Electrochromic devices (ECDs) can reversibly change their light transmittance via external voltage-induced redox reactions, and show potential applications in smart windows, electrochromic displays, switching mirrors, sensors, supercapacitors and so on[1]. To stabilize ECDs, the internal resistance of ECDs must be remarkably reduced, which requires anionic conductivity higher than 1 mS∙cm-1[2]. Liquid electrolytes usually deliver high conductivity and high switching speed[3], but ECDs using liquid electrolytes suffer from persistent problems, such as electrolyte leakage, flammability and chemical stability issues.
To overcome the above mentioned problems, gel polymer electrolytes (GPEs) have been developed; these GPEs generally show a much higher conductivity than solid polymer electrolytes (> 10-4vs 10-8 S∙cm-1)[4]. GPEs refer to a family of polymer-based electrolytes that are prepared by dissolving polymer matrices into liquid electrolytes or trapping liquid electrolytes into the network of polymer matrices[5]. The most commonly used matrices in GPEs are poly(ethylene oxide) (PEO)[6], poly(acrylonitrile) (PAN)[7], poly(vinylidene fluoride) (PVDF)[8], poly (vinylidene fluoride-hexafluoropropylene) (PVDF-HFP)[9] and polymethyl methacrylate (PMMA)[2,8], additionally, the common plasticizers are propylene carbonate (PC), ethylene carbonate (EC), ethyl methyl carbonate (EMC) or their mixtures. The crystalline structure of the matrices provides the backbone of the gel, and the amorphous phase of the matrices forms the continuous phase to transport ions in the GPEs.
It is well accepted that fillers play an important role in Li+ transport, but the apparent discrepancy in electrochemical performances can partially be ascribed to the difference in electrolyte materials (polymers, salt, filler type), along with their concentration and preparation conditions. The addition of nanoparticles (such as TiO2[10], CeO2[11], Al2O3[12], and palygorskite[13]) can not only improve the mechanical strength of GPEs, but also increase their conductivity by increasing the volume fraction of the amorphous phase. However, the ionic conductivity of these composite electrolytes ranges from 10-4 to 10-5 S·cm-1 at ambient temperature, which is still too low for commercial applications[14].
Fumed SiO2 has been widely adopted as a filler to improve the conductivity and mechanical stability of GPEs, because of its branched three-dimensional structure and the flexibly-tunable surface functionalities[10]. Currently, most studies are conducted on the development of hydrophilic fumed SiO2 as GPEs additive (with a large amount of silanol groups on the surface), because of its ionizing lithium salt and ability to increase the amorphous phase content of the polymer matrix. However, hydrophilic SiO2 with polar silanol groups tends to aggregate[15].Moreover, hydrophilic SiO2 presents a low compatibility with organic GPEs. Therefore, employing hydrophobic SiO2 may be a better choice for their application in GPEs due to its high dispersibility in organic matrices.
In this study, we investigate the effect of a hydrophobic fumed SiO2/PMMA/PC/LiClO4 GPE (H-SiO2 GPE) and their applications in ECDs. It is demonstrated that hydrophobic fumed SiO2 is more efficient in increasing the ionic conductivity of electrolytes, because of the hydrophobic- hydrophobic attractions between the hydrophobic groups on the SiO2 surfaces and solvents in the GPE.The ECD assembled with H-SiO2 GPE exhibits a fast switching speed (tbleaching=4 vs 8 s and tcoloring=14 vs 16 s). To compare, the effect of a hydrophobic fumed SiO2/LiClO4/ PC/liquid electrolyte is explored. This work demonstrates that the addition of hydrophobic fumed SiO2 in electrolytes provides great potential for the application in ECDs and other energy devices.
1 Experimental
1.1 Chemicals
Propylene carbonate (PC, 99%), lithium perchlorate (LiClO4, 99.9%), hydrophobic fumed SiO2 (Aladdin, 7-40 nm, pH=4.5), polymethyl methacrylate (PMMA, Aldrich, Mw≈1000, 000) were obtained and used.
1.2 Preparation of the liquid electrolytes and GPEs
The liquid electrolyte was prepared by dissolving 0.1 mol·L-1 LiClO4 in 7.41 mL propylene carbonate under stirring. Afterward, 0, 20, 50, 80 and 100 mg of hydrophobic fumed SiO2 was dispersed into 10 g of liquid electrolyte with ultrasonication. This process provided hydrophobic fumed SiO2/LiClO4/PC liquid electrolyte with 0, 0.2wt%, 0.5wt%, 0.8wt% and 1.0wt% fumed SiO2. Hydrophobic fumed SiO2/polymethyl methacrylate (PMMA)/PC/LiClO4 GPEs (H-SiO2 GPEs) were prepared by dissolving 1 g of PMMA (vacuum dried at 90 ℃ for 24 h) into 9 g of liquid electrolyte under stirring at 90 ℃.
1.3 Preparation of the WO3 nanopowder
WO3 nanoparticles were synthesized using the reported hydrothermal process[16]. In a typical experiment, 0.815 g of Na2WO4·2H2O was dissolved in 10 mL of deionized water. Then the Na2WO4 solution was acidified to pH 2.0 using HCl solution at room temperature. Afterward, the solution was transferred into a Teflon-lined autoclave and heat-treated at 180 ℃ for 24 h. Finally, the final products were sequentially washed with deionized water and ethanol to remove the sulfate ions and other remnants by centrifugation.
1.4 Assembly of ECDs based on different electrolytes
WO3 electrochromic films were deposited on clean ITO glasses by a wire-bar coating method, as reported in our previous work[17]. When using the liquid electrolytes, the ECDs were sealed with 3M tape while leaving one small gap in which the LiClO4/PC liquid electrolytes (with/without hydrophobic fumed SiO2) were injected into the device by a syringe. Finally, the injection ports were sealed with UV curing glue. In regard to the GPEs, GPEs with/without hydrophobic fumed SiO2 were cast into WO3 electrochromic layers, and finally, these ECDs were also sealed with 3M tape.
1.5 Characterization
Viscosity measurements were determined by rotary rheometer (HAAKE MARS60) under shear rate of 0.1- 100 s-1 at room temperature. The morphology of WO3 films was characterized by scanning electron microscope (SEM, S-4800, Hitachi, Tokyo, Japan) and transmission electron microscope (TEM, JEOL, JEM-2100, 200 kV). The morphologies of the gel electrolyte were characterized by FE-SEM (JEOL, JSM6700F, Tokyo). The crystal structure of the electrochromic film was required by X-ray diffraction (XRD; 3kW Bruker D8 Advance X-ray diffractometer with Cu Kα irradiation, λ=0.154 nm). The optical transmittance of the electrochromic devices was characterized by UV spectrophotometer (UH4150; Hitachi, Japan) at 633 nm. The ionic conductivity was measured by Mettler Seven compact conductivity meter. Electrochemical measurements were performed on electrochemical workstation (CHI760E, CHI Instruments, China). Electrochemical impedance spectroscopy (EIS) of the electrochromic film was recorded from 10-1 to 106 Hz at an amplitude of 5 mV.
2 Results and discussion
2.1 Phase characterization
The hydrophobicity of SiO2 is investigated by the contact angle test, as presented in Fig. S1. Regardless of how the needle moves, water droplets cannot adhere to the surface of hydrophobic fumed SiO2. Fig. 1 shows the FT-IR spectrum of the hydrophobic fumed SiO2. The broad peaks at 3446 and 1631 cm-1 are the -OH stretching and bending vibration peaks of adsorbed water, respectively. The peaks at 808 and 478 cm-1 are the symmetric stretching vibrations and bending vibrations of Si-O bonds. The strong and wide absorption band at 1110 cm-1 is the anti- symmetric stretching vibration peak of Si-O-Si. The peak at 979 cm-1 is the stretching vibration of Si-OH, indicating the possibility for further reaction as a proton donor[18]. Peaks at 2976, 2927, and 2856 cm-1 are the stretching vibration peaks of the -CH3, and -CH2- groups. Due to the existence of -CH3, -CH2- terminals, this fumed SiO2 shows hydrophobic properties and good dispersibility in organic matrices, as revealed in Fig. S2. The particle size distribution of hydrophilic fumed SiO2 shows two peaks in PC, and a strong peak in the range of 1000-10000 nm. These results indicate that hydrophilic fumed -SiO2 can agglomerate in PC. In contrast, hydrophobic fumed-SiO2 is mainly distributed between 100 and 1000 nm. It can be concluded that the hydrophobic fumed SiO2 has better uniformity in PC. The scanning electron micrographs of the prepared pure GPEs and
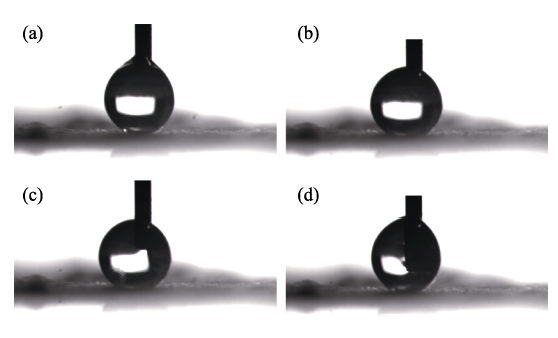
Fig. S1
Static water contact angle (CA) of hydrophobic fumed SiO2
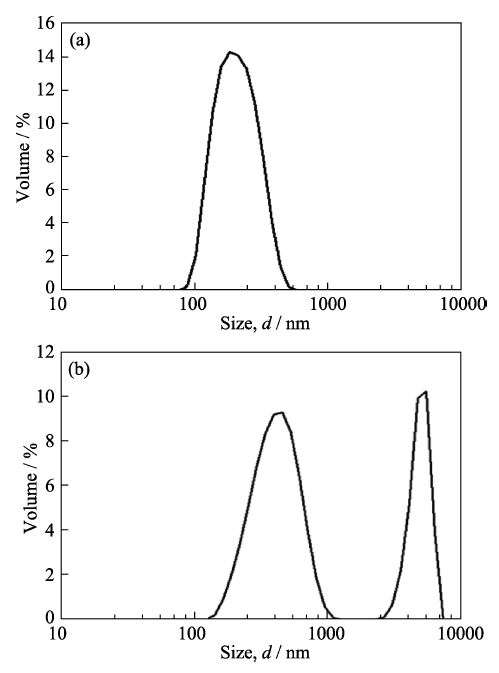
Fig. S2
Particle size of hydrophobic fumed silica (a) and hydrophilic fumed silica (b) in PC
2.2 Effect of fumed SiO2 on the Gel electrolyte and electrochromic devices
2.2.1 Measurements of the ionic conductivities of H-SiO2 GPEs
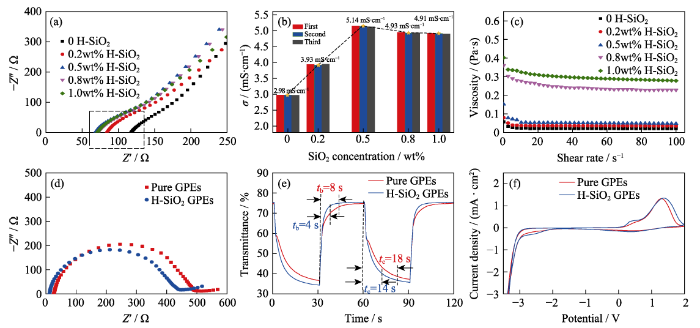
A high ionic conductivity facilitates the transportation of ions into and out of an EC film to initiate a color change, which is the key indicator of an electrolyte. The ionic conductivities of these GPEs are calculated by the formula σ = L/RbA, where L is the thickness of the GPE electrolyte, A is the contact area between the electrolyte and the electrode, and the bulk resistance (Rb) is the intercept of the curve and the Z’ axis in the AC impedances (Fig. 2(a))[19]. The measurement was conducted at 25 ℃ and 40% humidity. The experiments were repeated three times to reduce the measuring and experimental error, and an average value was obtained for the final results. The conductivity of the H-SiO2 GPE first increases and then decreases with an increasing concentration of fumed SiO2. The GPE shows a maximum conductivity of 5.14 mS∙cm-1 at 0.5wt% concentration of fumed SiO2 (Fig. 2(b)), which is clearly higher than the previously reported values in the literature (Table S1). The conductivity evolution of the GPEs with different SiO2 concentrations can be explained by the trade-off between the improved dissociation of lithium perchlorate and the increase in viscosity (Fig. 2(c)). Specifically, there are a large number of methyl groups on the surface of hydrophobic SiO2, which is the main reason for its hydrophobicity. These methyl groups can form mutual hydrophobic-hydrophobic attractions with the organic groups in organic solvents to promote the dispersion of SiO2, thus improving the dissociation of lithium perchlorate and providing the GPE a high transparency. Moreover, it is noted that the polarity difference between SiO2 with a large number of hydroxyl groups and an organic electrolyte can affect the dispersion of SiO2 in PMMA matrices[15], further decreasing the ionic conductivity (Table 1). Additionally, as the viscosity continues to increase, the polar end of the molecule is gradually adsorbed on the silica surface, which halts further network formation at this site and hinders the motion of Li+.
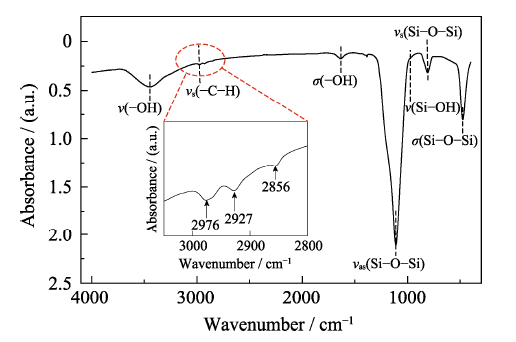
Fig. 1
FT-IR spectrum of hydrophobic fumed SiO2
Table 1 Ion conductivity of gel electrolytes from the literature
| Electrolyte | Ionic conductivity/(mS·cm-1) |
|---|---|
| PMMA/LiClO4/hydrophobic SiO2 (This work) | 5.14 |
| PMMA/LiClO4/hydrophilic SiO2[1] | 3.8 |
| P(BMA-St)/hydrophilic SiO2[2] | 2.15 |
| PEO/LiCF3SO3/TiO2[3] | 0.16 |
| PVDF/LiClO4/palygorskite[4] | 0.12 |
| PMMA/LiClO4/[Emim]BF4[5] | 2.9 |
| PVB/LiClO4[6] | 0.04 |
| PVDF-HFP/LiCF3SO3/ZrO2[7] | 1.78 |
| PAN/LiClO4/Li0.33La0.557TiO3[8] | 0.0605 |
H-SiO2 GPEs are shown in Fig. S3. H-SiO2 evenly distributes in the main body of the GPEs.
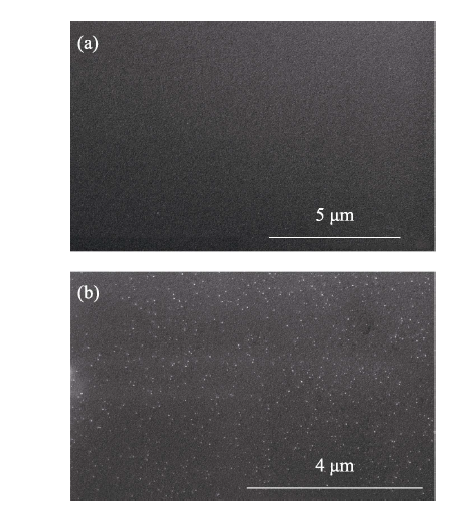
Fig. S3
SEM images of pure GPEs (a) and 0.5wt% H-SiO2 GPEs (b)
2.2.2 Electrochromic performances of ECDs based on H-SiO2 GPEs
To explore the effect of H-SiO2 GPEs on electrochromism, the ECDs were assembled by WO3 coated on ITO glass[17] and using GPEs with/without hydrophobic fumed SiO2. The specific details of WO3 are shown in Fig. S4. The electrochemical behaviors of the ECDs were characterized by electrochemical impedance spectroscopy in the colored state (EIS, Fig. 2(d)). Each of the EIS spectra is composed of a semicircle part at high frequencies and a diagonal part at low frequencies. The semicircle part reflects the charge transfer resistance between the electrochromic film and electrolyte. The diagonal part represents the Warburg impedance that is associated with the ion diffusion process. In other words, the larger the slope of the line is, the faster the ion transfer process. Therefore, it is obvious that the resistance is low and the ion diffusion is good with H-SiO2 GPEs.
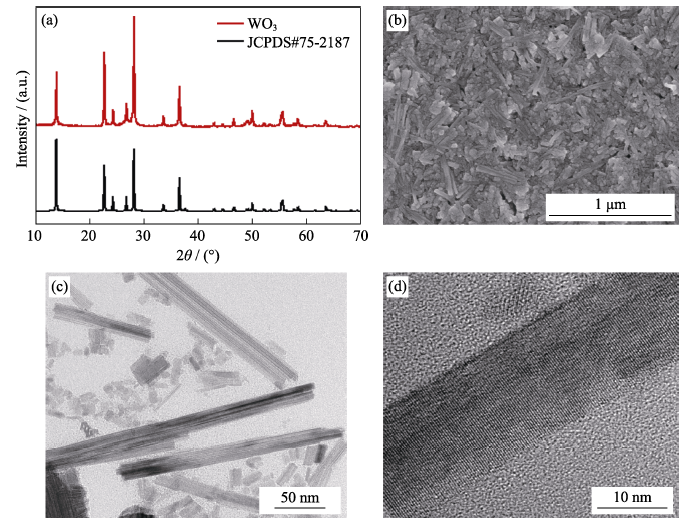
Fig. S4
XRD patterns of the as-prepared WO3(a), SEM image of WO3 films coated on ITO substrates(b), TEM images of WO3 dispersion (c) and typical nanorod (d) with high magnification
Fig. 2
Fig. 2
Nyquist plots (a), ionic conductivity (b) and viscosity (c) of PMMA-based GPEs with 0, 0.2wt%, 0.5wt%, 0.8wt% and 1.0wt% fumed SiO2. Electrochromic switching behaviors at 633 nm (e), colored state EIS (d) and CV curves (f) of ECDs contained PMMA-based GPEs with 0, 0.5wt% fumed SiO2. Scan rate: 100 mV/s
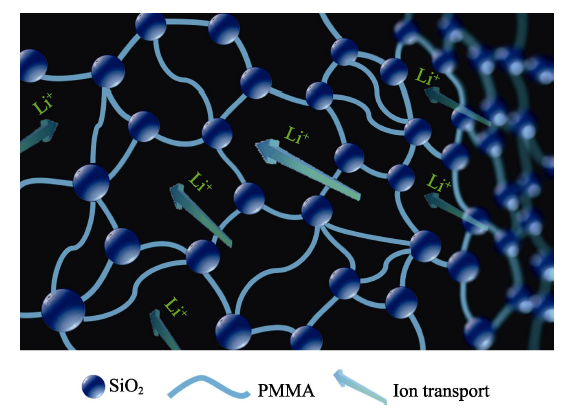
To further investigate the electrochromic performances of ECDs, as shown in Fig. 2(e). An ECD with H-SiO2 GPEs switches faster than that without SiO2. More precisely, the switching time sare 16/14 s (coloring) and 8/4s (bleaching) for ECDs with pure GPEs and H-SiO2 GPEs, respectively. Compared with other similar constructions of ECDs (using WO3, ITO glass and a gel electrolyte as electrochromic layers, substrates and ion transport layers, respectively), H-SiO2 GPEs exhibit faster speeds than previously reported results for PVdF- HFP-based (tcoloring=17 s/tbleaching=28 s)[20], PMMA-based (tcoloring=62.6 s/tbleaching=41.2 s)[21], ionic-liquid-based (tcoloring= 15 s/tbleaching=15 s)[22] and PVB-based (tcoloring=16.5 s/tbleaching= 9.5 s)[23] gel electrolytes. In addition, the transmittance modulation of ECDs increases from 38.3% to 41.2%, which is higher than that reported above (41.2% vs 35%[20]vs 24%[22] vs 18%[19]). The enhanced electrochromic performance can be attributed to the above mentioned high ionic conductivity and shear thinning behavior. The viscosity follows a linear correlation with respect to the shear rate. The viscosity as a function of shear rate for the GPE with various fumed silica contents is shown in Fig. 2(c). At a low shear rate, it exhibits a solid-like behavior with a high viscosity value due to the formation of a network structure by the induction of a siloxane linkage in the polymer matrix. At a high shear rate, because the orientation of the siloxane linkage is preferential parallel to the flow direction, the viscosity decreases. Shear thinning occurs because the bonds composing the network structure are physically weak, which can be disturbed by shear[24]. Thus, the H-SiO2 GPEs are proven to be physical gels with a three-dimensional network[14]. As presented in Fig. 3, hydrophobic fumed SiO2 can be attracted to the PMMA chains, forming a three-dimensional network structure, while providing ion transport channels so that ions can transport more quickly and easily to the interface of the WO3 films and electrolyte.
Fig. 3
Schematic diagram of the transportation of Li+ ion through H-SiO2GPEs with three-dimensional structure
In addition, the CV curves of the ECDs with different GPEs are shown in Fig. 2(f). The CV curve of the SiO2-free ECD has a cathode current peak at -3.3 V and an anodic current peak at 1.3 V. In contrast, the CV curves of the SiO2 containing ECDs show cathode current peaks at +0.37 V, and anodic current peaks at -2.6 V. These new peaks may be attributed to different binding sites on the WO3 film[25], indicating a better reactivity of the electrochromic WO3 films at a low bias voltage. According to a previous report[26], the introduction of cations can decrease the reaction voltage of WO3. The addition of fumed SiO2 enhances the concentration of Li+ near the WO3 film, thus leading to better electrochromic performance.
2.3 Effect of fumed SiO2 on the liquid electrolyte and electrochromic devices
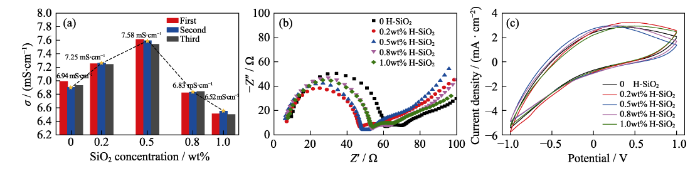
For a LiClO4/PC liquid electrolyte, the PC solvent has a strong solvation effect on Li+ cations, but the perchlorate anions are difficult to coordinate or solvate, limiting the achievement of high conductivity in this system. The conductivity of liquid electrolytes was measured by a conductivity meter, and the results show a similar trend to that of gel electrolytes. To increase the accuracy of data, the experiment was repeated three times (Fig. 4(a)). With SiO2 additives from 0 to 0.5wt%, the ionic conductivity of the liquid electrolyte increases up to a maximum of 7.58 mS∙cm-1, which is higher than hydrophilic fumed SiO2 or other additives as fillers (no more than 7.0 mS∙cm-1)[10,14]. The increase of conductivity can be attributed to the reasons discussed in the section on GPEs. The good dispersion of hydrophobic fumed SiO2 homogenizes the electrical field[27]. However, further increasing the SiO2 concentration leads to a decrease in conductivity, possibly due to the increase in electrolyte viscosity and the aggregation of fumed SiO2.
Fig. 4
Ionic conductivity of liquid electrolyte with 0, 0.2wt%, 0.5wt%, 0.8wt%, and 1.0wt% fumed SiO2 (a), and colored state Nyquist plots (b) and CV curves (c) of WO3 films in liquid electrolyte with 0, 0.2wt%, 0.5wt%, 0.8wt% and 1.0wt% fumed SiO2. Scan rate: 100 mV/s
The effects of the hydrophobic fumed SiO2 composite electrolyte on the electrochemical behaviors of WO3 films were characterized by electrochemical impedance spectroscopy in the colored state (EIS, Fig. 4(b)). As shown in Fig. 4(b), both the charge transfer resistance and ion diffusion resistance reach a minimum at a SiO2 concentration of 0.5wt%, which is consistent with the conductivity variation. Fig. 4(c) further presents the CV curves of the WO3 films in SiO2 electrolytes at different concentrations. In all of CV curves, clear cathodic current peaks appear with potential sweeping from +1 V to -1 V. Moreover, the colorless WO3 film appears blue, due to the reduction of W6+→W5+ after Li+ insertion. The film returns to be colorless after reverse sweeping the potential back to +1 V, suggesting the occurrence of the W5+→W6+ oxidation reaction accompanied by Li+ extraction. Therefore, the WO3 film demonstrates the largest CV curve in the highly conductive electrolyte containing the addition of 0.5wt% fumed SiO2, confirming the beneficial influence of the fumed SiO2 additive on the electrochemical reactions of the WO3 film.
3 Conclusion
In conclusion, the introduction of hydrophobic fumed SiO2 into GPEs can effectively increase the conductivity from 3.99 to 5.14 mS∙cm-1, because of the attractive force of hydrophobic groups. These interactions are beneficial for increasing the compatibility between SiO2 and GPEs, thereby promoting the dissociation of lithium perchlorate, and improving the ionic conductivity. The assembled ECDs using H-SiO2 GPEs exhibit lower cathode interface impedances, better electrochemical behaviors, and faster switching times, as compared with those of the unmodified GPEs (tbleaching=4 vs 8 s and tcoloring=14 vs 16 s). These observations are owing to that H-SiO2 GPEs form a three-dimensional network structure, thereby providing an ion transport channel. Similarly, the ionic conductivity of the LiClO4/PC/liquid electrolyte without/with hydrophobic fumed SiO2 increases from 6.94 mS∙cm-1 (without hydrophobic fumed SiO2) to 7.58 mS∙cm-1. This work demonstrates that the introduction of hydrophobic SiO2 has a positive effect on electrolytes, which is an important step for their application in ECDs.
Supporting materials:
High-conductivity Hydrophobic Fumed-SiO2 Composite Gel Electrolyte for High Performance Electrochromic Devices
ZHAO Qi1, QIAO Ke1, YAO Yongji1, CHEN Zhang1, CHEN Dongchu2, GAO Yanfeng1
1. School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China; 2. School of Materials Science and Energy Engineering, Foshan University, Foshan 528000, China
References:
[1] AHMAD S, AHMAD S, AGNIHOTRY S A. Nanocomposite electrolytes with fumed silica in poly(methyl methacrylate): thermal, rheological and conductivity studies. Journal of Power Sources, 2005, 140: 151-156.
[2] LIAO Y H, RAO M M, LI W S, et al. Fumed silica-doped poly(butyl methacrylate-styrene)-based gel polymer electrolyte for lithium ion battery. Journal of Membrane Science, 2010, 352: 95-99.
[3] VIGNAROOBAN K, DISSANAYAKE M A K L, ALBINSSON I, et al. Effect of TiO2 nano-filler and EC plasticizer on electrical and thermal properties of poly(ethylene oxide) (PEO) based solid polymer electrolytes. Solid State Ionics, 2014, 266: 25-28.
[4] YAO P, ZHU B, ZHAI H, et al. PVDF/palygorskite nanowire composite electrolyte for 4 V rechargeable lithium batteries with high energy density. Nano Letters, 2018, 18: 6113-6120.
[5] TANG Q, LI H, YUE Y, et al. 1-Ethyl-3-methylimidazolium tetrafluoroborate-doped high ionic conductivity gel electrolytes with reduced anodic reaction potentials for electrochromic devices. Materials & Design, 2017, 118: 279-285.
[6] ZHANG F, DONG G, LIU J, et al. Polyvinyl butyral-based gel polymer electrolyte films for solid-state laminated electrochromic devices. Ionics, 2017, 23: 1879-1888.
[7] PUGUAN J M C, CHINNAPPAN A, APPIAH-NTIAMOAH R, et al. Enhanced ionic conductivity and optical transmissivity of functionalized ZrO2/PVdF-HFP hybrid electrolyte for energy efficient windows. Solar Energy Materials and Solar Cells, 2015, 137: 265-273.
[8] LIU W, LEE S W, LIN D. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nature Energy, 2017, 2(5): 17035.
参考文献
All-inorganic solid-state electrochromic devices: a review
PMMA based gel electrolyte for EC smart windows
Constructing three-dimensional quasi- vertical nanosheet architectures from self-assemble two-dimensional WO3·2H2O for efficient electrochromic devices
Nanocomposite polymer electrolytes for lithium batteries
A conceptual review on polymer electrolytes and ion transport models
A promising TPU/PEO blend polymer electrolyte for all-solid-state lithium ion batteries
Electrospun hydrophilic fumed silica/polyacrylonitrile nanofiber-based composite electrolyte membranes
Preparation and characterizations of PMMA-PVDF based polymer composite electrolyte materials for dye sensitized solar cell
Study of PVDF-HFP/PMMA blended micro-porous gel polymer electrolyte incorporating ionic liquid [BMIM] BF4 for lithium ion batteries
DISSANAYAKE M A K L, ALBINSSON I,
Effect of TiO2 nano-filler and EC plasticizer on electrical and thermal properties of poly(ethylene oxide)(PEO) based solid polymer electrolytes
SATHIYA PRIYA A R,
Effect of nanoscale CeO2 on PVDF-HFP-based nanocomposite porous polymer electrolytes for Li-ion batteries
Electrochemical and spectroscopic study of the transport properties of composite polymer electrolytes
PVDF/palygorskite nanowire composite electrolyte for 4 V rechargeable lithium batteries with high energy density
Nanocomposite electrolytes with fumed silica in poly(methyl methacrylate): thermal, rheological and conductivity studies
Spinning of poly(ethylene terephthalate) fiber composites incorporated with fumed silica
Synthesis, assembly, and electrochromic properties of uniform crystalline WO3 nanorods
Printing of WO3/ITO nanocomposite electrochromic smart windows
M,
et al.Chemical interaction of different sized fumed silica with epoxy via ultrasonication for improved coating
Ion-conductive and transparent PVdF-HFP/silane-functionalized ZrO2 nanocomposite electrolyte for electrochromic applications
Organic-inorganic hybrid polymer electrolytes based on polyether diamine, alkoxysilane, and trichlorotriazine: synthesis, characterization, and electrochemical applications
1-Ethyl-3-methylimidazolium tetrafluoroborate-doped high ionic conductivity gel electrolytes with reduced anodic reaction potentials for electrochromic devices
Polymer electrolytes for electrochromic devices through solvent casting and Sol-Gel routes
Polyvinyl butyral-based gel polymer electrolyte films for solid-state laminated electrochromic devices
Composite polymer electrolytes based on poly(ethylene glycol) and hydrophobic fumed silica: dynamic rheology and microstructure
Imidazolium-based ionic liquid derivatives for application in electrochromic devices
Low-voltage, simple WO3-based electrochromic devices by directly incorporating an anodic species into the electrolyte
Solid polymer electrolytes with flexible framework of SiO2 nanofibers for highly safe solid lithium batteries