电致变色是指在电场作用下, 材料的颜色或光学性能发生可逆变化的现象[1,2,3,4]。具有电致变色性能的材料称为电致变色材料, 这些材料组装成的器件称为电致变色器件。目前, 电致变色技术在智能窗、显示器、防眩后视镜、电子纸、军事伪装等领域都有广泛的应用前景[5,6,7,8,9]。随着科技的不断发展, 电致变色技术的应用领域日新月异。相对于其它种类的显示器件, 电致变色显示器件具有色彩丰富、对比度高、无视盲角、断电后仍显色等优点[10]。最近在智能手机领域, 一加手机推出了首款概念手机OnePlus Concept One, 首次利用电致变色技术, 实现了“潜隐式后摄”的效果, 将摄像头巧妙地隐藏起来。Vivo手机厂商官方宣布了手机背盖的电致变色技术, 用户可根据自身喜好定义多种手机配色, 实现“千人千面”。这种依托于电致变色技术的手机或将引领智能手机行业的新潮流, 从而推动电致变色技术进一步走进大众视野。
1 电致变色材料与器件
1.1 电致变色材料
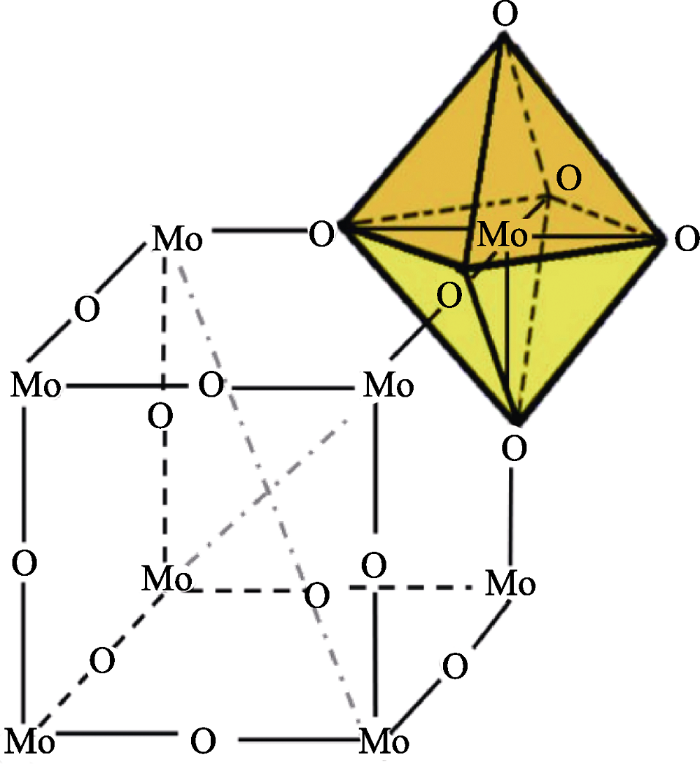
自20世纪60年代, Platt[11]在研究有机染料时首次发现电致变色现象以来, 研究人员对电致变色现象进行了大量的研究, 极大地丰富了电致变色材料。电致变色材料可分为有机电致变色材料与无机电致变色材料。有机电致变色材料颜色种类多、光学性能好、变色速率快, 但易老化和氧化, 典型代表有紫罗精类化合物[12,13]、导电聚合物[14,15]。无机电致变色材料优点是结构与性能稳定性好, 缺点是颜色单一, 典型代表有WO3[16]、MoO3[17]、NiO[18]等。根据着色电位的不同, 即发生电化学氧化还原反应时是氧化态着色还是还原态着色, 电致变色材料可分为阳极电致变色材料和阴极电致变色材料。
$\mathrm{MoO}_{3}(\text { Colorless })+x \mathrm{~A}^{+}+x \mathrm{e}^{-} \leftrightarrow \mathrm{A}_{x} \mathrm{MoO}_{3}(\text { Blue })$
图1
其中0<x<1; A+可以是Li+、Na+、H+等。
1.2 电致变色器件的结构与工作原理
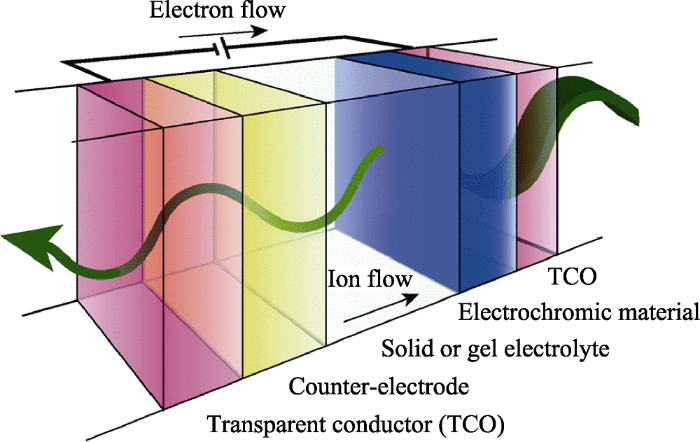
电致变色器件主要由基底层、透明导电(Transmittance Conducitive, TC)层、电致变色(Electrochromic, EC)层、电解质(Electrolyte, EL)层和离子存储(Ion Storage, IS)层组成[27,28], 如图2所示。基底层一般为玻璃或柔性的聚对苯二甲酸乙二醇酯(Polyethylene Terephthalate, PET), 透明导电层一般为氟掺杂氧化锡(Fluorine-doped Tin Oxide, FTO)或氧化铟锡(Indium Tin Oxide, ITO)。当在TC层上施加电压时, EL层中的离子迁移到EC层中, 同时电子从TC层传入EC层中, 导致电致变色材料发生离子注入和氧化还原反应, 出现着色(或褪色)现象。当施加反向电压时, 已经注入EC层中离子便会抽出, 同时电子传回TC层而发生褪色(或着色)现象。这便是电致变色器件工作的基本原理。
图2
1.3 电致变色器件的性能评价
(1)光调制幅度: 电致变色器件在褪色态与着色态之间的透过率之差。电致变色器件的光调制幅度越大, 说明该器件的颜色变化越明显。
(2)响应时间: 电致变色过程所花费的时间, 一般定义为着色态与褪色态之间的透过率差值达到90%时所对应的时间间隔。其中, 电致变色器件从着色态向褪色态转变的响应时间称为褪色响应时间, 从褪色态向着色态转变的响应时间称为着色响应时间。
(3)着色效率(Coloration Efficiency, CE): 单位面积内所消耗的电荷引起的光密度的变化量。$CE=\Delta\mathrm{OD}/(Q/A)$, $\Delta\mathrm{OD}=lg(T_{b}/T_{c})$, 其中$T_{b}$和$T_{c}$分别为电致变色器件在特定波长下褪色态和着色态的透过率, ∆OD为光密度变化量, Q为注入的电荷, A为电致变色器件的有效面积。
(4)循环寿命: 电致变色器件在着色态与褪色态之间反复变色的最大次数, 当不再满足变色需求时寿命终止。
电致变色层是电致变色器件的关键组成部分, 对电致变色器件的性能起到决定性作用。本文将对MoO3电致变色薄膜的制备方法、改性研究及MoO3基电致变色器件的研究进展进行综述, 并对目前存在的问题及发展前景进行分析与展望。
2 氧化钼薄膜的制备
2.1 气相法
2.1.1 化学气相沉积(CVD)
化学气相沉积(Chemical Vapor Deposition, CVD)[31]是指在可通气、加热的反应器中, 利用气体传输在固体上发生化学反应产生固态沉积物。即将一种或多种化合物放置在反应器中, 通气, 利用气体相互作用或在基底表面上的化学反应生成所需的薄膜。CVD法广泛应用于物质提纯、无机薄膜制备、纤维涂层等方面, 其优点是沉积成膜装置简单, 可以在各种衬底上制备元素及化合物薄膜。CVD法可以连续运行, 不用分批处理, 可以大规模制备样品;缺点是需要较高的温度、能量损耗大、沉积速率低。Gesheva等[32]采用CVD法制备氧化钼及钨钼氧化物薄膜, 其中钨钼氧化物具有较高的着色效率(110 mC·cm-2)。Ivanova等[33]采用低温CVD方法在150~200 ℃之间合成了α-MoO3薄膜以及MoO3- WO3薄膜, 合成的两种薄膜均具有电致变色性能, MoO3-WO3薄膜比纯金属氧化物MoO3和WO3薄膜具有更大的电流密度。
2.1.2 物理气相沉积(PVD)
物理气相沉积(Physical Vapour Deposition, PVD)法制备薄膜主要包括磁控溅射和真空蒸发镀膜。磁控溅射[34]是利用磁场与电场交互作用, 在真空条件下注入惰性气体, 惰性气体被电离后产生Ar+轰击靶材, 通过离子的动量传递, 靶上的原子、分子或离子被溅射, 在基片表面形成致密的薄膜。磁控溅射具有沉积速度快、溅射能量低、基板温度低的优点。根据制备条件的不同, 磁控溅射既可以制备晶态薄膜又可以制备非晶薄膜, 成膜均匀性好且与基板的附着力好。Usha等[35]以150 W的射频功率沉积Nb2O5 : MoO3薄膜, 制备的薄膜具有电致变色性能, 且当Nb2O5 : MoO3比例为85 : 15时, 薄膜的着色效率达到最大值230.3 cm2·C-1。
真空蒸发镀膜法[36,37]又叫热沉积法或热蒸发法, 是将原材料在真空室内加热, 使材料原子或分子从表面汽化逸出, 再到达衬底表面, 形成固态薄膜的办法。用该方法制备的氧化钼薄膜的电致变色性能与衬底温度、氧分压等因素密切相关。真空蒸发镀膜法设备简单、操作容易, 制备薄膜速度快、效率高, 薄膜生长机理简单。Miyata等[38]以纯度为99.99%的MoO3粉末为原料, 采用热蒸发法沉积MoO3薄膜, 研究了基板温度对MoO3薄膜性能的影响。结果表明, 基板温度低于200 ℃所形成的薄膜具有良好的电致变色性能, 而高于320 ℃得到的薄膜电致变色性能较差。Dixit等[39]以纯度为99.97%的MoO3粉末为原料, 采用可变电阻式高真空镀膜装置将MoO3粉末用热蒸发法沉积在ITO上, 研究了氧分压对MoO3薄膜生长的影响, 并测试其电致变色性能。结果表明, 在氧气分压为0.02 Pa、基板温度为150 ℃的条件下, 薄膜在442 nm的着色效率为23.98 cm2·C-1。
2.2 湿化学法
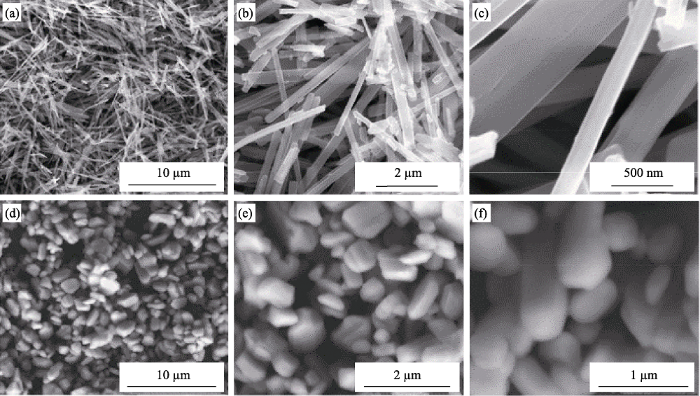
2.2.1 水/溶剂热法
图3
图4
2.2.2 溶胶-凝胶法
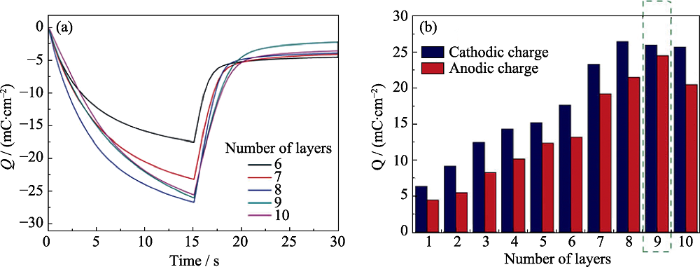
溶胶-凝胶法[46,47]是将含高化学活性组分的化合物经过溶液、溶胶、凝胶而固化, 再经热处理形成氧化物或其它化合物固体的方法。溶胶-凝胶法的优点是所需设备简单、成本低, 易在分子水平上控制薄膜的组分掺杂或复合, 适合大批量生产, 常用于氧化物的制备。Lemos等[26]使用溶胶-凝胶工艺和旋涂技术制备MoO3薄膜, 研究了薄膜厚度对其电化学性能的影响, 如图5所示。从图5(a)中可以看出电荷密度与薄膜的层数密切相关, 图5(b)表明当MoO3薄膜层数为9时, 电化学性能和电化学过程可逆性最佳, 其中阳极电荷(Qa)和阴极电荷(Qc)之比为0.98。Dhanasankar等[48]采用溶胶-凝胶法制备了铈掺杂的氧化钼薄膜, 纯氧化钼薄膜在第25圈和第60圈的阳极电流密度分别为1.90和0.65 mA·cm-2, 而掺杂铈的氧化钼薄膜在第25圈和第130圈的阳极电流密度分别为2.80和1.37 mA·cm-2。由此可见, 铈掺杂增强了氧化钼薄膜的电致变色性能, 并使其循环稳定性也得到了改善。
图5
2.2.3 电化学沉积法
电化学沉积法[49]是在含有电解质的电解池中, 以惰性电极为阴极, 在外加电压下发生氧化还原反应, 将电解池中的离子沉积在电极基底表面形成薄膜的方法。电化学沉积制备薄膜与电解液的组成、pH、温度等条件有关, 其优点包括可精准控制沉积厚度和沉积速度, 同时可以在异形结构件上沉积薄膜, 反应温度低, 成本低。温洋洋等[50]采用电化学沉积制备了氧化钼包覆碳纳米管复合纤维(MoOx/CNT), 该复合纤维具有明显的电化学活性, 电容量为19 F·g-1, 可用于电化学超级电容器的柔性电极。Zhuzhel’skii等[51]首先通过恒电流法, 在玻璃碳(Glass Carbon, GC)电极表面沉积了导电聚合物(PEDOT)薄膜, 然后以0.2 mol/L钼酸锂为电解液, 分别在GC和GC/PEDOT电极上沉积了氧化钼薄膜, 制备的薄膜表面光滑、厚度均匀。
2.3 喷雾热解法
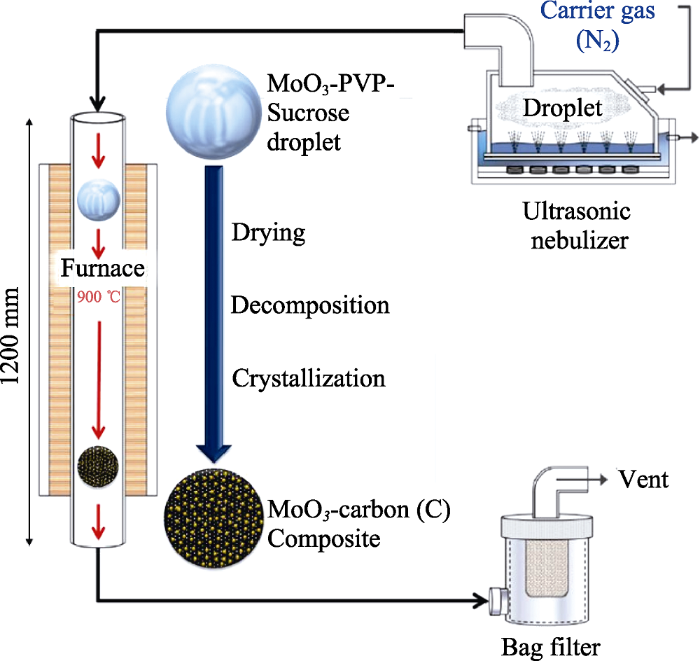
喷雾热解法[52,53]是通过金属前驱体的热分解来制备半导体氧化物和硫化物及金属纳米颗粒的方法。此方法制备的样品分散性好、纯度高、粒度均匀。Cho等[54]通过一步喷雾热解法将MoO3均匀分布在非晶态碳基体中。图6为反应装置示意图, 用超声波喷雾发生器产生MoO3液滴, 通过N2的流动将其带到石英管反应器, 在热壁反应器内经过干燥、分解和结晶, MoO3液滴在6 s内直接形成平均直径为0.7 μm的碳复合微球, 复合薄膜在经过100次循环后仍然有811 mAh·g-1的高比放电容量。Mousavi-zadeh等[55]在450 ℃的衬底温度下, 用喷雾热解法制备出未掺杂和Zn掺杂的MoO3薄膜, 并对其微观结构、形貌及光学性能进行了研究, 结果表明Zn的掺杂量增大至5at%时, MoO3晶粒尺寸减小至约60 nm, 薄膜的带隙增加; 随着Zn掺杂量的增加, 吸收光谱发生蓝移。
图6
2.4 其它方法
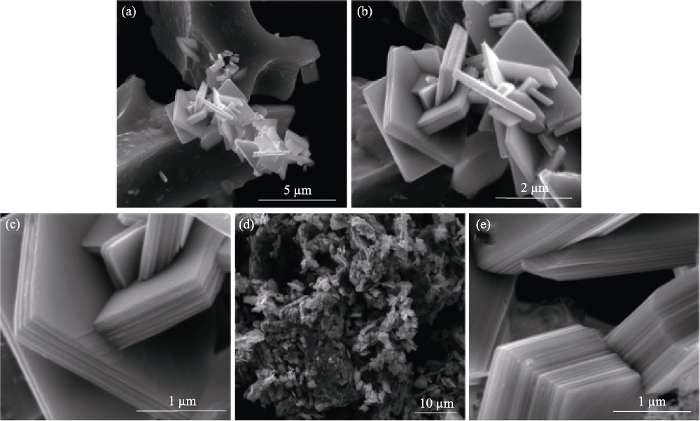
除上述常用方法, 研究者们还在积极寻找新的合成氧化钼薄膜方法。于海燕等[56]将水热法与电沉积法结合在FTO上制备出钼钛复合纳米薄膜, 复合薄膜的多孔结构便于离子扩散, 提高了电致变色性能。Martín-Ramos等[57]提出了一种通过层状MoS2/g- C3N4纳米杂化退火获得多层堆叠结构的α-MoO3晶体。首先, 通过钼酸盐、柠檬酸盐和硫脲反应获得MoS2, 然后将n(g-C3N4) : n(MoS2)=1 : 1混合, 分散在碳酸亚丙酯中, 经过超声、离心、沉淀, 150 ℃干燥24 h, 得到MoS2/g-C3N4复合材料; 650 ℃下煅烧, 得到无色(或绿色)α-MoO3多层堆叠结构的纳米板。如图7所示, SEM照片显示了α-MoO3多层堆叠的结构, 但与工业煅烧MoO3·H2O获得的MoO3形貌不同(图7(d))。此种方法与PVD和CVD方法相比, 结晶度提高, 并克服了在能量需求和设备以及常规液相合成技术方面的某些局限性。
图7
图7
具有多层堆叠结构的α-MoO3晶体在不同放大倍率下的SEM显微照片(a~c), 通过煅烧市售钼酸(MoO3·H2O)获得的MoO3晶体的SEM照片(d), 以及具有44层堆积的α-MoO3的SEM显微照片(e)[57]
Fig. 7
SEM images of α-MoO3 crystals with a multi-layer stack structure at different magnifications (a-c), SEM image of MoO3 crystals obtained by calcination of commercial molybdic acid (MoO3·H2O) (d), and SEM image of α-MoO3 stacking with 44 layers (e)[57]
3 氧化钼的改性
MoO3薄膜虽然具有变色响应时间短的优点, 但存在着色效率低、与基底结合不牢固等缺点。为此, 研究人员通过向氧化钼中掺杂离子以改变氧化钼的结构, 从而提高MoO3薄膜的电致变色性能。
3.1 单一离子掺杂MoO3
掺入离子可提高MoO3薄膜中电荷的注入量, 使得掺杂后的薄膜具有良好的可逆性能。章俞之 等[58]室温下采用溶胶-凝胶法制备了10mol% Li+掺杂的MoO3薄膜, 该薄膜具有良好的电致变色性能。与未掺杂的MoO3薄膜相比, Li+掺杂MoO3薄膜的光调制幅度为32.3%。Mahajan等[59]使用喷雾热解技术制备了Ti掺杂的MoO3薄膜, 随着Ti掺杂浓度的增加, MoO3薄膜逐渐由多晶态转变为无定形态, 且颗粒尺寸逐渐减小。当Ti掺杂浓度为9at%时, 制备薄膜呈无定形海绵状, 这种结构有利于离子的嵌入和脱嵌, 具有良好的电致变色性能。Layegh等[60]以七水合七钼酸铵和氯化铁作为前驱体, 通过溶胶-凝胶法分别制备了未掺杂和铁掺杂的氧化钼薄膜。与纯氧化钼电极相比, 提高铁掺杂浓度会显著增强薄膜的电化学性能。掺杂可以改变主体氧化物的形态和结构, 同时还可以改善其低电子电导率的问题。
3.2 Fe、Co共掺杂MoO3
图8
4 氧化钼电致变色器件
随着电致变色技术的发展, 尤其是近年来电致变色技术在手机上的应用, 电致变色领域成为人们关注的焦点。单一MoO3电致变色器件的制备依然是研究重点。王金敏课题组用两种不同方法制备了MoO3薄膜, 并组装了电致变色器件。一种方法是以MoS2为钼源, 采用超声波辅助剥离MoS2, 将其分散到乙醇溶液中, 再旋涂到ITO透明导电玻璃上, 经过煅烧得到MoO3薄膜[62]。由制备的MoO3薄膜组装成的电致变色器件在580 nm处的透过率调制幅度为16.2%, 着色响应时间为3.0 s, 褪色响应时间为9.0 s。另一种方法是以钼粉为钼源, 通过水热法在FTO透明导电玻璃上直接生长MoO3薄膜[63]。组装的电致变色器件在750 nm处的透过率调制幅度为13.3%, 着色与褪色响应时间分别为3.5和2.9 s。
由单一氧化钼薄膜制备的电致变色器件虽然可以变色, 但是光调制幅度较小、着色效率不高, 因此复合电致变色器件应运而生。氧化钼电致变色器件存在颜色变化单一、响应速度慢等缺陷。将氧化钼薄膜与其它电致变色薄膜组成复合变色器件可以改善这些缺陷, 使器件具有更好的电致变色性能[64]。
张阁等[65]先用水热法制备出六方相氧化钼(h-MoO3), 烘干得到MoO3样品, 与乙基纤维素、无水乙醇、松油醇充分搅拌直至无水乙醇挥发, 得到MoO3浆料, 旋涂在ITO表面, 加热, 制备得到氧化钼薄膜, 再用恒电压聚合法在ITO上制得聚吡咯薄膜, 将两者组装成纳米氧化钼/聚吡咯复合变色器件。测试结果表明, 随着电压的升高(-1.5 V→+1.5 V), 薄膜在481 nm处吸收峰的强度减弱, 光学对比度变化较大, 强度从0.62减小到0.55; 着色时间为35 s, 褪色时间为22 s; 着色态颜色为浅蓝色, 褪色态颜色为淡黄色。
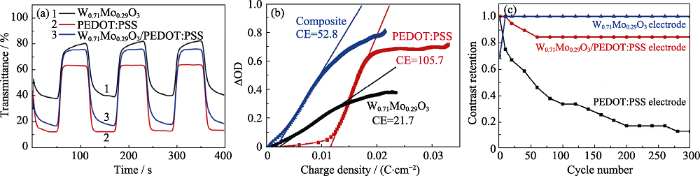
由于过渡金属氧化物电致变色器件电导率较差、电荷传输势垒较高。为改善这些缺点, 李海增等[66]引入共轭聚合物聚乙撑二氧噻吩-聚苯乙烯磺酸盐(PEDOT:PSS)制备得到有机-无机纳米复合薄膜。使用逐层喷涂沉积技术分别制备W0.71Mo0.29O3薄膜、W0.71Mo0.29O3/PEDOT:PSS薄膜、PEDOT:PSS薄膜。研究结果表明纳米复合电极的光学对比度优于纯PEDOT:PSS和W0.71Mo0.29O3电极, 比纯薄膜组合的线性预测对比度高23%。图9(a)动力学特征曲线表明, 以透过率变化90%所需的时间定义为切换时间, PEDOT:PSS着色时间和褪色时间分别为6.5和3 s。相同条件下纯W0.71Mo0.29O3电极着色时间为22.1 s, 褪色时间为13.9 s。而在W0.71Mo0.29O3电极中引入PEDOT:PSS可使响应时间缩短20% (着色时间17.9 s, 褪色时间10.5 s)。从图9(b)电极的着色效率曲线看出W0.71Mo0.29O3/PEDOT:PSS电极的着色效率为52.8 cm2·C-1, 是W0.71Mo0.29O3电极21.7 cm2·C-1的两倍。图9(c)为电极的循环稳定性曲线, 从图中可以看出W0.71Mo0.29O3/PEDOT:PSS电极在300个循环后的对比度保持率为84.2%。
图9
图9
W0.71Mo0.29O3薄膜、PEDOT:PSS薄膜和W0.71Mo0.29O3/PEDOT:PSS薄膜632.8 nm下的原位动力学特征曲线(a), 电极的着色效率曲线(b)和循环稳定性曲线(c)[66]
Fig. 9
In-situ kinetic properties measured at 632.8 nm for W0.71Mo0.29O3 film, PEDOT:PSS film and W0.71Mo0.29O3/PEDOT:PSS film (a), coloration efficiencies (b), and cycling stabilities (c) of the electrodes[66]
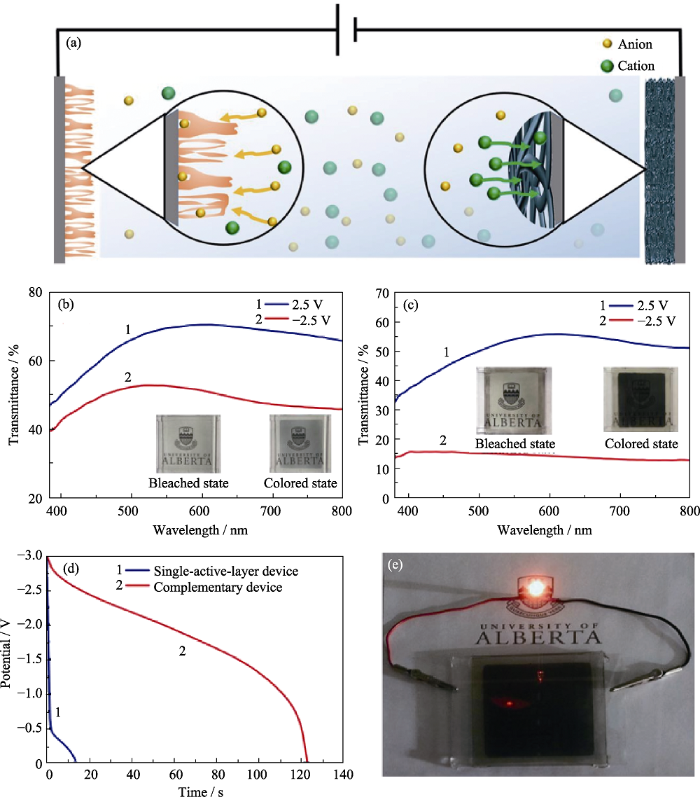
除了制备复合电致变色器件外, 一种既变色又储能的电致变色器件引起了人们的注意, 这种器件即双功能电致变色器件[67]。这类器件的电致变色材料从最初的普鲁士蓝(Prussian Blue, PB)[68], 发展到WO3[69]、钨钼复合氧化物[70]、聚吡咯[71]等。李海增等[70]通过一种自上而下的方法将Mo粉添加到HNO3溶液中, 搅拌、回流形成白色悬浮液, 离心、洗涤后将白色产物分散在去离子水中形成前驱体溶液, 制备得到MoO3胶体。再通过湿化学法将高度分散的MoO3胶体嵌入W0.71Mo0.29O3中, 形成MoO3-W0.71Mo0.29O3纳米复合材料, 经喷涂再得到MoO3-W0.71Mo0.29O3复合薄膜。以此为工作电极与喷涂的NiO对电极组装成互补型储能智能窗器件, 如图10所示。互补型电致变色电池在-2.5 V下着色1 min, 可为LED供电10 min。
图10
图10
互补型电致变色电池的示意图(a), 单活性层电致变色电池(b)和互补型电致变色电池的可见-近红外透射光谱(c), 单层器件和互补型器件的放电曲线(电流密度为0.05 mA·cm-2) (d), 以及互补型电致变色电池在-2.5 V着色后可以使LED点亮10 min以上(e)[70]
Fig. 10
Schematic diagram of a complementary electrochromic battery (a), visible-near-infrared transmission spectra of single active layer electrochromic battery (b) and complementary electrochromic batteries (c), discharge curves (current density is 0.05 mA·cm-2) of single-layer device and complementary device (d), and complementary electrochromic batteries lighting up the LED for 10 min after being colored at -2.5 V (e)[70]
5 结束语
近年来, MoO3电致变色材料在响应时间和着色态特点方面的优势, 使得MoO3在电致变色材料方面的研究愈发丰富。但是MoO3电致变色薄膜着色效率较低、与基底附着不牢固, 使得MoO3电致变色器件的使用寿命较短。为提高其电致变色性能, 研究人员探索出三条改进路线: 1) 在已有制备方法的基础上, 不断探索新的MoO3薄膜的制备方法, 或者采用两种已有的不同方法制备MoO3薄膜; 2) 对MoO3进行掺杂, 不同的掺杂离子会产生不同的形貌结构, 对应的电致变色性能也会受到不同程度的影响, 因此研究不同离子掺杂对MoO3电致变色性能的影响是一个重要方向; 3) 与其它有机或无机电致变色材料组装成互补型电致变色器件。目前关于MoO3的互补型电致变色器件研究较少, 互补型电致变色器件不仅有良好的电致变色性能, 而且可以呈现出更加丰富的颜色变化, 拓展了MoO3电致变色材料的实际应用范围。目前MoO3电致变色材料还没有得到广泛应用, 今后可借鉴以上思路来丰富MoO3电致变色材料与器件的研究方向, 以获得性能优异的大面积电致变色器件, 使得MoO3电致变色材料的应用更为广泛。
参考文献
Organic-inorganic hybrid electrochromic materials, polysilsesquioxanes containing triarylamine, changing color from colorless to blue
Four kinds of soluble monomers, containing triarylamine (TAA) group with reactive siloxane group, were synthesized under mild conditions via the reaction between 3-(triethoxysilyl)propyl isocyanate (TEOSPIC) and four TAA derivatives, respectively. Then the corresponding colorless organic-inorganic hybrid materials (PSSOs) were derived from the hydrolytic condensation of the monomers. PSSOs revealed good solubility in polar solvents on account of the effect of propeller-like TAA unit as well as the auxo-action effect of the flexible chain within the monomers. The structural characteristics of these PSSOs were identified by (1)H NMR, (29)Si NMR, FT-IR spectroscopies and X-ray diffraction (XRD). The morphology, dynamic changes of the transmittance and current before and after electro-oxidizing reaction were studied, and didn't show significant change suggesting good stability of the PSSOs. Meanwhile, these PSSOs performed high contrast of optical transmittance change up to 84% with the highest coloration efficiency to 241 cm(2).C(-1). Furthermore, electrofluorescent properties of PSSOs were investigated with high-contrast.
Towards full-colour tunability of inorganic electrochromic devices using ultracompact fabry- perot nanocavities
Intercalation-based inorganic materials that change their colours upon ion insertion/extraction lay an important foundation for existing electrochromic technology. However, using only such inorganic electrochromic materials, it is very difficult to achieve the utmost goal of full-colour tunability for future electrochromic technology mainly due to the absence of structural flexibility. Herein, we demonstrate an ultracompact asymmetric Fabry-Perot (F-P) nanocavity-type electrochromic device formed by using partially reflective metal tungsten as the current collector and reflector layer simultaneously; this approach enables fairly close matching of the reflections at both interfaces of the WO3 thin layer in device form, inducing a strong interference. Such an interference-enhanced device that is optically manipulated at the nanoscale displays various structural colours before coloration and, further, can change to other colours including blue, red, and yellow by changing the optical indexes (n, k) of the tungsten oxide layer through ion insertion.
Fusing electrochromic technology with other advanced technologies: a new roadmap for future development
Facile solution synthesis of tungsten trioxide doped with nanocrystalline molybdenum trioxide for electrochromic devices
A facile, highly efficient approach to obtain molybdenum trioxide (MoO3)-doped tungsten trioxide (WO3) is reported. An annealing process was used to transform ammonium tetrathiotungstate [(NH4)2WS4] to WO3 in the presence of oxygen. Ammonium tetrathiomolybdate [(NH4)2MoS4] was used as a dopant to improve the film for use in an electrochromic (EC) cell. (NH4)2MoS4 at different concentrations (10, 20, 30, and 40 mM) was added to the (NH4)2WS4 precursor by sonication and the samples were annealed at 500 degrees C in air. Raman, X-ray diffraction, and X-ray photoelectron spectroscopy measurements confirmed that the (NH4)2WS4 precursor decomposed to WO3 and the (NH4)2MoS4-(NH4)2WS4 precursor was transformed to MoO3-doped WO3 after annealing at 500 degrees C. It is shown that the MoO3-doped WO3 film is more uniform and porous than pure WO3, confirming the doping quality and the privileges of the proposed method. The optimal MoO3-doped WO3 used as an EC layer exhibited a high coloration efficiency of 128.1 cm(2)/C, which is larger than that of pure WO3 (74.5 cm(2)/C). Therefore, MoO3-doped WO3 synthesized by the reported method is a promising candidate for high-efficiency and low-cost smart windows.
Enhanced lithium electrochromism of atmospheric pressure plasma jet-synthesized tungsten/ molybdenum oxide films for flexible electrochromic devices
Enhanced lithium electrochromic performances of mixed organo-tungsten oxide (WxOyCz)/organo-molybdenum oxide (MoxOyCz) films by a rapid codeposition onto 40 Ω/□ flexible polyethylene terephthalate/indium tin oxide substrates at a short exposed duration of 23 s using an atmospheric pressure plasma jet (APPJ) at various mixed concentrations of hexacarbonyl precursors [W(CO)6 and Mo(CO)6] are investigated. The flexible organo-tungsten–molybdenum oxide (WMoxOyCz) films demonstrated noteworthy electrochromic performance for 200 cycles of reversible Li+ ion intercalation and deintercalation in a 1 M LiClO4–propylene carbonate electrolyte by the switching measurements of potential sweep from −1 to 1 V at a scan rate of 50 mV/s and the potential step at −1 and 1 V, even after being bent 360o around a 2.5-cm diameter rod for 1,000 cycles. The optical modulation (ΔT) of 61.3 % for MoOyCz films at a wavelength of 795.6 nm was significantly improved up to 72.5 % for WMoxOyCz films cosynthesized with an APPJ.]]>
Photoelectrochromic windows and displays
Plasmonic metasufaces with conjugated polymers for flexible electronic paper in color
A flexible electronic paper in full color is realized by plasmonic metasurfaces with conjugated polymers. An ultrathin large-area electrochromic material is presented which provides high polarization-independent reflection, strong contrast, fast response time, and long-term stability. This technology opens up for new electronic readers and posters with ultralow power consumption.
Progress in the preparation and application of nanostructured manganese dioxide
Electrochemistry and rapid electrochromism control of MoO3/V2O5 hybrid nanobilayers
High-contrast and fast electrochromic switching enabled by plasmonics
Electrochromism, a possible change of color producible in dyes by an electric field
Piezochromism and hydrochromism through electron transfer: new stories for viologen materials
Electroless metallization of dielectric SiLK surfaces functionalized by viologen
AIEE-active and electrochromic bifunctional polymer and device composed thereof synchronously achieving electrochemical fluorescence switching and electrochromic switching
Highly regiosymmetric homopolymer based on dioxythiophene for realizing water- processable blue-to-transmissive electrochrome
A highly regiosymmetric homopolymer based on a diethyl malonate derivatized 3,4-propylenedioxythiophene (ProDOT) monomer was synthesized through FeCl3 oxidative polymerization and postpolymerization functionalization to realize a water-processable blue-to-transmissive switching electrochromic polymer (WPECP-blue). As an electrochromic material, the polymer has a high electrochromic contrast DeltaTmax=56% at 580 nm and a relatively fast switching speed t95=1.8 s, and shows only contrast loss of 11% (from 56% to 45%) at square wave potential step of 5 s over 11,000 switching cycles, making it a desirable candidate for electrochromic applications such as windows and displays.
A novel electrophotographic system
Controllable synthesis and surface modification of molybdenum oxide nanowires: a short review
Lattice and electronic structure variations in critical lithium doped nickel oxide thin film for superior anode electrochromism
Tuneable dielectric and optical characteristics of tailor-made inorganic electro-chromic materials
Controlled growth of MoO3 nanorods on transparent conducting substrates
MoO3 nanorods with well-defined crystalline structure have been grown in situ on Fluorine doped Tin Oxide glass (FTO) by magnetron sputtering and subsequent oxidation treatment. Moreover, the morphologies and the crystalline structures of MoO3 products could be rationally tailored by adjusting the annealing temperature. More specifically, the calcination operated at 500 degrees C for 6 h leads to the formation of uniform MoO3 nanorods with an average diameter of 200 nm, and length of up to 800 nm, whereas only irregular nanoparticles or nanoplates have been obtained when the temperature was higher or lower than 500 degrees C. (C) 2014 Elsevier B.V.
High efficient reduction of graphene oxide via nascent hydrogen at room temperature
Field emission from MoO3 nanobelts
Facile scalable synthesis of MoO2 nanoparticles by new solvothermal cracking process and their application to hole transporting layer for CH3NH3PbI3 planar perovskite solar cells
MoO3 nanoparticles distributed uniformly in carbon matrix for supercapacitor applications
A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts
Influence of molybdenum trioxide thin film thickness on its electrochemical properties
Electrochromic energy storage devices
Research progress of inorganic all-solid-state electrochromic devices
Electrochromic Materials and Devices
Advances in inorganic all-solid-state electrochromic materials and devices
e.g., electric field, temperature, illumination, and atmosphere). Among them, electrochromic materials are expected to be widely used in smart windows, screen displays, multi-functional energy storage devices and other fields due to their characteristics such as large adjustment range, fast response rate, high coloring efficiency and good cycle stability. However, compared with semi-solid-state electrochromic devices that are difficult to package and organic electrochromic materials that are prone to denaturation and failure, inorganic all-solid-state electrochromic materials and devices have better comprehensive application. This paper focuses on the typical inorganic all-solid-state electrochromic materials and devices, presents a brief review on the current preparation methods of each structure layer of electrochromic devices and compares its advantages and disadvantages, introduces in detail the main alternative electrochromic materials and its key performance evaluation index, and explains the principle of several representative electrochromic devices, proposes to use transparent flexible electrodes with both high light transmittance, low surface resistance and excellent bending fold to replace the traditional rigid substrate in order to realize multi-field responsible device application development. Finally, the application prospect of inorganic all-solid-state electrochromic devices is prospected from the perspective of performance bottleneck, process difficulty and industrialization opportunity, which provides reference for the industrialization process of electrochromic devices.]]>
Chemical vapor deposition and its application in surface modification of nanoparticles
Crystallization of chemically vapor deposited molybdenum and mixed tungsten/ molybdenum oxide films for electrochromic application
Electrochromic properties of atmospheric CVD MoO3 and MoO3-WO3 films and their application in electrochromic devices
Catalytic growth of ZnO nanostructures by r.f. magnetron sputtering
Structural, optical and electrochromic properties of Nb2O5:MoO3 (95:5, 90:10, and 85:15) thin films prepared by RF magnetron sputtering technique
Characterization of nanostructured ZnO thin films deposited through vacuum evaporation
This work presents a novel technique to deposit ZnO thin films through a metal vacuum evaporation technique using colloidal nanoparticles (average size of 30 nm), which were synthesized by our research group, as source. These thin films had a thickness between 45 and 123 nm as measured by profilometry. XRD patterns of the deposited thin films were obtained. According to the HRSEM micrographs worm-shaped nanostructures are observed in samples annealed at 600 degrees C and this characteristic disappears as the annealing temperature increases. The films obtained were annealed from 25 to 1000 degrees C, showing a gradual increase in transmittance spectra up to 85%. The optical band gaps obtained for these films are about 3.22 eV. The PL measurement shows an emission in the red and in the violet region and there is a correlation with the annealing process.
Effect of heat treatment on characteristics of nanocrystalline ZnO films by electron beam evaporation
Physical properties of evaporated molybdenum oxide films
Effect of oxygen partial pressure on the growth of molybdenum trioxide thin films
Conventional and microwave hydrothermal synthesis and application of functional materials: a review
Swift tuning from spherical molybdenum microspheres to hierarchical molybdenum disulfide nanostructures by switching from solvothermal to hydrothermal synthesis route
Herein, we report the synthesis of metallic molybdenum microspheres and hierarchical MoS2 nanostructures by facile template-free solvothermal and hydrothermal approach, respectively. The morphological transition of the Mo microspheres to hierarchical MoS2 nanoflower architectures is observed to be accomplished with change in solvent from ethylenediamine to water. The resultant marigold flower-like MoS2 nanostructures are few layers thick with poor crystallinity while spherical ball-like molybdenum microspheres exhibit better crystalline nature. This is the first report pertaining to the synthesis of Mo microspheres and MoS2 nanoflowers without using any surfactant, template or substrate in hydro/solvothermal regime. It is opined that such nanoarchitectures of MoS2 are useful candidates for energy related applications such as hydrogen evolution reaction, Li ion battery and pseudocapacitors. Inquisitively, metallic Mo can potentially act as catalyst as well as fairly economical Surface Enhanced Raman Spectroscopy (SERS) substrate in biosensor applications.
Development application and of hydrothermal method
Design of NiO flakes@CoMoO4 nanosheets core-shell architecture on Ni foam for high- performance supercapacitors
Flexible transparent molybdenum trioxide nanopaper for energy storage
A flexible transparent molybdenum trioxide nanopaper, assembled via ultralong molybdenum trioxide nanobelts, displays an excellent average transmittance of approximately 90% in the visible region. The free-standing nanopaper electrode delivers an outstanding specific capacitance of 1198 F g(-1) and shows an excellent long-term stability performance over 20 000 cycles with a retention rate of 99.1%.
Rational interaction between the aimed gas and oxide surfaces enabling high-performance sensor: the case of acidic α-MoO3 nanorods for selective detection of triethylamine
Electrochromic properties of Sol-Gel prepared hybrid transition metal oxides-a short review
Tuning texture and morphology of mesoporous TiO2 by non-hydrolytic Sol- Gel syntheses
Enhanced electrochromism in cerium doped molybdenum oxide thin films
Fabrication and electrochromic properties of NiO electrodeposit films
Fabrication of molybdenum oxides/carbon nanotube composite fibers by electrochemical deposition and its electrochemical behavior
Electrochemical deposition of molybdenum oxide into films of poly (3,4-ethylenedioxythiophene) conducting polymer on glassy carbon substrates
Low-cost plasmonic solar cells prepared by chemical spray pyrolysis
TiO2 thin films by ultrasonic spray pyrolysis as photocatalytic material for air purification
In this study, we showed that the TiO2 thin films deposited onto window glass are practicable for air purification and self-cleaning applications. TiO2 films were deposited onto window glass by ultrasonic spray pyrolysis method. Different deposition temperatures were used in the range of 250-450 degrees C. The structural, morphological, optical properties and surface chemical composition were investigated to understand probable factors affecting photocatalytic performance and wettability of the TiO2 thin films. The TiO2 thin films were smooth, compacted and adhered adequately on the substrate with a thickness in the range of 100-240 nm. X-ray diffraction patterns revealed that all the TiO2 thin films consisted of anatase phase structure with the mean crystallite size in the range of 13-35 nm. The optical measurements showed that the deposited films were highly transparent (approx. 85%). The wettability test results showed that the TiO2 thin films sprayed at 350 degrees C and 450 degrees C and annealed at 500 degrees C for 1 h were superhydrophilic. The photocatalytic activity of the films was tested for the degradation of methyl tert-butyl ether (MTBE) in multi-section plug-flow reactor. The TiO2 film deposited at 350 degrees C exhibited the highest amount of conversion of MTBE, approximately 80%.
Large scale process for low crystalline MoO3-carbon composite microspheres prepared by one-step spray pyrolysis for anodes in lithium-ion batteries
Synthesis and ethanol sensing characteristics of nanostructured MoO3:Zn thin films
Novel MoO3-TiO2 composite nanorods films with improved electrochromic performance
α-MoO3 crystals with a multilayer stack structure obtained by annealing from a lamellar MoS2/g-C3N4 nanohybrid
Synthesis and electro- photochromic properties of lithium-doped MoO3 films
Structural, morphological, optical and electrochromic properties of Ti-doped MoO3 thin films
Experimental and theoretical study of Fe doping as a modifying factor in electrochemical behavior of mixed-phase molybdenum oxide thin films
Nanostructured Fe, Co-codoped MoO3 thin films
Hydrothermal growth, device preparation and electrochromic properties of nano-molybdenum oxide film
Preparation of MoO3 thin film by MoS2 oxidation method, device assembly and electrochromic properties
Complementary hybrid electrodes for high contrast electrochromic devices with fast response
Fast switching 'transparent-to-black' electrochromic devices are currently under investigation as potential candidates in modern applications like e-papers or with additional functionality as ultracompact iris or switchable neutral filter in camera systems. However, recent electrochromic devices show either a lack of contrast or slow response times. To overcome these deficiencies we focus on a careful material composition of the colouring hybrid electrodes in our device. We have established a nanoporous Sb-doped SnO[Formula: see text] electrode as supporting electrode for chemisorbed electrochromic tetraphenylbenzidine molecules due to its good conductivity in the redox potential range of the molecule. This hybrid electrode was combined with a modified nanoporous TiO[Formula: see text] / viologen electrode to realize a high performance, complementary electrochromic device. Fast switching time constants of 0.5 s and concurrently high change in optical density [Formula: see text]OD = 2.04 at 605 nm confirm our successful concept. The achieved colouration efficiency of 440 cm[Formula: see text] C[Formula: see text] exceeds every high contrast device presented so far.
Preparation of molybdenum oxide/polypyrrole composite membrane and study on its discoloration properties
Solution-processed interfacial PEDOT:PSS assembly into porous tungsten molybdenum oxide nanocomposite films for electrochromic applications
Electrochromic devices (ECDs) have received increased attention for applications including optoelectronics, smart windows, and low-emission displays. However, it has been recognized that the ECDs with transition-metal oxide (TMO) electrodes possess a high charge transport barrier because of their poor electrical conductivity, which limits their electrochromic performance. In this work, we addressed this limitation by utilizing a conjugated polymer to fabricate an organic-inorganic nanocomposite film that decreases the charge transport barrier of typical TMO electrodes. Using a conventional spray-layer-by-layer (spray-LbL) deposition technique, we demonstrate an electrochromic film composed of porous layers of tungsten molybdenum oxide (W0.71Mo0.29O3) nanorods permeated with an interconnected conductive layer of poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS). The introduction of PEDOT:PSS is shown to significantly reduce the charge transport barrier, allowing the nanocomposite W0.71Mo0.29O3/PEDOT:PSS electrode to exhibit significantly improved electrochromic switching kinetics compared with the deposited W0.71Mo0.29O3 films. Furthermore, the optical contrast of the nanocomposite electrode was observed to be superior to both pure PEDOT:PSS and W0.71Mo0.29O3 electrodes, with a performance that exceeded the linearly predicted contrast of combining the pure films by 23%. The enhanced performance of the PEDOT:PSS-intercalated porous W0.71Mo0.29O3 nanocomposite electrodes and the facile synthesis through a spray-LbL method demonstrate a viable strategy for preparing fast assembling high-performance nanocomposite electrodes for a wide variety of electrochemical devices.
Enhanced electrochromic and energy storage performance in mesoporous WO3 film and its application in a bi-functional smart window
Construction of multifunctional photoelectrochemical energy devices is of great importance to energy saving. In this study, we have successfully prepared a mesoporous WO3 film on FTO glass via a facile dip-coating sol-gel method; the designed mesoporous WO3 film exhibited advantages including high transparency, good adhesion and high porosity. Also, multifunctional integrated energy storage and optical modulation ability are simultaneously achieved by the mesoporous WO3 film. Impressively, the mesoporous WO3 film exhibits a noticeable electrochromic energy storage performance with a large optical modulation up to 75.6% at 633 nm, accompanied by energy storage with a specific capacity of 75.3 mA h g-1. Furthermore, a full electrochromic energy storage window assembled with the mesoporous WO3 anode and PANI nanoparticle cathode is demonstrated with large optical modulation and good long-term stability. Our research provides a new route to realize the coincident utilization of optical-electrochemical energy.
A bi-functional device for self-powered electrochromic window and self-rechargeable transparent battery application
Electrochromic smart windows are regarded as a good choice for green buildings. However, conventional devices need external biases to operate, which causes additional energy consumption. Here we report a self-powered electrochromic window, which can be used as a self-rechargeable battery. We use aluminium to reduce Prussian blue (PB, blue in colour) to Prussian white (PW, colourless) in potassium chloride electrolyte, realizing a device capable of self-bleaching. Interestingly, the device can be self-recovered (gaining blue appearance again) by simply disconnecting the aluminium and PB electrodes, which is due to the spontaneous oxidation of PW to PB by the dissolved oxygen in aqueous solution. The self-operated bleaching and colouration suggest another important function of the device: a self-rechargeable transparent battery. Thus the PB/aluminium device we report here is bifunctional, that is, it is a self-powered electrochromic window as well as a self-rechargeable transparent battery.
Single-crystalline tungsten oxide quantum dots for fast pseudocapacitor and electrochromic applications
Nanohybridization of molybdenum oxide with tungsten molybdenum oxide nanowires for solution-processed fully reversible switching of energy storing smart windows
A self-rechargeable electrochromic battery based on electrodeposited polypyrrole film