甲硝唑(Metronidazole)是一种常用的抗生素, 临床上主要用于抗滴虫、阿米巴原虫以及抗菌[1], 化学结构比较复杂, 属于难生物降解的有机物质[2]。通过污水排放、医疗固废排放或代谢等途径进入环境后, 容易在环境中富集, 给各种环境生物造成严重的影响[3]。含甲硝唑废水的治理方法有多种, 如用活性炭吸附[4]、零价铁催化降解[5]、紫外光降解[6]、生物降解[7,8]、传统的芬顿降解和光芬顿降解等[9,10,11]。有些方法虽然降解得较为彻底, 但耗时很长; 有些方法则只是将污染物与环境分离, 并没有破坏污染物; 有些则对处理条件要求高, 所以寻找更好的处理方法和材料依然很迫切。Shemer等[12]以H2O2为氧化剂, 比较了UV/H2O2、H2O2/Fe2+和UV/H2O2/Fe2+在初始pH 3.5条件下对1 mg/L甲硝唑降解, 分别达到64%、76%和94%的去除效果, 证明均相紫外光- Fenton方法的处理效果明显优于单一紫外光活化和单一均相Fenton方法。熊振湖等[13]在初始pH 4和1 mg/L甲硝唑的条件下, 测得UV/H2O2/Fe2+对甲硝唑的降解率为95.8%, 优于UV/H2O2(36.2%)和H2O2/Fe2+(66.6%), 并且优于典型的UV/TiO2光催化法(85.5%)。Wang等[14]制备了磁性g-C3N4/MnFe2O4/ graphene复合催化剂, 在可见光照射下用它活化过二硫酸盐(S2O82-), 对20 mg/L甲硝唑的降解率最高达到94.5%。可见, 利用光催化和催化H2O2氧化协同降解甲硝唑, 可以充分发挥两类高级氧化法的优势, 得到良好的处理效果。
利用光催化H2O2氧化降解有机污染物, 关键是制备高效催化剂。ZnO与TiO2相似[15], 是宽带隙半导体材料(~3.2 eV), 具有晶型丰富、形貌和尺寸易于调控、无毒和制备成本较低等优势, 适合作为光催化剂和光-H2O2活化催化剂, 多年来备受关注[16]。研究发现, 通过掺杂或复合其它物质可提高ZnO的催化性能。Gholami等[17]将ZnO纳米棒负载在生物炭表面, 可以显著改善其对格美沙星(Gemifloxacin)类药物的超声催化降解性能。基于此, 我们设计用松木碱解液与锌盐发生复分解反应, 制备Zn(OH)2前驱体, 再通过水热转化和热解处理, 制备ZnO-生物炭复合催化剂。但是, 在松木碱解过程中, 由于碱解导致玻璃三颈瓶的玻璃溶解, 意外得到Zn2SiO4- ZnO-生物炭复合材料, 发现其具有良好的可见光- Fenton催化活性。根据文献检索, 纳米结构Zn2SiO4不仅对重金属离子有良好的吸附性能[18], 而且对亚甲基蓝具有比ZnO更好的紫外光催化降解性能[19], 但其在环境净化中的应用很少被人们关注。本文报道Zn2SiO4-ZnO-生物炭复合材料及其对甲硝唑的可见光催化H2O2降解性能, 为新型光催化剂的研制和抗生素废水的治理提供依据。
1 实验方法
1.1 材料与仪器
松木粉(华北油松), 取自邢台市某木器加工厂, 用去离子水洗涤, 干燥后机械研磨, 过孔径0.15 mm的筛网。氯化锌(ZnCl2)、氢氧化钠(NaOH)和无水乙醇(C2H5OH)均为分析纯试剂, 购于天津永大化学试剂股份有限公司。甲醇(CH3OH)为色谱纯级试剂, 购于天津赛浮瑞科技有限公司。甲硝唑(C6H9N3O3)也为分析纯级产品, 采购于北京索莱宝科技有限公司, 未经纯化直接使用。实验用水均为本单位自制的去离子水(电导率≤1 μS•cm-1)。
WHL-25A恒温干燥箱(天津市泰斯特仪器有限公司); SHB-III循环水真空泵(郑州长城科工贸有限公司); PHS-3S型pH计(天津市科密欧化学试剂有限公司); MS-PB磁力搅拌器(上海鼎科科学仪器有限公司); PLS-SXE300D氙灯光源(北京泊菲莱科技有限公司); L-3000高效液相色谱仪(北京普源精电科技有限公司); 5B-2F型COD (Chemical Oxygen Demand)速测仪(兰州连华环保科技有限公司)。
1.2 催化剂的制备
(1)松木碱解液的制备:称取3.00 g松木屑, 转移到250 mL装有球形冷凝器的三口烧瓶中, 加入60 mL、1.0 mol/L的NaOH水溶液, 在磁力搅拌下加热至回流并保持12 h, 自然冷却至室温, 得到松木碱解液。
(2)复合锌前驱体的制备:计量松木碱解液的体积, 取样, 用标准HCl溶液标定其OH-浓度。根据松木碱解液的体积和OH-浓度, 控制[Zn2+] : [OH-]= 1 : 2, 慢慢加入ZnCl2溶液, 然后在室温下陈化4 h, 形成复合锌前驱体(包括Zn(OH)2、ZnSiO3和Zn2SiO4)。
(3)复合锌前驱体/生物质的水热转化:将上述含复合锌前驱体与松木碱解物的悬浊液全部转移到200 mL聚四氟乙烯内衬的水热反应釜中, 在180 ℃恒温干燥箱内反应12 h, 冷却至室温, 将沉淀抽滤并用乙醇和蒸馏水(1 : 1)混合液洗涤3次, 将洗涤后的产物在70 ℃下干燥6 h, 研磨过100目(149 μm)筛, 得到复合催化剂, 标记为SOB-3-1, 其中, S代表Zn2SiO4 (zinc silicate)、O代表ZnO (zinc oxide)、B代表生物炭(biochar), 第一个数字“3”表示反应中加入的松木粉为3 g, 第二个数字“1”表示反应中使用的NaOH浓度为1.00 mol/L。
按照上述制备方法, 保持松木粉质量不变, 改变NaOH浓度为2.00、3.00和4.00 mol/L, 可分别得到复合催化剂SOB-3-2、SOB-3-3和SOB-3-4。当固定NaOH浓度为4.00 mol/L条件下, 改变松木粉的使用质量为2和4 g时, 分别得到复合催化剂SOB-2-4和SOB-4-4。
以4.00 mol/L的NaOH溶液代替松木碱解液, 重复步骤(2)和(3)得到的纯ZnO样品作为对比催化剂。
1.3 催化剂的表征
采用Kubo-X1000型比表面积&孔径分析仪(北京彼奥德电子技术有限公司)测试复合催化剂的比表面积、孔容和平均孔径; 利用S-4800型冷场发射扫描电子显微镜(Hitachi Limited, Japan)测试复合催化剂的形貌, 并利用其附带的INCA Energy 350能谱仪测定表面区的元素组成; 通过Nicolet 6700型傅里叶红外光谱分析仪(Theromo Fisher, America)测量试样的FT-IR谱图, 用KBr压片, 扫描范围是4000~ 400 cm-1; 使用D8ADVANCE型X射线衍射仪(Bruker AXS, Germany)测定复合催化剂的晶相结构; 在FLS980型荧光光谱仪上测定样品的光致发光光谱(PL), 激发波长为365 nm, 测定其在450~850 nm范围的发光信号; 采用Cary 5000型紫外可见分光光度计测定紫外-可见漫反射光谱(UV-Vis DRS), 测定参照物为BaSO4。
1.4 吸附及催化降解实验
在吸附实验中, 控制甲硝唑初始浓度为300 mg/L, SOB-3-4和ZnO的用量为0.4 g/L。取50 mL的甲硝唑溶液置于100 mL的烧杯中, 溶液pH调至3.0, 加入催化剂, 在恒温(30 ℃)的条件下磁力搅拌。在吸附时间t为0.5、2、4、6、8、10、15、20、30、45和60 min时, 各取0.2 mL溶液, 用0.45 μm的微孔过滤器过滤后, 测定甲硝唑浓度。
在全部降解实验中甲硝唑溶液的使用量都为50.0 mL, 反应温度为(30±2)℃、氙灯光源固定在距反应器液面10 cm的上方, 功率为300 W, 磁力搅拌。在不同复合催化剂的H2O2光催化活性实验中, 控制甲硝唑初始浓度为300 mg/L、催化剂用量为 0.4 g/L、H2O2投加浓度为40 mmol/L及反应体系的初始pH为3.0。在研究反应条件影响的实验中, 均使用SOB-3-4样品, 仅改变其中一个变量(H2O2投加浓度、催化剂用量、甲硝唑初始浓度及反应体系的初始pH), 而其它条件保持一致。
每次实验开始时, 将反应器放入暗箱中, 先搅拌30 min, 使其达到吸附平衡, 用注射器取第一个试样, 其浓度作为催化降解过程的初始浓度; 然后滴加H2O2溶液(30wt%), 同时打开光源, 反应开始计时, 每隔20 min取样, 用0.45 μm的微孔过滤器过滤后, 用HPLC法测量试样浓度, 并按式(1)计算去除率。
式中, η为甲硝唑的降解率(%), C0为H2O2光催化反应的初始甲硝唑浓度(mg/L), C为反应到t时刻时甲硝唑的浓度(mg/L)。
1.5 分析方法
采用L-3000型高效液相色谱仪测定溶液中甲硝唑的浓度。使用的色谱柱为EC-C18 (4.6 mm× 100 mm, 2.7 μm), 柱温控制在30 ℃, 流动相为体积比80 : 20的水-甲醇混合液, 流速为0.8 mL/min, 进样体积为20.0 μL。检测器波长设定为277 nm。采用5B-2F型COD (Chemical Oxygen Demand)速测仪测定甲硝唑溶液的COD浓度。
2 结果与讨论
2.1 催化剂可见光催化H2O2降解甲硝唑活性的比较
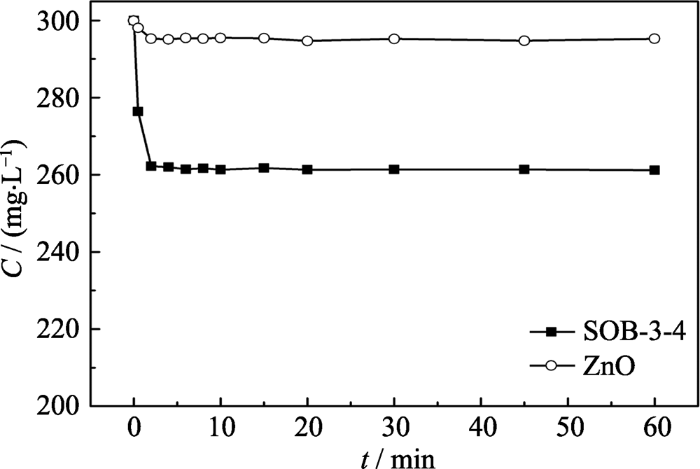
图1示出了在pH=3.0及非光照条件下SOB-3-4和ZnO对甲硝唑的吸附动力学曲线, 可以看出, 复合催化剂和对比催化剂对甲硝唑地吸附很快, 在实验设定的30 min内都可达到吸附平衡; SOB-3-4的吸附性能大于ZnO, 但其吸附率也仅为12.9%, 因此必须借助催化氧化作用有效去除甲硝唑。
图1
图1
催化剂对甲硝唑的吸附动力学曲线
Fig. 1
Adsorption kinetic curves of metronidazole on the catalysts
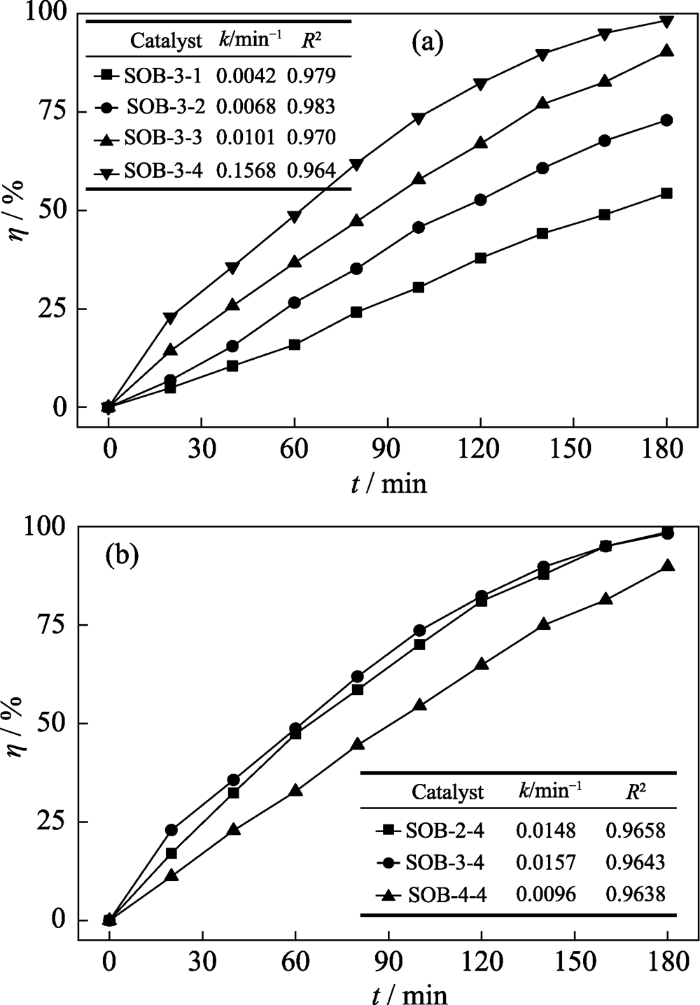
分别测定不同NaOH浓度和不同松木粉使用量得到的两组复合催化剂样品的可见光催化H2O2降解甲硝唑的动力学曲线, 并用准一级动力学方程拟合得到速率常数, 如图2(a,b)所示。从图2(a)可以看出, 松木粉用量相同时, 随着NaOH浓度的升高, 所得复合催化剂光催化H2O2降解甲硝唑的速率、η和k单调地增大, 这表明SOB对甲硝唑降解反应的光催化活性随NaOH浓度的增大而提高。但是, 若继续提高NaOH浓度(>4 mol/L), 会使松木碱解液十分粘稠, 不利于复合催化剂的制备。图2(b)的结果表明, 在NaOH浓度为4 mol/L的条件下, 松木粉用量为2和3 g所得样品的可见光催化H2O2活性接近, SOB-3-4的k略大于SOB-2-4; 当松木粉用量增大到4 g时, 所得样品的k反而减小, 可见光催化H2O2活性降低。比较而言, 以SOB-3-4可见光催化H2O2降解甲硝唑的催化活性最优。
图2
图2
光催化H2O2降解甲硝唑的动力学分析
Fig. 2
Degradation kinetics of metronidazole by H2O2 photocatalysis
(a) Effect of NaOH concentration; (b) Effect of pine wood flour dosage
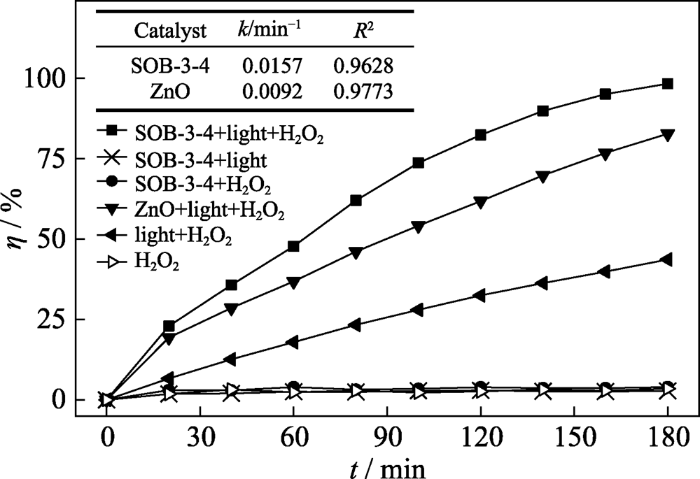
将SOB-3-4与纯ZnO降解甲硝唑的可见光催化活性以及不加H2O2(单纯可见光催化)和未施光照(单独催化H2O2)条件下的降解效果进行比较, 结果如图3所示, 可以看出, 在相同条件下SOB-3-4降解甲硝唑的可见光催化H2O2降解活性明显优于ZnO, 其速率常数是ZnO的1.71倍。这说明用松木碱解液代替单纯无机碱(NaOH)作Zn2+的沉淀剂, 可显著提高产物的可见光催化H2O2降解活性; 同时也表明, 该复合催化剂在可见光和H2O2协同作用下具有催化活性, 但不具备单独可见光催化活性或单独催化H2O2活性。
图3
图3
不同反应体系降解甲硝唑效果比较
Fig. 3
Comparison of metronidazole degradation in different reaction systems
此外, 对SOB-3-4可见光催化降解甲硝唑反应前后(0和180 min)溶液的COD进行了分析。结果表明, 其COD分别为296.4和57.3 mg/L, 矿化率为80.7%, 证明在光催化过程中甲硝唑能够被氧化降解[6]。
2.2 SOB和ZnO的表征结果
2.2.1 XRD表征结果
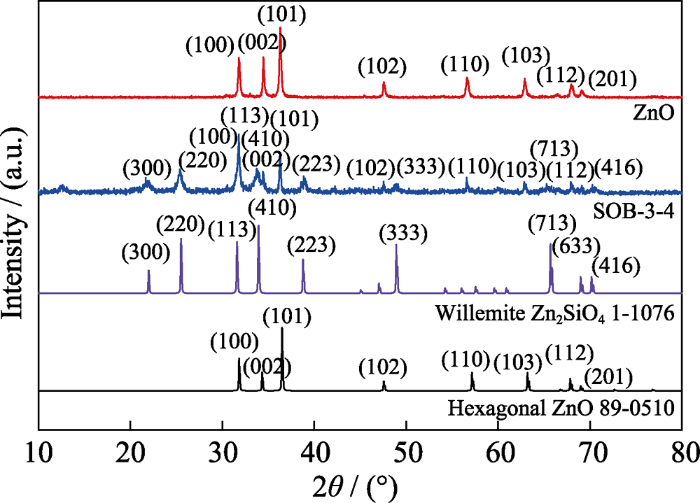
用上述实验优化得到的SOB-3-4样品与ZnO进行对比分析, 以揭示松木碱解液作沉淀剂产生的作用。图4为SO B-3-4和ZnO的XRD图谱, 由图可见, 用NaOH溶液作沉淀剂得到的ZnO的衍射峰尖锐且强, 说明其结晶状态良好, 并可归属为六方晶相ZnO (JCPDS 89-0510)[20]; 而用松木碱解液得到的SOB的图谱有较高的衍射背底, 衍射线宽化且谱峰数增多, 说明其结晶状态变差, 检索结果显示它由硅锌矿(Zn2SiO4, JCPDS 1-1076)和六方晶相ZnO组成。分析SOB-3-4的制备过程:在步骤(1)中热碱液会与容器玻璃内表面反应生成Na2SiO3, 并与松木粉发生碱性降解及剥皮等反应[21]; 在步骤(2)中ZnCl2将分别与剩余的游离OH-及SiO32-反应, 生成Zn(OH)2和ZnSiO3复合锌前驱体(见反应方程式(2, 3)); 在步骤(3)中Zn(OH)2前驱体发生水热转化形成六方晶相ZnO(见反应方程式(4)), 同时Zn(OH)2和ZnO均可与ZnSiO3反应生成硅锌矿型Zn2SiO4(见反应方程式(5, 6))[22]。Zn2SiO4和ZnO的晶体成核与生长过程会受到松木生物质碱性解离物的影响, 因而结晶状态变差及晶体形貌改变。
图4
2.2.2 SEM表征
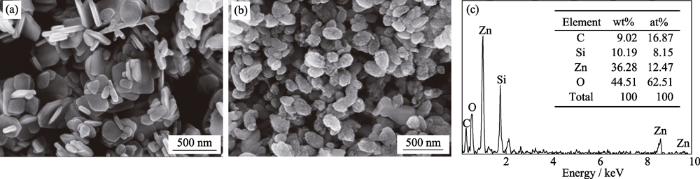
图5(a, b)分别示出了ZnO和SOB-3-4样品颗粒的SEM照片, 对比样品可以看出: ZnO主要是由准六角形颗粒相互堆叠而成, 颗粒表面光滑, 尺寸不均匀, 约分布在50~450 nm之间; SOB-3-4样品含有大量的枣核状颗粒和少量的多边形颗粒, 多边形颗粒表面光滑, 具有ZnO的特征, 而枣核状颗粒表面粗糙, 由极小的纳米颗粒组装而成, 类似于介晶颗粒[23], 并且尺寸相对均匀, 平均尺寸约为250 nm× 180 nm (长轴×短轴), 推测其为Zn2SiO4。根据非经典结晶(Non-classical crystallization)理论[24], 在水热反应条件下, 被有机聚合物包覆的无机纳米粒子作为结晶基元, 容易通过介观自组装和融合机制形成介晶。由前述分析可知, 在制备SOB-3-4的水热体系中含有大量的松木生物质有机聚合物, 这些生物质大分子容易包覆在新生的Zn2SiO4纳米晶核表面, 然后通过典型的介观自组装-融合机制形成枣核状Zn2SiO4介晶颗粒。
图5
图5
(a)ZnO的SEM照片, SOB-3-4的(b)SEM照片和(c)EDS谱图
Fig. 5
(a) SEM image of ZnO, (b) SEM image and (c) EDS results of SOB-3-4
由图5(c)的EDS分析结果可以得出, SOB-3-4主要含有O、Zn、Si和C四种元素, Zn与Si的原子数比为1.53, 小于Zn2SiO4的化学计量比(2), 而O与Si的原子数比为7.67, 显著大于Zn2SiO4的化学计量比(4)。样品中C的含量达到16.87at%, 说明碱解形成的松木生物质在水热条件下发生碳化, 形成了生物炭[25]。由于生物炭, 特别是水热碳化得到的生物炭含有丰富的羟基、羰基和羧基等氧官能团, 所以与Zn2SiO4和ZnO相比, 生物炭的存在对Zn和Si的原子组成造成“稀释效应”, 而对O的原子组成主要产生“增浓效应”。因此, SOB-3-4主要是由Zn2SiO4介晶构成, 其次是ZnO微晶和松木生物炭, 是一种三元复合材料。
2.2.3 FT-IR分析
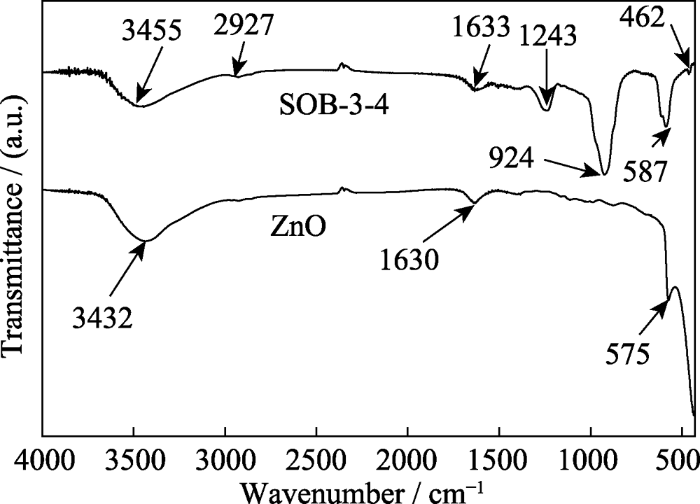
图6是ZnO和SOB-3-4样品的FT-IR谱图。比较发现, SOB-3-4的谱图中存在三个特征峰, 分别归属于ZnO表面化学吸附水H-O-H伸缩振动 (3432 cm-1)、H-O-H弯曲振动(1630 cm-1)和Zn-O伸缩振动(575 cm-1), 但其谱峰的中心位置发生了不同程度的蓝移且谱峰的强度变弱。SOB-3-4的谱图比ZnO多4个峰, 其中2927 cm-1处的弱峰、1243 cm-1处的中强峰可分别归属于生物炭的C-H及C-O-C的不对称伸缩振动峰, 位于924 cm-1的强峰和462 cm-1的弱峰分别归属于Zn2SiO4的O-Si-O伸缩振动峰和弯曲振动峰。FT-IR谱图的表征结果与SEM/EDS分析结果一致。
图6
2.2.4 BET测定
表1 不同催化剂的比表面积、孔容及孔径
Table 1
| Catalyst | Specific surface area/(m2•g-1) | Pore volume/ (cm3•g-1) | Pore size/nm |
|---|---|---|---|
| SOB-3-4 | 31.29 | 0.32 | 20.40 |
| ZnO | 24.39 | 0.24 | 19.74 |
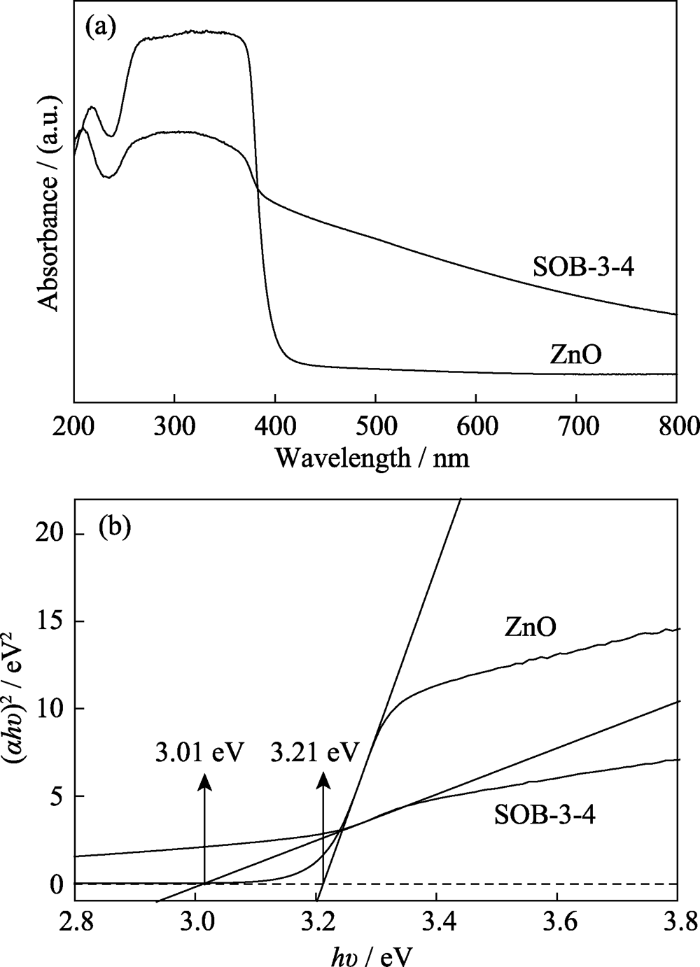
2.2.5 UV-Vis DRS测定
图7
图7
SOB-3-4和ZnO的紫外-可见漫反射光谱(a)及其对应的(αhν)2与光子能量关系图(b)
Fig. 7
UV-Vis diffuse reflectance spectra of SOB-3-4 and ZnO (a), and corresponding relationship between (αhv)2 and photonic energy(b)
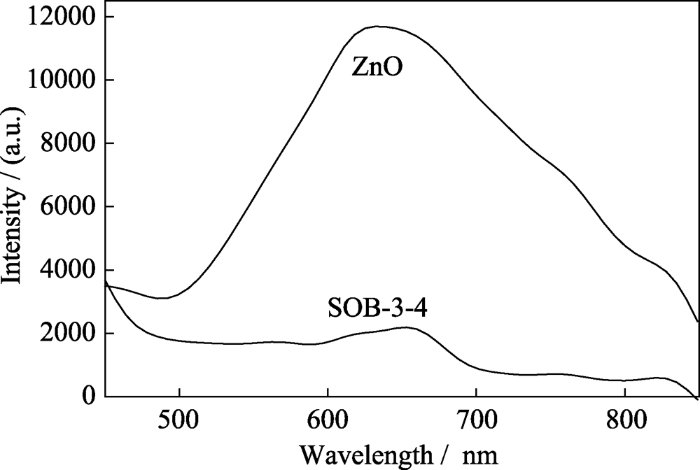
2.2.6 PL光谱分析
图8
2.3 反应条件对复合催化剂活性的影响
2.3.1 pH的影响
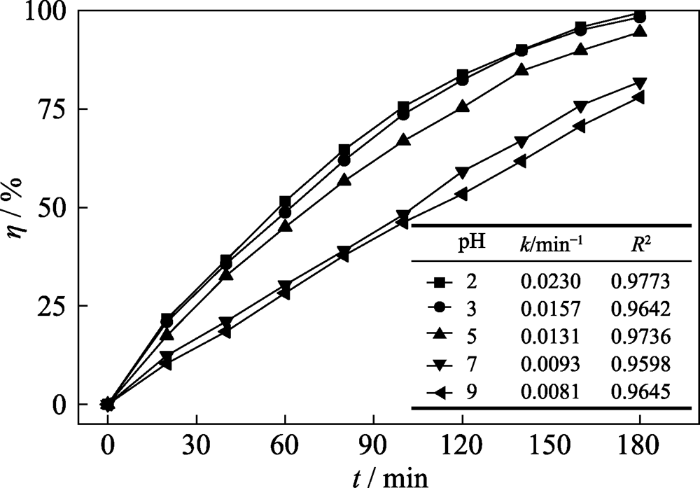
SOB-3-4是由Zn2SiO4、ZnO和松木生物炭构成的三元复合材料, 其表面电荷受介质pH的影响, 同时pH也改变甲硝唑的赋存形态, 从而影响自由基的产生和甲硝唑的降解。研究考察了SOB-3-4投加量为0.4 g/L、甲硝唑初始浓度为300 mg/L、H2O2使用浓度为40 mmol/L时, 溶液初始pH对甲硝唑降解率的影响。如图9所示, 随pH升高甲硝唑的降解曲线下移, η和k减小。当pH=9.0 时, η为78.0%、k为8.1×10-3 min-1, 而在pH=2.0条件下, η与k分别升至99.4%和2.3×10-2 min-1。说明酸性环境有利于甲硝唑的H2O2光催化降解, 这一效应与Fe-Zn- oxide/hydrochar催化体系相似[28], 这主要是由于酸性环境有利于•OH的产生。
图9
图9
SOB-3-4在不同pH条件下对甲硝唑的降解率
Fig. 9
Effect of pH on metronidazole degradation over SOB-3-4
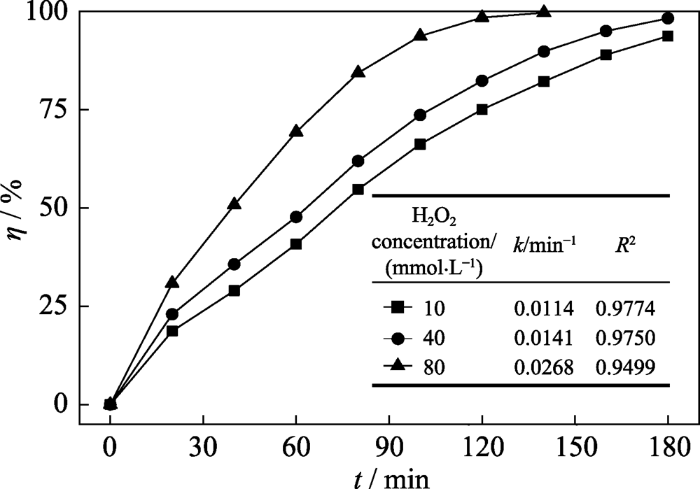
2.3.2 H2O2浓度的影响
图10
图10
H2O2浓度对SOB-3-4的甲硝唑降解性能的影响
Fig. 10
Effect of H2O2 concentration on the degradation efficiency of metronidazole over SOB-3-4
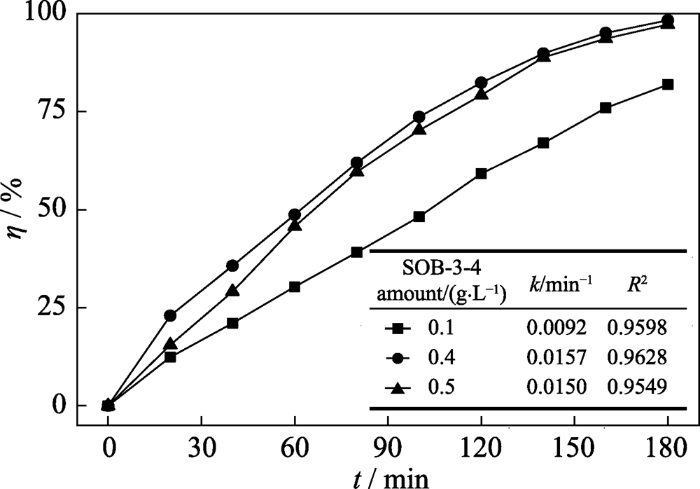
2.3.3 催化剂用量的影响
图11
图11
SOB-3-4的用量对甲硝唑的降解效能的影响
Fig. 11
Effect of SOB-3-4 amount on the degradation efficiency of metronidazole
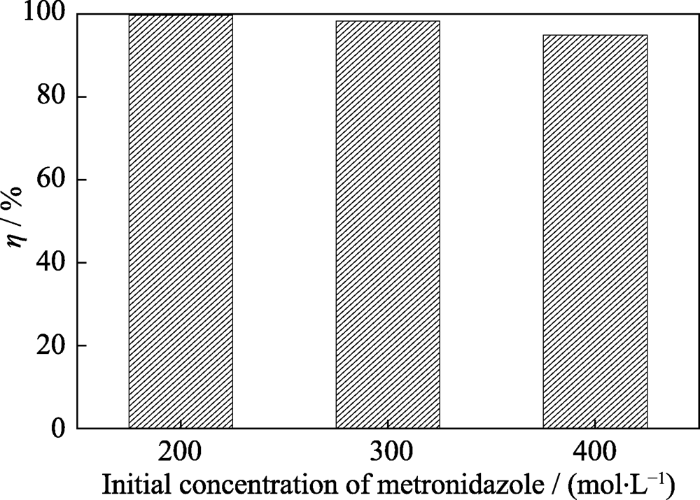
2.3.4 甲硝唑初始浓度的影响
在初始pH为3、催化剂用量为0.4 g/L、H2O2投加浓度为40 mmol/L及反应时间为3 h的条件下, 考察了初始甲硝唑浓度对其光催化H2O2降解效果的影响, 结果见图12。从图中可以看出, 随着甲硝唑初始浓度的升高, 其降解率逐渐降低。这是因为甲硝唑浓度越高, 参与竞争利用催化剂活性位点和•OH的目标物分子数越多, 被氧化降解的甲硝唑分子数不会减少, 但其降解率会下降。
图12
图12
甲硝唑浓度对其光芬顿降解效果的影响
Fig. 12
Effect of initial concentration of metronidazole on the degradation over SOB-3-4
3 结论
1) 在玻璃容器中先用NaOH溶液回流处理松木粉得到松木碱解液, 使之与锌盐反应形成复合锌前驱体, 再经水热转化, 成功制备出Zn2SiO4-ZnO-生物炭三元复合材料(SOB-x-y)。该材料由硅锌矿型Zn2SiO4、六方晶相ZnO和松木生物炭构成, Zn2SiO4呈枣核状介晶颗粒;
2) 与纯六方晶相ZnO相比, Zn2SiO4-ZnO-生物炭复合催化剂的比表面积和孔容增大、带隙能减小、荧光发射强度显著减弱, 其比表面积为31.29 m2/g, 孔容为0.32 cm3/g, 带隙能为3.01 eV;
3) Zn2SiO4-ZnO-生物炭复合催化剂对甲硝唑的光催化H2O2降解过程符合准一级动力学方程, 其光催化活性明显优于ZnO, 并且随NaOH浓度的增大而提高, 随松木粉用量的增大先增大后减小, 以SOB-3-4的催化活性最优;
4) SOB-3-4光催化H2O2降解甲硝唑的速率常数和降解率受体系pH、H2O2浓度、催化剂用量及甲硝唑初始浓度的影响。单因素试验结果表明, 甲硝唑降解反应的速率常数随pH的降低而增大, 随H2O2浓度的升高而增大, 随催化剂用量的增加先增大后减小; 随甲硝唑初始浓度的升高其降解率逐渐降低;
5) 当初始pH为3、催化剂用量为0.4 g/L、H2O2投加浓度为80 mmol/L及甲硝唑初始浓度为 300 mg/L时, 速率常数为2.68×10-2 min-1, 反应3 h后降解率达到99.70%。
参考文献
Effects of dissolved organic matter (DOM) on photodegradation of metronidazole
Primary biodegradation of veterinary antibiotics in aerobic and anaerobic surface water simulation systems
Abstract
The primary aerobic and anaerobic biodegradability at intermediate concentrations (50–5000 μg/l) of the antibiotics olaquindox (OLA), metronidazole (MET), tylosin (TYL) and oxytetracycline (OTC) was studied in a simple shake flask system simulating the conditions in surface waters. The purpose of the study was to provide rate data for primary biodegradation in the scenario where antibiotics pollute surface waters as a result of run-off from arable land. The source of antibiotics may be application of manure as fertilizer or excreta of grazing animals. Assuming first-order degradation kinetics, ranges of half-lifes for aerobic degradation of the four antibiotics studied were 4–8 days (OLA), 9.5–40 days (TYL), 14–104 days (MET) and 42–46 days (OTC). OLA and OTC were degraded with no initial lag phase whereas lag phases from 2 to 34 days (MET) and 31 to 40 days (TYL) were observed for other substances. The biodegradation behaviour was influenced by neither the concentrations of antibiotics nor the time of the year and location for sampling of surface water. Addition of 1 g/l of sediment or 3 mg/l of activated sludge from wastewater treatment increased the biodegradation potential which is believed to be the result of increased bacterial concentration in the test solution. Biodegradation was significantly slower in tests conducted in absence of oxygen. Assessments of the toxic properties of antibiotics by studying the influence on the biodegradation rates of 14C-aniline at different concentrations of antibiotics showed that no tests were conducted at toxic concentrations.
Kinetic study of the adsorption of nitroimidazole antibiotics on activated carbons in aqueous phase
The adsorption kinetics of four nitroimidazoles, Dimetridazole (DMZ), Metronidazole (MNZ), Ronidazole (RNZ) and Tinidazole (TNZ), were studied on three activated carbons: two commercial carbons from Sorbo-Norit (S) and Merck (M) and a third prepared by chemical activation of petroleum coke (C). Experimental data of the corresponding adsorption kinetics were analyzed by applying pseudo-first and pseudo-second-order models and a general diffusion model. Application of pseudo-first and pseudo-second-order kinetic models verified the following: (i) The kinetic model used that better predicts the adsorption rates depends of both the adsorbent and adsorbate studied. (ii) Nitroimidazole adsorption rate decreases in the order MNZ>DMZ>RNZ>TNZ; therefore, in the case of MNZ, molecular size does not appear to be a determining factor in the process. (iii) Nitroimidazole adsorption rate on carbons increases in the order C
Removal of nitroimidazole antibiotics from aqueous solution by adsorption/bioadsorption on activated carbon
The objective of the present study was to analyse the behaviour of activated carbon with different chemical and textural properties in nitroimidazole adsorption, also assessing the combined use of microorganisms and activated carbon in the removal of these compounds from waters and the influence of the chemical nature of the solution (pH and ionic strength) on the adsorption process. Results indicate that the adsorption of nitroimidazoles is largely determined by activated carbon chemical properties. Application of the Langmuir equation to the adsorption isotherms showed an elevated adsorption capacity (X(m)=1.04-2.04 mmol/g) for all contaminants studied. Solution pH and electrolyte concentration did not have a major effect on the adsorption of these compounds on activated carbon, confirming that the principal interactions involved in the adsorption of these compounds are non-electrostatic. Nitroimidazoles are not degraded by microorganisms used in the biological stage of a wastewater treatment plant. However, the presence of microorganisms during nitroimidazole adsorption increased their adsorption on the activated carbon, although it weakened interactions between the adsorbate and carbon surface. In dynamic regime, the adsorptive capacity of activated carbon was markedly higher in surface water and groundwater than in urban wastewaters.
Degradation of metronidazole by nanoscale zero-valent metal prepared from steel pickling waste liquor
Aqueous metronidazole degradation by UV/H2O2 process in single-and multi-lamp tubular photoreactors: kinetics and reactor design
Biological degradation of pharmaceuticals in municipal wastewater treatment: proposing a classification scheme
A simple classification scheme is suggested to characterize the biological degradation of micropollutants such as pharmaceuticals, musk fragrances and estrogens during wastewater treatment. The scheme should be a basis for the discussion about potential removal efficiencies. Hence, the biological degradation of 25 pharmaceuticals, hormones and fragrances was studied in batch experiments at typical concentration levels using activated sewage sludge originating from nutrient-eliminating municipal wastewater treatment plants. Since pseudo first-order degradation kinetics was observed for all compounds down to ng L(-1) levels, the removal rates can be predicted for various reactor configurations. Therefore dilution of wastewater (e.g. by extraneous water) is expected to reduce the degree of biological removal. Wastewater segregation and treatment at the source are therefore to be favoured for elimination of persistent micropollutants over centralized end-of-pipe treatment. For reactor configurations typical for nutrient removal in municipal wastewater, the derived formula for predicting removal allows the identification of three groups of micropollutants according to their degradation constant k(biol): compounds with k(biol)<0.1 L g(SS)(-1)d(-1) are not removed to a significant extent (<20%), compounds with k(biol)>10 L g(SS)(-1)d(-1) transformed by >90% and in-between moderate removal is expected. Based on the degradation of a heterogeneous group of 35 compounds (including literature data), state of the art biological treatment schemes for municipal wastewater are not efficient in degrading pharmaceuticals: only 4 out of 35 compounds are degraded by more than 90% while 17 compounds are removed by less than 50%.
Fate of pharmaceutical and personal care products (PPCPs) during anaerobic digestion of sewage sludge
Abstract
The behaviour of 13 pharmaceutical and personal care products (PPCPs) has been studied during anaerobic digestion of sewage sludge: two musks (Galaxolide and Tonalide), one tranquilliser (Diazepam), one anti-epileptic (Carbamazepine), three anti-phlogistics (Ibuprofen, Naproxen and Diclofenac), two antibiotics (Sulfamethoxazole and Roxithromycin), one X-ray contrast medium (Iopromide) and three oestrogens (Estrone, 17β-oestradiol and 17α-ethinyloestradiol). Two parallel processes have been carried out, one in mesophilic range (37 °C) and the other in thermophilic range (55 °C). The influence of temperature and sludge retention time (SRT) has been analysed. Among the substances considered, the higher removal efficiencies were achieved for the antibiotics, natural oestrogens, musks and Naproxen. For the other compounds, the values ranged between 20% and 60%, except for Carbamazepine, which showed no elimination. The removal of oestrogens, Diazepam and Diclofenac occurred after sludge adaptation. In general, no influence of SRT and temperature on PPCPs removal was observed.
Considering the difficulty of obtaining reliable PPCPs concentrations, especially those corresponding to the fractions sorbed onto sludge, a methodology to validate the experimental data has been developed and successfully applied.
Oxidative degradation of aox and cod by different advanced oxidation processes: a comparative study with two samples of a pharmaceutical wastewater
Enhanced degradation of metronidazole by sunlight via photo-Fenton process under gradual addition of hydrogen peroxide
Safe and efficient degradation of metronidazole using highly dispersed beta-FeOOH on palygorskite as heterogeneous Fenton-like activator of hydrogen peroxide
Degradation of the pharmaceutical metronidazole via UV, Fenton and photo-Fenton processes
Abstract
Degradation rates and removal efficiencies of Metronidazole using UV, UV/H2O2, H2O2/Fe2+, and UV/H2O2/Fe2+ were studied in de-ionized water. The four different oxidation processes were compared for the removal kinetics of the antimicrobial pharmaceutical Metronidazole. It was found that the degradation of Metronidazole by UV and UV/H2O2 exhibited pseudo-first order reaction kinetics. By applying H2O2/Fe2+, and UV/H2O2/Fe2+ the degradation kinetics followed a second order behavior. The quantum yields for direct photolysis, measured at 254 nm and 200–400 nm, were 0.0033 and 0.0080 mol E−1, respectively. Increasing the concentrations of hydrogen peroxide promoted the oxidation rate by UV/ H2O2. Adding more ferrous ions enhanced the oxidation rate for the H2O2/Fe2+ and UV/H2O2/Fe2+ processes. The major advantages and disadvantages of each process and the complexity of comparing the various advanced oxidation processes on an equal basis are discussed.
Comparison of metronidazole degradation by different advanced oxidation processes in low concentration aqueous solutions
Visible-light-driven photocatalytic removal of antibiotics by newly designed C3N4@MnFe2O4-graphene nanocomposites
Newly designed magnetic g-C3N4/MnFe2O4/graphene (C3N4@MnFe2O4-G) composites with enhanced photocatalytic activity were successfully synthesized. The photocatalytic behavior of C3N4@MnFe2O4-G was assessed in photo Fenton-like degradation of antibiotic pollutants, including metronidazole, amoxicillin, tetracycline and ciprofloxacin, using persulfate (S2O8(2-)) as an oxidant under visible light illumination. The C3N4@MnFe2O4-G composites show a superior catalytic activity with 94.5% removal of metronidazole that was almost 3.5 times as high as that of the pure g-C3N4, which could be attributed to the synergistic promoting effect of the favorable adsorptivity, enhanced light absorption intensity, high migration efficiency of charge carriers and longer lifetime of separated electron-hole pairs derived from the formation of the heterojunction between the g-C3N4 and MnFe2O4. Moreover, the self-redox properties of iron and manganese atoms in MnFe2O4 induced by S2O8(2-) were particularly beneficial for the generation of SO4(-). The quenching tests and electron spin resonance (ESR) display that h(+), O2(-), SO4(-) and OH are responsible for the antibiotics decomposition. The heterogeneous photocatalyst could be easily recovered by an extra magnetic field and reused several times without any obvious deterioration in catalytic activity. According to the investigation of active species and identified intermediates, the possible photocatalytic mechanism and reaction pathways have been proposed.
Piezo-phototronic effect enhanced UV/visible photodetector based on fully wide band gap Type-II ZnO/ZnS core/shell nanowire array
A high-performance broad band UV/visible photodetector has been successfully fabricated on a fully wide bandgap ZnO/ZnS type-II heterojunction core/shell nanowire array. The device can detect photons with energies significantly smaller (2.2 eV) than the band gap of ZnO (3.2 eV) and ZnS (3.7 eV), which is mainly attributed to spatially indirect type-II transition facilitated by the abrupt interface between the ZnO core and ZnS shell. The performance of the device was further enhanced through the piezo-phototronic effect induced lowering of the barrier height to allow charge carrier transport across the ZnO/ZnS interface, resulting in three orders of relative responsivity change measured at three different excitation wavelengths (385, 465, and 520 nm). This work demonstrates a prototype UV/visible photodetector based on the truly wide band gap semiconducting 3D core/shell nanowire array with enhanced performance through the piezo-phototronic effect.
Review on the improvement of the photocatalytic and antibacterial activities of ZnO
Sonocatalytic activity of biochar-supported ZnO nanorods in degradation of gemifloxacin: synergy study, effect of parameters and phytotoxicity evaluation. Ultrasonics -
Fine tuning of the dimensionality of zinc silicate nanostructures and their application as highly efficient absorbents for toxic metal ions
Low-temperature hydrothermal synthesis of Zn2SiO4 nanostructures and the novel photocatalytic application in wastewater treatment
Solvent-induced growth of ZnO particles at low temperature
A novel process for synthesis of zinc silicate
In situ synergistic crystallization-induced synthesis of novel Au nanostar-encrusted ZnO mesocrystals with high-quality heterojunctions for high-performance gas sensors
Zinc oxide nanoparticles: chemical mechanisms and classical and non-classical crystallization
Among all the functional materials, ZnO plays an outstanding role in terms of chemical and physical properties, but also in terms of morphological variety and the number of reported synthesis approaches. Complex shapes and hierarchical architectures make ZnO a perfect example to study chemical and crystallization mechanisms. In this review article, we will discuss the nucleation and growth of ZnO nanostructures in liquid media by classical and non-classical (i.e., particle-based) crystallization pathways. We elaborate the chemical conditions and parameters that are responsible for the occurrence of one or the other pathway.
Hydrothermal carbonization for energy-efficient processing of sewage sludge: a review
Influence of MnO2 on the photocatalytic activity of P-25 TiO2 in the degradation of methyl orange
Hydrothermal synthesis and photocatalytic performance of Bi2WO6/ZnO heterojunction photocatalysts
A series of Bi2WO6/ZnO heterojunction photocatalysts with different Bi2WO6 concentrations (1wt%, 2wt%, 4wt%, 8wt%) were prepared by hydrothermal method at different temperatures(120℃-220℃). The prepared pure ZnO, Bi2WO6 and Bi2WO6/ZnO heterojunction photocatalysts were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), Fourier transform infrared spectroscope (FT-IR), UV-Vis diffuse reflectance spectra, and photoluminescence (PL) spectroscope, respectively. The photocatalytic activity of the prepared catalysts was evaluated by photocatalytic degradation of acid orange II under UV light (λ=365nm) irradiation. Results show that the photocatalytic activity of Bi2WO6/ZnO heterojunction photocatalysts is superior to that of pure ZnO and Bi2WO6. The optimal concentration of Bi2WO6 is 4wt% and the best hydrothermal temperature is 150℃. Under the above conditions, the prepared composite catalyst shows about 2.4 times in activity of the pure ZnO. The formation of Bi2WO6/ZnO heterojunction could effectively suppress the recombination of the photo generated electron and hole pairs and result in an increase in photocatalytic activity. Moreover, more surface OH groups over the Bi2WO6/ZnO heterojunction photocatalysts will further benefit the improvement of photocatalytic activity.
Heterogeneous photo-Fenton degradation of organic pollutants with amorphous Fe-Zn-oxide/hydrochar under visible light irradiation
Dark and photoassisted Fe 3+-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide
Heterogeneous Fenton-like degradation of Rhodamine 6G in water using CuFeZSM-5 zeolite catalyst prepared by hydrothermal synthesis
In this study, heterogeneous Fenton-like degradation of reactive azo dye Rhodamine 6G in water was investigated over a CuFeZSM-5 zeolite catalyst prepared by hydrothermal synthesis. At initial pH of 3.4, a color removal of 100% was achieved after a reaction time of 45 min. TOC elimination was measured to be 51.8% after 2 h of oxidation. Initial decolorization rate was described by an equation of -r(A0) = 4.56 x 10(2) e(-24.83/RT)C(R6G,0)C(0.35)(H2O2,0) where R is in kJ/mol. The leaching of iron and copper cations from zeolite structure into the solution during oxidation was dependent on pH strongly. The regulation of pH from 6.5 (dye solution pH) to 3.4, increased leaching for iron from 0.7 to 0.8 mg/dm3 and for copper from 1.4 to 2.1 mg/dm3. The copper was totally leached from the catalyst during the process at pH 3.4.