synthesize MAX phases are hot pressing (HP) and spark plasma sintering (SPS). Compared to HP and SPS, the molten salt method is a simple and cost-effective route for preparing MAX phase powders. As a high-temperature ionic solvent, the molten salt bath offers high solvation power and liquid environment for reactants that will greatly facilitate the mass transport and nucleation processes, thus need lower synthesis temperature and bold time[23]. Some MAX phases (e.g. Ti3SiC2, Ti3AlC2, V2AlC, Cr2AlC) have been synthesized by molten salt method[24,25,26,27,28]. In the present work, we synthesized a MAX phase of Sc2SnC in molten salts environment where the Sc element belongs to rare earth. The crystal structure and chemical composition were confirmed by XRD and SEM-EDS, respectively. Furthermore, the structure stability, electronic structure and mechanical properties of Sc2SnC are also be investigated via density functional theory (DFT). ...
Cr2AlC powders prepared by molten salt method
1
2008
... Now, the common methods to synthesize MAX phases are hot pressing (HP) and spark plasma sintering (SPS). Compared to HP and SPS, the molten salt method is a simple and cost-effective route for preparing MAX phase powders. As a high-temperature ionic solvent, the molten salt bath offers high solvation power and liquid environment for reactants that will greatly facilitate the mass transport and nucleation processes, thus need lower synthesis temperature and bold time[23]. Some MAX phases (e.g. Ti3SiC2, Ti3AlC2, V2AlC, Cr2AlC) have been synthesized by molten salt method[24,25,26,27,28]. In the present work, we synthesized a MAX phase of Sc2SnC in molten salts environment where the Sc element belongs to rare earth. The crystal structure and chemical composition were confirmed by XRD and SEM-EDS, respectively. Furthermore, the structure stability, electronic structure and mechanical properties of Sc2SnC are also be investigated via density functional theory (DFT). ...
Molten salt synthesis of MAX phases in the Ti-Al-C system
1
2018
... Now, the common methods to synthesize MAX phases are hot pressing (HP) and spark plasma sintering (SPS). Compared to HP and SPS, the molten salt method is a simple and cost-effective route for preparing MAX phase powders. As a high-temperature ionic solvent, the molten salt bath offers high solvation power and liquid environment for reactants that will greatly facilitate the mass transport and nucleation processes, thus need lower synthesis temperature and bold time[23]. Some MAX phases (e.g. Ti3SiC2, Ti3AlC2, V2AlC, Cr2AlC) have been synthesized by molten salt method[24,25,26,27,28]. In the present work, we synthesized a MAX phase of Sc2SnC in molten salts environment where the Sc element belongs to rare earth. The crystal structure and chemical composition were confirmed by XRD and SEM-EDS, respectively. Furthermore, the structure stability, electronic structure and mechanical properties of Sc2SnC are also be investigated via density functional theory (DFT). ...
Preparation of Ti3SiC2 powders by the molten salt method
1
2013
... Now, the common methods to synthesize MAX phases are hot pressing (HP) and spark plasma sintering (SPS). Compared to HP and SPS, the molten salt method is a simple and cost-effective route for preparing MAX phase powders. As a high-temperature ionic solvent, the molten salt bath offers high solvation power and liquid environment for reactants that will greatly facilitate the mass transport and nucleation processes, thus need lower synthesis temperature and bold time[23]. Some MAX phases (e.g. Ti3SiC2, Ti3AlC2, V2AlC, Cr2AlC) have been synthesized by molten salt method[24,25,26,27,28]. In the present work, we synthesized a MAX phase of Sc2SnC in molten salts environment where the Sc element belongs to rare earth. The crystal structure and chemical composition were confirmed by XRD and SEM-EDS, respectively. Furthermore, the structure stability, electronic structure and mechanical properties of Sc2SnC are also be investigated via density functional theory (DFT). ...
Molten salt shielded synthesis (MS3) of Ti2AlN and V2AlC MAX phase powders in open air
1
2020
... Now, the common methods to synthesize MAX phases are hot pressing (HP) and spark plasma sintering (SPS). Compared to HP and SPS, the molten salt method is a simple and cost-effective route for preparing MAX phase powders. As a high-temperature ionic solvent, the molten salt bath offers high solvation power and liquid environment for reactants that will greatly facilitate the mass transport and nucleation processes, thus need lower synthesis temperature and bold time[23]. Some MAX phases (e.g. Ti3SiC2, Ti3AlC2, V2AlC, Cr2AlC) have been synthesized by molten salt method[24,25,26,27,28]. In the present work, we synthesized a MAX phase of Sc2SnC in molten salts environment where the Sc element belongs to rare earth. The crystal structure and chemical composition were confirmed by XRD and SEM-EDS, respectively. Furthermore, the structure stability, electronic structure and mechanical properties of Sc2SnC are also be investigated via density functional theory (DFT). ...
First principles methods using CASTEP
1
2005
... Density functional theory (DFT) calculations were programmed in the CASTEP code[29,30], using the generalized gradient approximation (GGA) as implemented in the Perdew-Breke-Ernzerhof (PBE) functional[31,32]. Phonon calculations were carried out to evaluate the dynamical stability using the finite displacement approach, as implemented in CASTEP[33,34]. The equation E= (Ebroken-Ebulk)/S[13] was adopted to calculate the cleavage energy E, where Ebulk and Ebroken represent the total energies of bulk MAX and the cleaving structures respectively with a 1 nm vacuum separation in the corresponding M and A atomic layers, and S is the cross- sectional surface area of the MAX phase materials. The Rietveld refinement of powder XRD pattern of Sc2SnC was by Total Pattern Solution (TOPAS-Academic V6) software. ...
First-principles simulation: ideas, illustrations and the CASTEP code
1
2002
... Density functional theory (DFT) calculations were programmed in the CASTEP code[29,30], using the generalized gradient approximation (GGA) as implemented in the Perdew-Breke-Ernzerhof (PBE) functional[31,32]. Phonon calculations were carried out to evaluate the dynamical stability using the finite displacement approach, as implemented in CASTEP[33,34]. The equation E= (Ebroken-Ebulk)/S[13] was adopted to calculate the cleavage energy E, where Ebulk and Ebroken represent the total energies of bulk MAX and the cleaving structures respectively with a 1 nm vacuum separation in the corresponding M and A atomic layers, and S is the cross- sectional surface area of the MAX phase materials. The Rietveld refinement of powder XRD pattern of Sc2SnC was by Total Pattern Solution (TOPAS-Academic V6) software. ...
Generalized gradient approximation made simple
1
1996
... Density functional theory (DFT) calculations were programmed in the CASTEP code[29,30], using the generalized gradient approximation (GGA) as implemented in the Perdew-Breke-Ernzerhof (PBE) functional[31,32]. Phonon calculations were carried out to evaluate the dynamical stability using the finite displacement approach, as implemented in CASTEP[33,34]. The equation E= (Ebroken-Ebulk)/S[13] was adopted to calculate the cleavage energy E, where Ebulk and Ebroken represent the total energies of bulk MAX and the cleaving structures respectively with a 1 nm vacuum separation in the corresponding M and A atomic layers, and S is the cross- sectional surface area of the MAX phase materials. The Rietveld refinement of powder XRD pattern of Sc2SnC was by Total Pattern Solution (TOPAS-Academic V6) software. ...
Soft self-consistent pseudopotentials in a generalized eigenvalue formalism
1
1990
... Density functional theory (DFT) calculations were programmed in the CASTEP code[29,30], using the generalized gradient approximation (GGA) as implemented in the Perdew-Breke-Ernzerhof (PBE) functional[31,32]. Phonon calculations were carried out to evaluate the dynamical stability using the finite displacement approach, as implemented in CASTEP[33,34]. The equation E= (Ebroken-Ebulk)/S[13] was adopted to calculate the cleavage energy E, where Ebulk and Ebroken represent the total energies of bulk MAX and the cleaving structures respectively with a 1 nm vacuum separation in the corresponding M and A atomic layers, and S is the cross- sectional surface area of the MAX phase materials. The Rietveld refinement of powder XRD pattern of Sc2SnC was by Total Pattern Solution (TOPAS-Academic V6) software. ...
Ab initio force-constant method for phonon dispersions in alkali metals
1
1995
... Density functional theory (DFT) calculations were programmed in the CASTEP code[29,30], using the generalized gradient approximation (GGA) as implemented in the Perdew-Breke-Ernzerhof (PBE) functional[31,32]. Phonon calculations were carried out to evaluate the dynamical stability using the finite displacement approach, as implemented in CASTEP[33,34]. The equation E= (Ebroken-Ebulk)/S[13] was adopted to calculate the cleavage energy E, where Ebulk and Ebroken represent the total energies of bulk MAX and the cleaving structures respectively with a 1 nm vacuum separation in the corresponding M and A atomic layers, and S is the cross- sectional surface area of the MAX phase materials. The Rietveld refinement of powder XRD pattern of Sc2SnC was by Total Pattern Solution (TOPAS-Academic V6) software. ...
First-principles determination of the soft mode in cubic ZrO2
1
1997
... Density functional theory (DFT) calculations were programmed in the CASTEP code[29,30], using the generalized gradient approximation (GGA) as implemented in the Perdew-Breke-Ernzerhof (PBE) functional[31,32]. Phonon calculations were carried out to evaluate the dynamical stability using the finite displacement approach, as implemented in CASTEP[33,34]. The equation E= (Ebroken-Ebulk)/S[13] was adopted to calculate the cleavage energy E, where Ebulk and Ebroken represent the total energies of bulk MAX and the cleaving structures respectively with a 1 nm vacuum separation in the corresponding M and A atomic layers, and S is the cross- sectional surface area of the MAX phase materials. The Rietveld refinement of powder XRD pattern of Sc2SnC was by Total Pattern Solution (TOPAS-Academic V6) software. ...
Theoretical prediction, synthesis, and crystal structure determination of new MAX phase compound V2SnC
2
2020
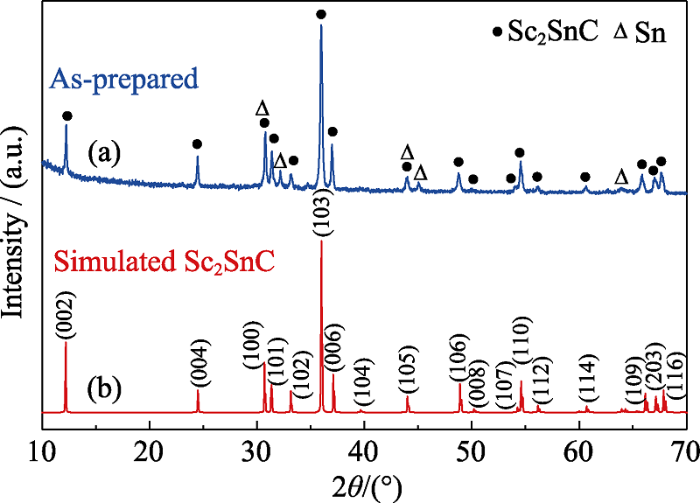
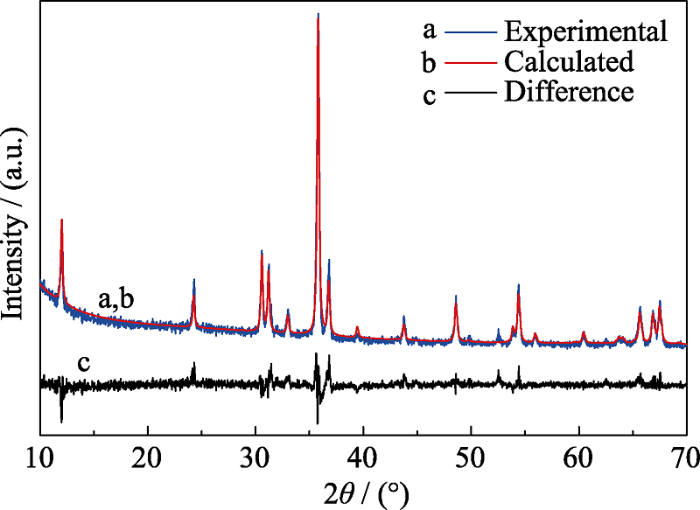
... XRD pattern is important for phase identification and structure analysis. Because no XRD pattern of Sc2SnC was available in previous literatures, the Rietveld refinement of powder XRD pattern of Sc2SnC was conducted. As shown in Fig. 2, the blue crosses represent the experimental diffraction profile (the Sn metal was remove by FeCl3 solution), while the red solid line denotes the theoretical pattern. The theoretical Bragg diffraction positions of Sc2SnC are marked as red line. The gray curve is the deviation between calculated and experimental XRD patterns. The obtained reliability factors are Rp=8.56% and Rwp=11.19%, respectively, indicating good agreement between model and measured data. The space group of Sc2SnC is P63/mmc (194), and the lattice constants measured from XRD pattern are a=0.33692 nm and c=1.46374 nm, respectively. The difference between theoretical calculation and the Rietveld refinement is probably ascribed to the existence of defects in the crystal structure, as the case of V2SnC in the previous report[35]. The atomic positions of Sc2SnC determined from the Rietveld refinement are listed in Table 1. ...
... The relative small value of C33 indicates that the compound is more compressible along the c-axis compared to other studied compounds; while low C44 indicates being subject to shear deformation along $[11\bar{2}0]$ (0001); and small C66 probably means lower resistance to shear in the <110> direction[20,35,37]. The low shear deformation of Sc2SnC is also reflected from shear modulus G, which represents the resistance to shape change of the polycrystalline material[38]. The calculated value of G/B >0.5 indicates that the phase is brittle in nature following Pugh’s criterion. Furthermore, the obtained value of v (0.238) for Sc2SnC shows that it locates at the boundary between covalent and ionic materials. The calculated band structure of Sc2SnC and the projected density of states (DOS) of Sc, Sn, and C atoms with k-points are shown in Fig. 4(c, d), respectively. Similar to other MAX phases and MAX phase-like compounds, Sc2SnC exhibits metallic nature, and the overlapping between valence and conduction bands across the Fermi level also reveals the presence of metallic bonding, which can be treated as the origin of the quasi-ductility of Sc2SnC (Fig. 4(c)). From Fig. 4(d), it can be observed that the Sc-3d electrons are mainly contributing to the DOS at the Fermi level, and should be involved in the conduction properties, while the minor contributions come from Sn5p electrons. ...
On the stability of crystal lattices
1
1940
... The structural analysis of Sc2SnC phase was carried out via DFT calculations. Fig. 4(a) shows the ternary- layered carbide crystal structure of Sc2SnC; and the calculated ΔHform (Sc2SnC) is -0.7167 eV, indicating the stability of Sc2SnC phase. The lattice parameters, elastic constants and polycrystalline elastic modulus of Sc2SnC, as well as for other Sn-containing MAX phases are listed in Table 2. From the DFT calculation result, i.e. a= 0.33686, c=1.46532 nm, is very close to experimental results. The mechanical stability of Sc2SnC is justified from the Born stability criteria[36]: C11>0, C11-C12>0, C44>0, (C11-C12)C33-2C13>0. Besides, the dynamical stability of Sc2SnC can also be identified from the phonon dispersion curves in Fig. 4(b). The results performed by theoretical calculations are consistent with experiments. However, compared with other MAX phases (listed in Table 2), it is found that they have lower values of elastic constants (i.e. C11, C33, C44, and C66). ...
Mechanical, electronic, and optical properties of Bi2S3 and Bi2Se3 compounds: first principle investigations
1
2014
... The relative small value of C33 indicates that the compound is more compressible along the c-axis compared to other studied compounds; while low C44 indicates being subject to shear deformation along $[11\bar{2}0]$ (0001); and small C66 probably means lower resistance to shear in the <110> direction[20,35,37]. The low shear deformation of Sc2SnC is also reflected from shear modulus G, which represents the resistance to shape change of the polycrystalline material[38]. The calculated value of G/B >0.5 indicates that the phase is brittle in nature following Pugh’s criterion. Furthermore, the obtained value of v (0.238) for Sc2SnC shows that it locates at the boundary between covalent and ionic materials. The calculated band structure of Sc2SnC and the projected density of states (DOS) of Sc, Sn, and C atoms with k-points are shown in Fig. 4(c, d), respectively. Similar to other MAX phases and MAX phase-like compounds, Sc2SnC exhibits metallic nature, and the overlapping between valence and conduction bands across the Fermi level also reveals the presence of metallic bonding, which can be treated as the origin of the quasi-ductility of Sc2SnC (Fig. 4(c)). From Fig. 4(d), it can be observed that the Sc-3d electrons are mainly contributing to the DOS at the Fermi level, and should be involved in the conduction properties, while the minor contributions come from Sn5p electrons. ...
Relations between the elastic moduli and the plastic properties of polycrystalline pure metals
1
1954
... The relative small value of C33 indicates that the compound is more compressible along the c-axis compared to other studied compounds; while low C44 indicates being subject to shear deformation along $[11\bar{2}0]$ (0001); and small C66 probably means lower resistance to shear in the <110> direction[20,35,37]. The low shear deformation of Sc2SnC is also reflected from shear modulus G, which represents the resistance to shape change of the polycrystalline material[38]. The calculated value of G/B >0.5 indicates that the phase is brittle in nature following Pugh’s criterion. Furthermore, the obtained value of v (0.238) for Sc2SnC shows that it locates at the boundary between covalent and ionic materials. The calculated band structure of Sc2SnC and the projected density of states (DOS) of Sc, Sn, and C atoms with k-points are shown in Fig. 4(c, d), respectively. Similar to other MAX phases and MAX phase-like compounds, Sc2SnC exhibits metallic nature, and the overlapping between valence and conduction bands across the Fermi level also reveals the presence of metallic bonding, which can be treated as the origin of the quasi-ductility of Sc2SnC (Fig. 4(c)). From Fig. 4(d), it can be observed that the Sc-3d electrons are mainly contributing to the DOS at the Fermi level, and should be involved in the conduction properties, while the minor contributions come from Sn5p electrons. ...
Theoretical study of mechanical, electronic, chemical bonding and optical properties of Ti2SnC, Zr2SnC, Hf2SnC and Nb2SnC
0
2009