目前WLED用红光荧光粉的激活离子主要有Eu3+ (4f7-4f7禁戒跃迁)、Eu2+ (4f65d1-4f75d0允许跃迁)和Mn4+ (3d3-3d3禁戒跃迁)。Eu3+激活红光荧光粉(如Y2O3:Eu3+)主要激发跃迁为电荷迁移带跃迁, 在近紫外光区(<365 nm), 无法应用于蓝光LED泵浦的WLED。Eu2+激活硫化物如Sr1-xCaxS:Eu2+(λem:600~ 650 nm, 光谱半高宽FWHM:1800~2000 cm-1)的研究较早[4], 但其化学性质不稳定, 易与水和封装材料反应。目前商业显示级WLED所用红光荧光粉主要为Eu2+激活氮化物, 如(Ba,Sr)2Si5N8:Eu2+(λem:590~ 625 nm, FWHM:2050~2600 cm-1)和(Ca,Sr)AlSiN3: Eu2+ (λem:610~660 nm, FWHM:2100~2500 cm-1)[1,5]。其量子效率很高, 可被蓝光LED有效激发, 化学稳定性好。但其激发光谱超过500 nm, 而与绿/黄粉共封装时产生光谱重吸收, 部分发射光子(>650 nm)落在人眼明视觉敏感度较低波长范围; 合成需要高温高压低氧环境(>1700 ℃, ~1 MPa, N2), 条件苛刻。2014年, Schnick等报道了SrLiAl3N4:Eu2+ (λem=650 nm, FWHM=1180 cm-1)[6]和Sr[Mg3SiN4]: Eu2+ (λem=615 nm, FWHM=1170 cm-1)[7], 其发光光谱半高宽较窄, 且发光热猝灭小, 用于低色温高显色WLED (如色温< 3000 K, 显色指数>90)封装时, 可比传统低色温高显色WLED的流明效率提高4%~12%; 待其化学稳定性提高和成本降低后, 在商业红粉中的占比有望增大。近年来, 美国GE公司将Mn4+激活K2SiF6(KSF)荧光粉用于WLED并商业化。Mn4+在KSF中表现出以630 nm为主峰、来自于ν6/ν4/ν3振动模式的发射峰[8,9], 每个发光峰都很窄(FWHM<5 nm), 内量子效率高达80%[10]。由于K2SiF6:Mn4+的窄带发光特征, 目前其主要应用于液晶显示背光源, 难以实现氮化物红光荧光粉封装所得WLED的高显色指数而不能在多领域完全取代后者。1972年欧司朗/ GE公司[11]报道了K2SiF6:Mn4+材料, 2009年GE申请了其与LED的组合使用专利(US7497973B2等)。目前我国没有掌握KSF类荧光粉核心专利, 产销受制于人。
在K2SiF6中, Mn-F键的高电负性、低极化率和低共价性使2Eg→4A2g跃迁表现较高能量。F-作为双给配体, 一方面向Mn4+提供p轨道成σ键, 另一方面又提供p轨道成π键, 有效中和Mn4+的正电子而形成稳定配体[8]。由于KSF的窄带发光特征, 其发光光谱经过滤光片及液晶面板后依然有很高色纯度[12]; 利用KSF搭配β-Sialon窄带绿粉, 已可实现接近OLED面板色域。但KSF类荧光粉(指以K2SiF6为代表的A2MF6氟化物, A为Li+/K+/Na+/Rb+/Cs+/NH4+; M为 Si4+/Ge4+/Ti4+/Zr4+/Sn4+等)也存在以下劣势: (1)制备过程用到强腐蚀性氢氟酸, 环保压力大, 需要专门耐强酸设备; 即使采用两步湿化学法也无法避免出现杂相(如KHF2)[10,13]; (2)化学稳定性相对差, 高温(>150 ℃)下易分解失效; (3)在潮湿环境下, Mn4+易潮解为Mn3+ (如形成KMnF4·H2O和K2MnF5·H2O)[13], 与Mn4+形成竞争吸收而降低荧光粉量子效率; (4) Mn4+在KSF类基质中处于八面体反转中心, R线跃迁强度非常弱, 对蓝光吸收强度低[14], 易在大功率LED辐照下出现吸收饱和。当前对于Mn4+激活氟化物荧光粉的研究主要有新组分开发、绿色制备及表面包覆等。比如除KSF外, 相继开发了多种氟化物荧光粉, 包括A2MF6:Mn4+ (A为Li+/K+/Na+/Rb+/ Cs+/NH4+; M为 Si4+/Ge4+/Ti4+/Zr4+/Sn4+)、A3MF6:Mn4+ (A为Li+/K+/Na+, M为Al3+/Ga3+)和AXF6(6H2O):Mn4+ (A为Ba2+/Zn2+, X为Si4+/Ge4+/Ti4+/Sn4+)等[15]。
近年来也出现了一系列氧氟化物荧光粉的报道。Mn4+激活氧氟化物荧光粉是一类化学稳定性更高、组分新颖、具有F-/O2-配位而形成畸变八面体的红光荧光粉, 有望部分解决KSF类荧光粉的不足。本文将从合成制备、晶体结构和荧光性质方面综述目前Mn4+激活氧氟化物荧光粉的研究进展。
1 Mn4+离子的能级与荧光性质
1.1 Mn4+离子的基态和激发态能级
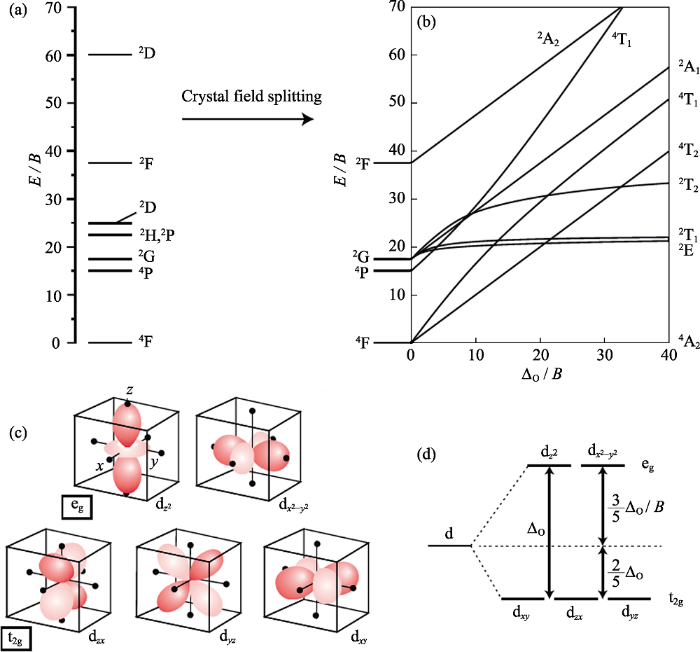
图1给出d轨道的五种简并轨道的相对空间形状、其在八面体晶体场中的劈裂、d3自由离子能级及描述d3离子在八面体晶体场中能级劈裂的Tanabe- Sugano图[16]。当Mn4+掺入固体晶格时, 受晶体场影响, d轨道发生能级劈裂。在八面体晶体场中, ${{d}_{{{z}^{2}}}}$和${{\text{d}}_{{{x}^{2}}-{{y}^{2}}}}$电子云极大值与配体离子电子云迎头相碰而受到较大推斥, 使该轨道能量升高; 而dxy, dyz, dxz电子云极大值插在配体之间, 受到的推斥作用较小, 轨道能量升高较少。因此d轨道能级劈裂为两组: 一组能量高于Es能级, 称为eg轨 道; 另一组能量低于Es能级, 称为t2g轨道(如图1(d)所示)。
图1
图1
d3离子的自由离子能级(C = 4.5B) (a), 描述d3离子在八面体晶体场中能级劈裂的Tanabe-Sugano图(C = 4.5B) (b), 八面体晶体场中五种d轨道相对于配体的取向(黑点表示配体离子)(c)和d轨道在八面体晶体场中的晶体场劈裂(d)[16]
Fig. 1
Energy levels arising from a d3 configuration for a free transition metal ion (C=4.5B) (a), Tanabe-Sugano diagram for the d3 electron configuration in an octahedral crystal field (C=4.5B) (b), orientation of the five d-orbitals with respect to the ligands of an octahedral complex (black dots showing the ligands around the transition metal ion) (c), and crystal field splitting for the d-orbitals in an octahedral crystal field (d)[16]
eg和t2g轨道间能级差称为晶体场分裂能ΔO。在八面体晶体场中, ΔO较大, Mn4+的3个d电子可稳定占据3个t2g轨道, 因此Mn4+倾向占据八面体格位。ΔO取决于过渡金属离子的氧化态、配体离子电荷及两者间键长[9]。过渡金属离子氧化态越高, 其所受晶体场劈裂一般越大; 而配位离子电荷增多或两者间键长变短, 则晶体场劈裂变大。Tanabe和Sugano[17]在考虑d电子和晶体场间的相互作用后计算了d3自由离子在八面体晶体场中的劈裂, 示于图1(b)。Mn4+自由离子能级在八面体晶体场中劈裂为二个或多个能级, 由群论决定。由于过渡金属离子在晶体场中劈裂后能级的能量受晶体场强度和d轨道电子间相互作用的共同影响; 因此, Tanabe-Sugano图的横纵坐标都除以Racah参数B(描述d轨道电子间相互作用, 一般为500~1000 cm-1)并假定C≈4.5B。由于Mn4+具有较多正电荷而常受到强晶体场作用; 在八面体晶体场中, 其最低激发态能级为2Eg(2G); 该能级在Tanabe-Sugano图中几乎为一条平线, 不受晶体场劈裂强度变化的影响。
当Mn4+掺杂到晶体材料中时, 除受晶体场效应影响外, 还受电子云扩展效应(Nephelauxetic effect)影响[18]。电子云扩展效应指金属阳离子与配体离子成键后, 其电子云比自由离子时的电子云更为扩散而引起光谱性质变化的效应。由于金属离子的正电荷被配位键中的负电荷中和而降低, d轨道会有轻微扩展, 使得d轨道电子间排斥作用减弱而能量降低, 表现为Racah参数B和C在晶体中比其在自由离子时有所减小。d轨道电子的离域扩展受金属离子与配体离子间所形成化学键的影响, 该化学键中共价键成分越多, 离域扩展效应越显著, Racah参数减小越显著。Mn4+最低激发态能级2Eg几乎与晶体场效应无关, 其能量仅由Racah参数B和C决定。
1.2 Mn4+离子能级跃迁与荧光性质
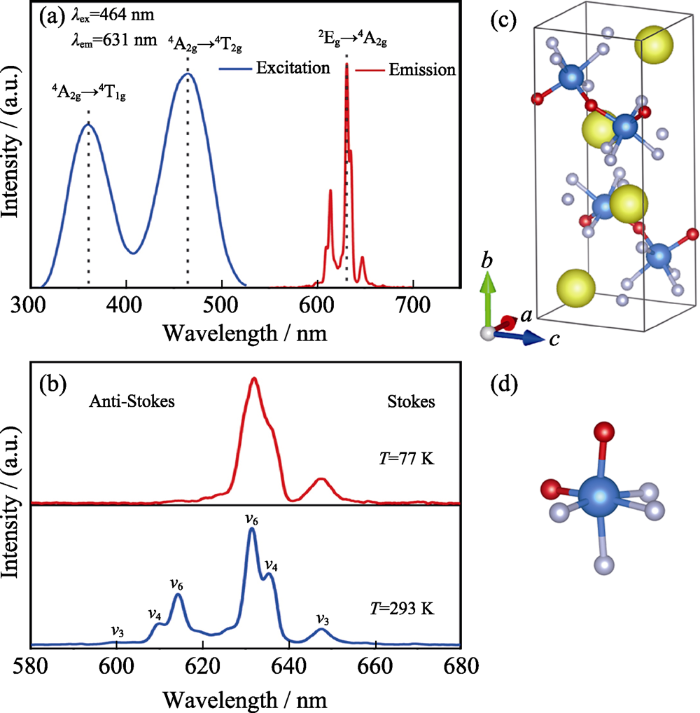
Mn4+的激发是由4F组态的4A2g(电子构型t2g3)能级跃迁到4T2g或4T1g(电子构型t2g2eg1)能级; 根据自旋选择定则, 此激发跃迁为自旋允许跃迁。在K2SiF6:Mn4+中, 此跃迁吸收表现为宽峰, 分别位于300~400 nm和400~500 nm; 由图1(b)可以看出, 该跃迁能量与晶体场强度密切相关。而2Eg(2G)→4A2g(4F)发光跃迁为自旋和宇称双重禁戒; 由于电子与声子耦合作用而部分解禁, 表现为610~650 nm范围多个锐线窄带Stokes和anti-Stokes发射峰。
除d-d跃迁外, 在Mn4+激活荧光粉中还存在着配体离子与Mn4+之间的电荷转移跃迁。电子从配体离子转移到过渡金属离子过程, 伴随偶极矩的巨大变化, 为完全允许跃迁, 因此荧光强度很高。此外, Mn4+和配体离子间的化学键合在电荷迁移激发时发生显著变化; 由于化学键合的变化, 电荷迁移态和基态势能曲线间存在很大的偏移而导致宽带吸收。根据过渡金属离子和配体离子类型的不同, 电荷迁移吸收带可在紫外或可见光区。
2 Mn4+激活氧氟化物荧光粉
2.1 Mn4+激活氧氟化物红光荧光粉的潜在优势
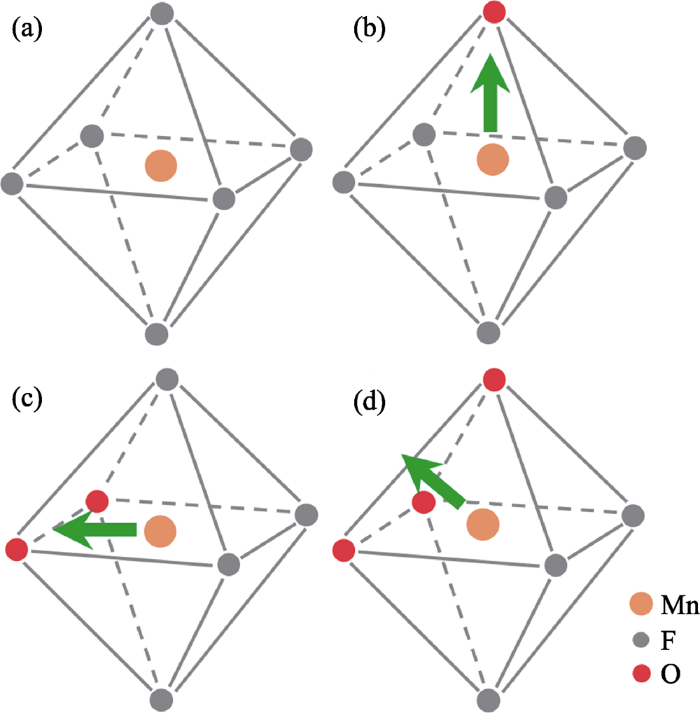
氧氟化物作为Mn4+掺杂基质具有以下重要潜在优势: (1)氧氟化物通常具有比氟化物更好的化学稳定性, 且其制备过程不一定需要HF参与; (2)由于Mn-O/Mn-F键长不同而形成具有较大畸变和低点群对称性的配位八面体, 且由于O和F原子质量不同而热振动振幅和频率不同, 因此更容易产生Jahn-Teller效应而放宽Mn4+ d-d电偶极跃迁选律限制, 得到强R线发光, 并提高对蓝光LED的吸收效率; (3) [MnF6-xOx]可以结合Mn-F离子键特性使R线和相关声子振动带在630~650 nm红光区以得到较高流明效率。当Mn4+处于八面体场反转中心(图2(a))时, R线发光强度非常弱。一旦Mn4+所处环境的局部对称性降低而失去反转对称中心, 则宇称选择规则将通过高能量奇宇称能态(如3d24p)的混入而放宽, 使R线跃迁成为电偶极允许跃迁。氧氟化物中, 阳离子与O2-/F-离子间dπ-pπ轨道成键作用不同, 其八面体配位结构形成天然大畸变(图2(b)~(d)); 当Mn4+掺杂其中时, 其R线发光强度将可能远超来自于反对称振动模式ν6(T2u bending)/ν4(T1u bending)/ ν3(T1u stretching)的发光峰, 而成为Mn4+发光光谱中最强峰。
图2
图2
规则八面体配位和畸变八面体配位
Fig. 2
Regular octahedron coordination and distorted octahedra coordination
(a) Point symmetry of Oh; (b) Central cation shifting to a vertex, C4v; (c) Central cation shifting to an edge, C2v; (d) Central cation shifting to a face, C3v
2.2 Mn4+激活氧氟化物红光荧光粉的制备、晶体结构与荧光性质
目前国内外报道了七种Mn4+激活氧氟化物荧光粉, 分别为R2WO2F4:Mn4+ (R=Na, Cs), Cs2NbOF5: Mn4+, BaNbOF5:Mn4+, Sr2ScO3F:Mn4+, BaTiOF4: Mn4+, Mg28Ge7.55O32F15.04:Mn4+和LiAl4O6F:Mn4+。按所含八面体配位阳离子电子构型可分为d0离子(W6+:[Xe]4f145d0, Nb5+:[Kr]4d0, Sc3+:[Ar]3d0, Ti4+: [Ar]3d0), d10离子(Ge4+:[Ar]3d10)和s0离子(Al3+: [Ne]3s0)三类, 列于表1。
表1 目前所报道的Mn4+激活氧氟化物荧光粉
Table 1
| Cation | Phosphor host | Peaking wavelength/nm | (R-line/ν6 intensity ratio)/% | T50%/K | Ref. |
|---|---|---|---|---|---|
| d0 | Na2WO2F4 | 619 | 125 | 340 | [21-22] |
| Cs2WO2F4 | 632 | 5 | 350 | [23] | |
| Cs2NbOF5 | 632 | 10 | - | [24-25] | |
| BaNbOF5 | 629 | 10 | - | [26] | |
| Sr2ScO3F | 690 | - | 320 | [27] | |
| BaTiOF4 | 632 | 5 | - | [28] | |
| d10 | Mg28Ge7.55O32F15.04 | 657 | - | 700 | [29] |
| s0 | LiAl4O6F | 662 | 5-10 | - | [30] |
Mn4+离子4A2g→4T2g激发跃迁能量与晶体场劈裂相关, 而2Eg→4A2g发光跃迁能量主要取决于电子云扩展效应。目前学术界主流观点认为晶体场劈裂取决于配位键键长和八面体畸变, 而电子云扩展效应与Mn4+-配体离子间离子键性/共价键性和八面体畸变有关。因此, 下文在介绍各氧氟化物荧光粉时, 也考察了Mn4+在上述氧氟化物中的微观配位环境。
2.2.1 含d0离子配位八面体的R2WO2F4:Mn4+
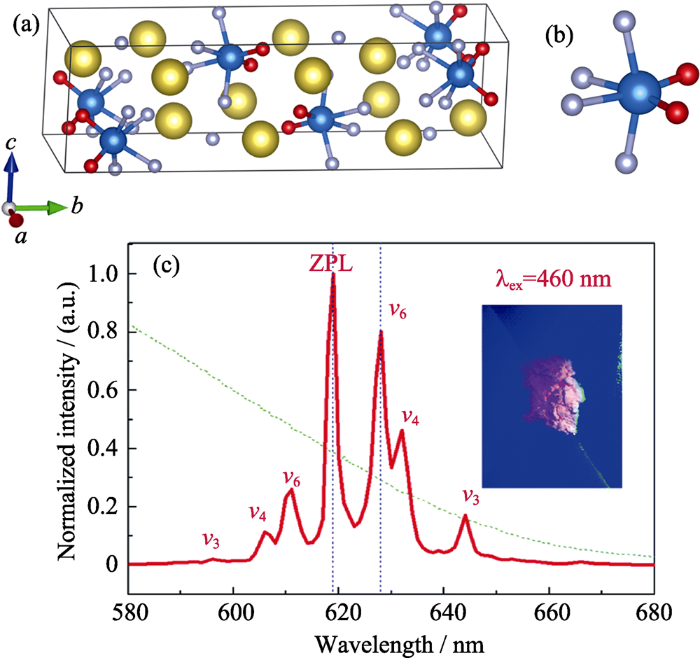
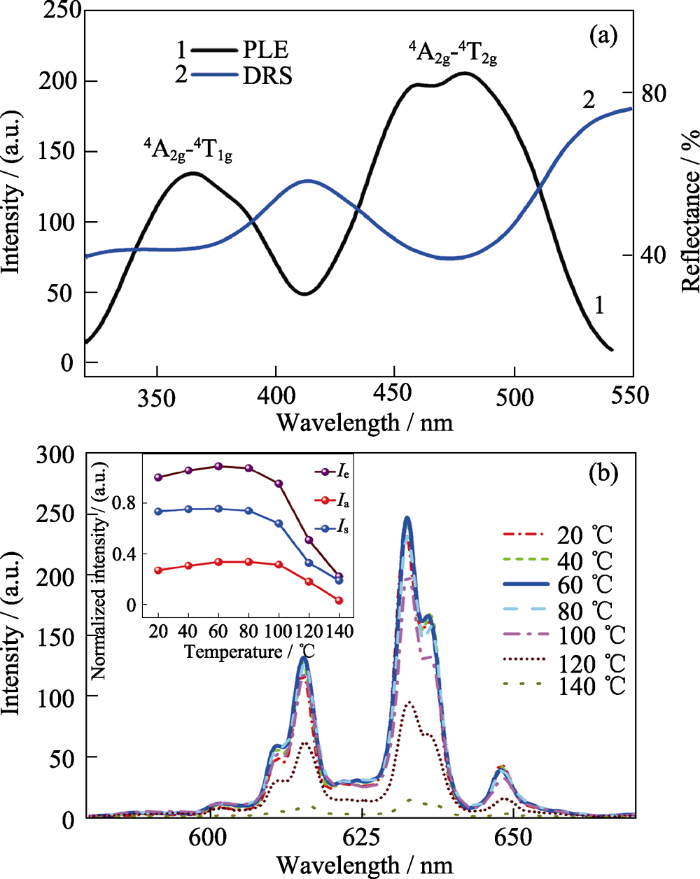
Hu等[21]采用共沉淀法制备了Na2WO2F4:Mn4+。首先将Na2WO4·2H2O在HF溶液中经磁力搅拌溶解, 然后放入适量实验室自制的K2MnF6, 并逐滴加入作为沉淀剂的甲醇, 所得沉淀物经洗涤、离心、干燥得到Na2WO2F4:Mn4+。如图3所示, 在Na2WO2F4中, W6+与2个O2-+4个F-成键形成[WO2F4]2-, 其点群对称操作为C2v; 当Mn4+取代W6+时, 其2Eg→4A2g跃迁的R线位于619 nm处[21]且R线发光强度远高于ν6/ν4/ν3振动模式发光强度(约为ν6声子振动发光峰强度的125%)。考虑到Mn4+在Na2WO2F4中R线发光能量与其在K2SiF6中的一致, 推测Mn4+在Na2WO2F4中仍与6个F-成键形成[MnF6]2-八面体。Mn4+在Na2WO2F4中, 在低温到室温区间的积分发光强度几乎不变, 而从室温开始其积分发光强度骤减, 在340 K时减弱为低温发光强度的一半[21]。
图3
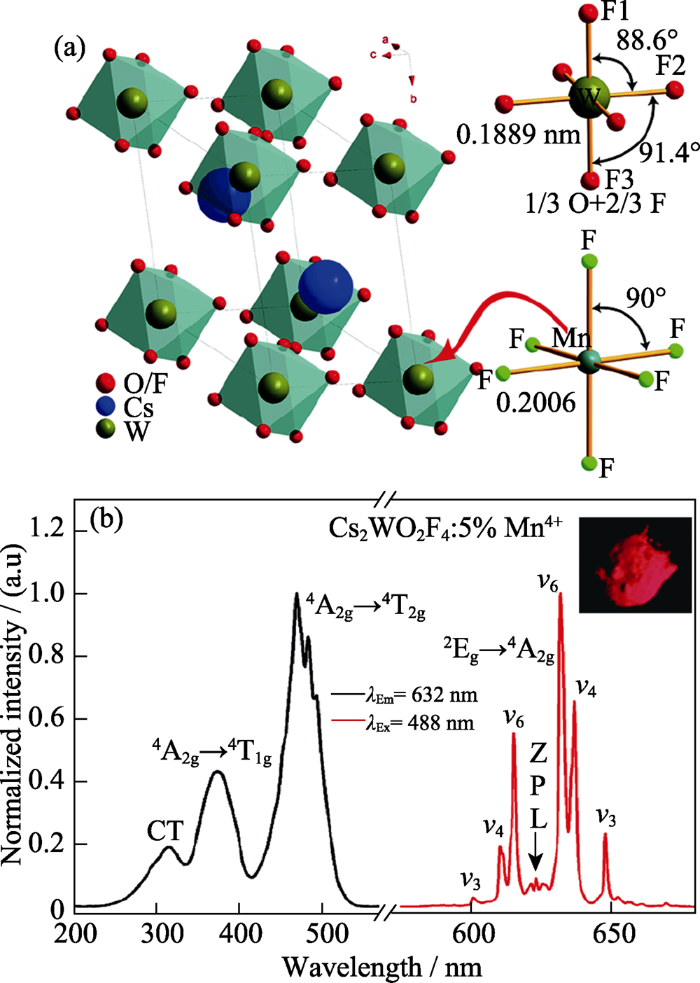
Cai等[23]采用湿化学法制备了Cs2WO2F4:Mn4+。首先将Cs2CO3和WO3经700 ℃煅烧10 h得到Cs2WO4, 然后将其溶解于HF酸溶液中并加入适量K2MnF6, 在通风橱内50 ℃干燥12 h, 所得产物经乙醇洗涤数次, 得到Cs2WO2F4:Mn4+。
在Cs2WO2F4中, O2-/F-占据同一晶体学格位(如图4(a)所示), 六个W-(O,F)键键长相同; [WO2F4]2-八面体所具有的较小畸变, 来自于W-(O,F)键角的不同, 如图4(a)所示。当Mn4+取代W6+时, 由于需要电荷平衡而不能形成[MnO2F4]2-, 而是与其在K2MnF6中一样与六个F-形成[MnF6]2-八面体[23], 且形成的[MnF6]具有较小的八面体畸变。因此, 如图4(b)所示, Mn4+在Cs2WO2F4中其2Eg→4A2g跃迁(R线)位于620 nm处[23]且R线跃迁强度很弱, 与Mn4+ 在K2SiF6中的发光性质非常接近。但两者的激发光谱有明显不同, 计算得到的晶体场强度10Dq分别为21050 cm-1 (Cs2WO2F4:Mn4+)和23900 cm-1 (K2SiF6:Mn4+)[23]。在Cs2WO2F4所含[WO2F4]八面体中, W-O/F键键长为0.1889 nm, 比K2SiF6中所含Si-F键的键长(0.1682 nm)长; 预期Mn4+在Cs2WO2F4中所形成的Mn-F键也比其在K2SiF6中所形成Mn-F键长, 晶体场强度10Dq与金属离子-配体离子间键长成反比, 因此, Mn4+取代Cs2WO2F4中W6+离子时, 其所受晶体场强度较其取代K2SiF6中的Si4+时减弱, 因此4A2g→4T2g跃迁激发波长红移, 但2Eg→4A2g发光跃迁几乎不受晶体场效应影响而保持一致。Mn4+在Cs2WO2F4中的积分发光强度, 在10~280 K范围, 随温度升高而增强, 之后在280~ 430 K范围, 随温度升高而迅速降低。在约350 K时, 其积分发光强度减弱为最高积分发光强度时的一 半[23], 因此热猝灭温度为350 K。
图4
图4
(a) Cs2WO2F4的晶体结构, 其含有具有较小畸变的W(O,F)6配位八面体, 右下所示为Mn4+在K2MnF6中的微观配位八面体; (b) Cs2WO2F4:Mn4+的激发与发射光谱(插图为其在365 nm激发下照片)[23]
Fig. 4
(a) Unit cell of Cs2WO2F4 which contains slightly- distorted [W(O,F)6] octahedra, with the bottom-right showing the local coordination of Mn4+ in K2MnF6; (b) Excitation and emission spectra of Cs2WO2F4:Mn4+ with inset showing the phosphor image under 365 nm light[23]
2.2.2 含d0离子配位八面体的Cs2NbOF5:Mn4+
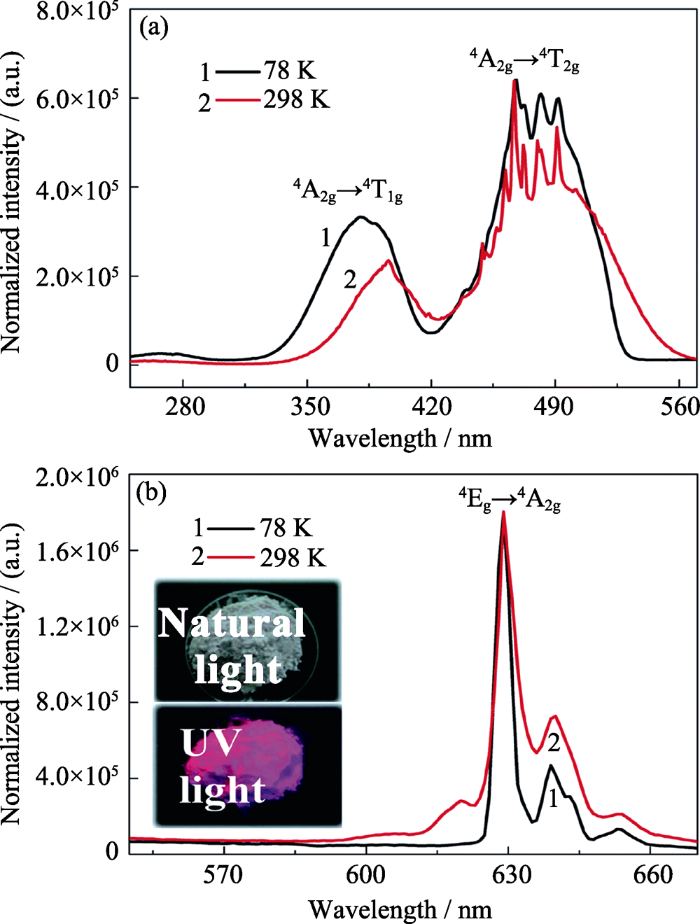
在Cs2NbOF5中, Nb5+与5个F-+1个O2-形成畸变[NbOF5]2-八面体, 且[NbOF5]彼此孤立存在[31]; 其晶体结构与Cs2ZrF6相同[31], 但目前尚没有详细的关于[NbOF5]2-八面体中键长和键角的报道。如图5所示, Mn4+在Cs2NbOF5中, 相比于其在K2SiF6中时, 4A2g→4T2g跃迁激发波长红移; 其R线发光强度很弱, 而最强发光峰来自于ν6振动模式(632 nm)[24,25]。这可能与Cs2NbOF5结构所含[NbOF5]2-八面体呈孤立出现有关; 当Mn4+取代Nb5+时, 为了补偿电价失衡, O2-被F-取代而形成[MnF6]2-八面体, 且由于晶体结构中该八面体孤立存在而不易保持畸变, 因此呈现具有反转对称中心的[MnF6]2-八面体。
图5
图5
Cs2NbOF5:Mn4+的激发光谱(PLE)与漫反射光谱(DRS) (a)和Cs2NbOF5:Mn4+的变温发光光谱(b)[24]
Fig. 5
PLE and DRS spectra of the Cs2NbOF5:Mn4+ phosphor (a) and temperature-dependent emission spectra of Cs2NbOF5:Mn4+ (b)[24] with the inset showing the intensity evolution of the integrated emission (Ie), the stokes emission (Is) and the anti-stokes emmission (Ia)
2.2.3 含d0离子配位八面体的BaNbOF5:Mn4+
Dong等[26]用离子交换再结晶法制备了BaNbOF5:Mn4+。将适量BaF2、Nb2O5、K2MnF6在磁力搅拌下溶解于HF酸溶液中, 将所得沉淀物离心收集并用乙醇洗涤数次得到BaNbOF5:Mn4+。与Cs2WO2F4和Cs2NbOF5晶体结构的配位八面体连接方式类似, 在BaNbOF5中Nb5+与5个F-+1个O2-形成畸变的彼此孤立存在的[NbOF5]2-八面体[32]。目前尚没有该化合物中详细的关于[NbOF5]2-八面体中键长和键角的报道。如图6所示, 当Mn4+掺杂其中时, 其R线发光强度很弱, 而最强发光峰来自于ν6振动模式(629 nm)[26], 与其在Cs2NbOF5中的发光特性一致。在BaNbOF5结构中, [NbOF5]2-八面体孤立存在, [NbOF5]2-八面体中的O2-与Ba2+成键, 但当Mn4+取代Nb5+进行电荷补偿时, 该O2-被F-取代而形成畸变较小的[MnF6]2-八面体, 因此R线发光强度依然很弱。其4A2g→4T2g跃迁激发波长具有较低能量, 位于480 nm (20833 cm-1)处。
图6
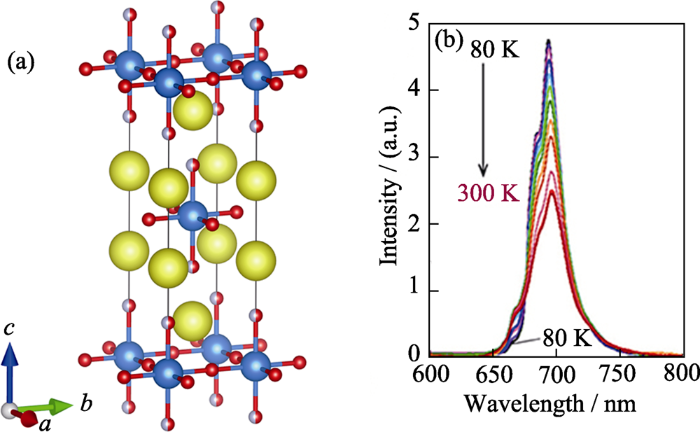
2.2.4 含d0离子配位八面体的Sr2ScO3F:Mn4+
图7
2.2.5 含d0离子配位八面体的BaTiOF4:Mn4+
Liang等[28]用水热和离子交换法制备了BaTiOF4:Mn4+。首先制备K2MnF6作为Mn源, 然后用水热方法以BaF2和H2TiF6为原料制备了BaTiOF4; 将BaTiOF4与适量K2MnF6混合并加入乙醇和HF酸混合溶液, 过夜磁力搅拌后, 收集沉淀物并干燥, 即得BaTiOF4:Mn4+。图8(c)所示为BaTiOF4的晶体结构, 其晶胞中含有一种Ti4+晶体学格位。由于Ti-O键的键长短于Ti-F键键长, 所以Ti4+形成[TiO2F4]畸变八面体配位结构; 如图8(d)所示, Ti偏离八面体反转中心而偏向该八面体的一条边。在BaTiOF4中, 只有Ti4+格位可供Mn4+占据; 且该氧氟化物基质与前述几种的不同在于, 当Mn4+取代Ti4+时为等价取代, 因此非常可能在取代Ti4+后保持原来的配位环境不变, 即形成[MnO2F4]。但从图8(a)所示BaTiOF4: Mn4+的激发和发射光谱可以看出, 其最强发光峰位于631 nm处, 来自于ν6振动模式, 而R线发光强度很弱。因此, Mn4+在BaTiOF4中掺杂后所形成的微观配位环境极有可能是具有反转中心的[MnF6]八面体, 而非预期的[MnO2F4]八面体。当冷却到77 K时, 其发射光谱中只表现出ν6/ν4/ν3振动模式的Stokes发光峰(图8(b))。其积分发光强度从20 ℃至120 ℃之间逐渐增强, 而在其后骤降, 在160 ℃时降为室温发光强度的一半[28]。
图8
图8
BaTiOF4:Mn4+的室温激发与发射光谱(a), BaTiOF4:Mn4+的室温和低温发光光谱(b), BaTiOF4的晶胞(c)和[Ti2OF4]畸变八面体(d)[28]
Fig. 8
Excitation and emission spectra of BaTiOF4:Mn4+ at room temperature (a), emission spectra of BaTiOF4:Mn4+ at 77 K and 293 K (b), unit cell of BaTiOF4 (c), and distorted octahedron coordination of [Ti2OF4] (d)[28]Ba: yellow; Ti: blue; O: red; F: gray
2.2.6 含d10离子配位八面体的Mg28Ge7.55O32F15.04:Mn4+
商业Mn4+激活荧光粉中, 除用于WLED的K2SiF6:Mn4+外, 还有一种用于高压汞蒸气灯和荧光灯的Mg28Ge7.55O32F15.04:Mn4+。其可由MgO、MgF2和GeO2经高温固相反应法制备: 将上述原料研磨均匀后转移到带盖的铂坩埚中在1100 ℃下煅烧1 h, 再次研磨后于1200 ℃下煅烧16 h即得[33]。
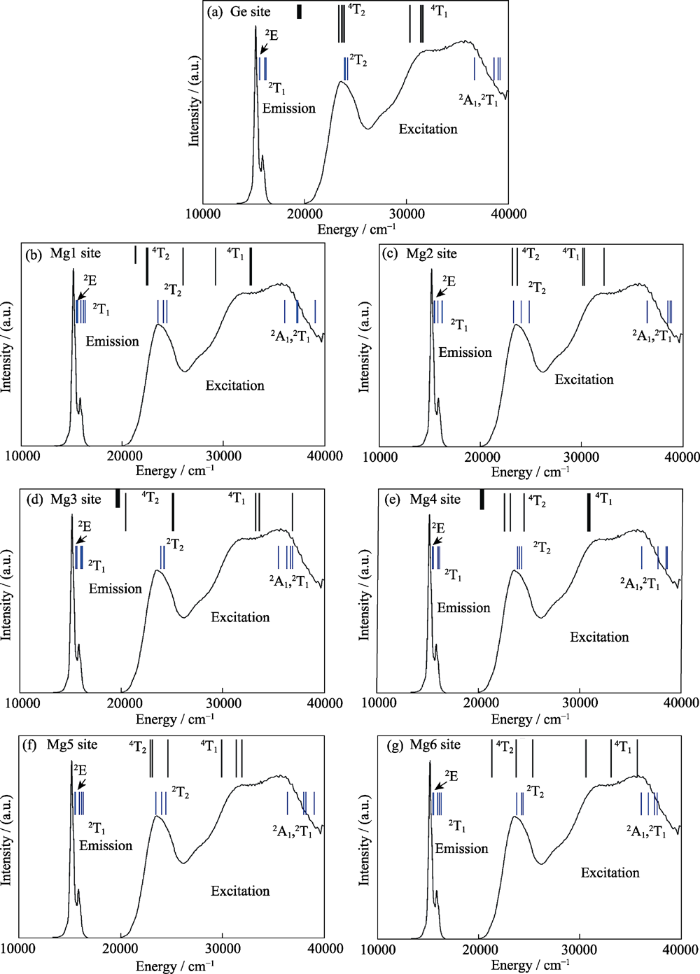
Mg28Ge7.55O32F15.04晶胞中共含六种Mg2+和三种Ge4+格位。其中, Ge1与6个O2-形成八面体配位, 而Ge2或Ge3分别与O2-或F-形成四面体配位; 且在这三种Ge格位中, 实际上只有38.8%的Ge3格位有Ge4+离子占据。因此Mn4+只可能占据Ge1格位[29]。六种Mg2+格位都形成八面体配位, 但其成键情况不同, 分别为: Mg1/Mg3/Mg6与4个O2-+2个F-成键, Mg2与6个O2-成键, Mg4与3个O2-+3个F-成键, Mg5与5个O2-+1个F-成键[29]。Mn4+取代Ge4+时为等价取代, 而取代Mg2+时需产生间隙阴离子或阳离子空位等进行电荷补偿。Brik等[29]通过电荷交换模型研究了Mn4+在Mg28Ge7.55O32F15.04的倾向占据格位: 通过计算4T2g和4T1g三重态在上述七种八面体格位中的劈裂并与实测4A2g→4T2g和4A2g→4T1g激发光谱相比较(图9), 排除了Mn4+占据Mg1/Mg3/Mg4/Mg6格位的可能; 再考虑到电荷平衡后认为Mn4+在该化合物中最倾向占据Ge1而次倾向于占据Mg2格位。考虑到此两个格位都只与六个O2-形成八面体, 因此Mn4+在该基质中的微观配位依然是[MnO6]。Mn4+在该基质中最大发光峰波长为657 nm[29], 其热猝灭温度高达700 K, 远高于K2SiF6:Mn4+荧光粉558 K的热猝灭温度[34]。在Mg28Ge7.55O32F15.04中4A2g→4T2g跃迁能量较高(23640 cm-1), 在假定势能曲线的曲率和平衡位置不随晶体结构改变的情况下, 较高的4T2g能级位置使得发生非辐射跃迁所需活化能较高[34]。
图9
2.2.7 含s0离子配位八面体的LiAl4O6F:Mn4+
Wang等[30]用高温固相反应法制备了LiAl4O6F: Mn4+。以LiF、Al2O3、MnCO3为原料, 充分研磨后于750~800 ℃下煅烧8 h即得。如图10(a)所示, LiAl4O6F结构中同时存在AlO4四面体和AlO6八面体; 当掺杂Mn4+时, 其取代占据AlO6八面体中Al3+格位[30]。这与Mn4+在其他铝酸盐如Sr4Al14O25、CaAl12O19、BaMgAl10O17等中占据AlO6八面体时配位情况相似, 即形成[MnO6]八面体配位; 由于Mn-O键的高共价键性, 因此Mn4+在LiAl4O6F中其2Eg→4A2g跃迁在662 nm附近(图10(b))。该荧光粉的热猝灭性较差, 加热到398 K时, 其发光强度约降至室温发光强度的一半。
图10
3 总结与展望
氧氟化物中所含有的配位八面体具有天然大畸变, 有助于放宽宇称跃迁选律, 因此Mn4+激活氧氟化物荧光粉, 有望作为一类组分新颖、化学稳定性好、具有更高d-d跃迁几率和强R线发光的暖白光WLED用红光荧光粉, 具有研究价值。
在目前所报道的七种Mn4+激活氧氟化物荧光粉中, Mn4+在微观配位上仍是与六个F-形成[MnF6]八面体或与六个O2-形成[MnO6]八面体, 并未同时与O2-/F-离子形成化合键; 而且只在Na2WO2F4这一种氧氟化物基质中实现了强R线发光。此外, 目前所报道的七种Mn4+激活含d0/d10/s0离子氧氟化物荧光粉仍无法与商业K2SiF6:Mn4+荧光粉相媲美且研究结果不足, 表现在如下六个方面: 1)当前Mn4+激活氧氟化物荧光粉的研究主要集中在不等价取代较大离子半径阳离子如W6+/Nb5+等, 而在Al3+/Ge4+等小离子半径取代氧氟化物中的研究较少。Mn4+所取代阳离子的离子半径会影响其晶体场效应10Dq的大小, 进而影响4T2g能级位置。2)当前所报道的Mn4+激活氧氟化物荧光粉的耐热猝灭性普遍较差(这可能与4T2g能级位置较低有关)。3)Mn4+掺杂在氧氟化物中所形成的微观配位体没有明确确定, 甚至Mn4+到底是否能够形成同时与F-和O2-配位的畸变八面体都不确定。Mn4+在同时与O2-/F-成键配位时其光谱特性(2Eg→4A2g跃迁能量、R线发光强度与ν6/ν4/ν3振动模式发光强度的比例)与O2-/F-离子的排列和数量关系之间的制约关系更是不明。4)Mn4+掺杂氧氟化物荧光粉的化学稳定性(基质吸潮变质和所掺杂Mn4+离子吸潮变价)是否优于Mn4+激活氟化物(尤其是在氧氟化物中形成[MnF6]配位时), 没有相关研究结果报道。5)目前Mn4+掺杂商业氟化物红光荧光粉的内量子效率很高, 但因d-d跃迁的宇称禁戒特性而导致外量子效率不高。理论上通过降低Mn4+局域配位结构对称性可放宽宇称禁戒选律、提高d-d跃迁几率, 进而提高外量子效率。但目前所研究的Mn4+激活氧氟化物荧光粉, 其内/外量子效率鲜有报道。6)商业K2SiF6:Mn4+氟化物荧光粉的荧光寿命τ约为8 ms, 远长于商业Eu2+激活氮化物荧光粉μs级寿命, 用于需要快速响应的背光源时可能会出现画面残影现象; Mn4+在氧氟化物中具有畸变八面体配位的低对称性格位取代, 其荧光寿命如何变化, 目前鲜有报道。
如今, 理论计算和机器学习等已成为材料研究的一种重要的先行研究手段。能否通过密度泛函理论等计算方法, 计算Mn4+在与O/F同时配位时的稳定性及其发光能量与O/F数量和相对空间位置的关系, 值得关注。尤其是现在Mn4+的跃迁发光能量在630~650 nm之间没有报道, Mn4+在什么样的氧氟化物基质中能否实现这种发光能量, 值得探索。此外, 作者认为, 未来Mn4+激活氧氟化物荧光粉研发应予以关注的研究内容有: 1)合成制备多种新型Mn4+激活氧氟化物红光荧光粉, 掌握其可控制备方法及Mn4+的稳定有效掺杂方法; 2)探索氧氟化物基质中不同O/F配位(相对排列和数量关系)对Mn4+ 2Eg→4A2g跃迁能量和能级劈裂的影响规律; 3)探明氧氟化物基质中Mn4+ 2Eg→4A2g热致猝灭的发生机理和影响因素; 4)了解Mn4+激活氧氟化物的化学稳定性和使用稳定性, 为其实际使用提供实验依据; 5)测试所研发Mn4+激活氧氟化物荧光粉的内/外量子效率, 研发荧光寿命短、高吸收效率和量子效率的氧氟化物荧光粉。
参考文献
Down-conversion nitride materials for solid state lighting: recent advances and perspectives
Advances in solid state white lighting technologies witness the explosive development of phosphor materials (down-conversion luminescent materials). A large amount of evidence has demonstrated the revolutionary role of the emerging nitride phosphors in producing superior white light-emitting diodes for lighting and display applications. The structural and compositional versatility together with the unique local coordination environments enable nitride materials to have compelling luminescent properties such as abundant emission colors, controllable photoluminescence spectra, high conversion efficiency, and small thermal quenching/degradation. Here, we summarize the state-of-art progress on this novel family of luminescent materials and discuss the topics of materials discovery, crystal chemistry, structure-related luminescence, temperature-dependent luminescence, and spectral tailoring. We also overview different types of nitride phosphors and their applications in solid state lighting, including general illumination, backlighting, and laser-driven lighting. Finally, the challenges and outlooks in this type of promising down-conversion materials are highlighted.
Progress in discovery and structural design of color conversion phosphors for LEDs
Critical red components for next-generation white LEDs
Warm white LEDs with a high color rendering index and a low correlated color temperature have undergone rapid development. In this regard, red-emitting materials-such as fluoride phosphors, namely, A2MF6:Mn(4+) (A = K, Na, and Cs; M = Si, Ge, Zr, Sn, and Ti) and XSiF6:Mn(4+) (X = Ba or Zn), nitridoaluminate phosphor (Sr[LiAl3N4]:Eu(2+)), and nanocrystals of cesium lead iodide perovskite (CsPbI3)-have been extensively investigated recently. These compounds generate narrow emissions in the visible red spectral region that are highly perceived by the human eye and lead to excellent chromatic saturation of the red spectra. This paper describes the structure, luminescence properties, morphologies, thermal features, and moisture resistance of critical red components, as well as their limitations for practical applications. This Perspective also provides a basis for future development and scientific challenges in optical research.
Preparation and luminescent properties of (Ca1-xSrx)S:Eu 2+ red-emitting phosphor for white LED
Optical properties of (oxy)nitride materials: a review
Narrow-band red-emitting Sr[LiAl3N4]:Eu 2+ as a next-generation LED-phosphor material
To facilitate the next generation of high-power white-light-emitting diodes (white LEDs), the discovery of more efficient red-emitting phosphor materials is essential. In this regard, the hardly explored compound class of nitridoaluminates affords a new material with superior luminescence properties. Doped with Eu2+, Sr[LiAl3N4] emerged as a new high-performance narrow-band red-emitting phosphor material, which can efficiently be excited by GaN-based blue LEDs. Owing to the highly efficient red emission at lambda(max) similar to 650 nm with a full-width at half-maximum of similar to 1,180 cm(-1) (similar to 50 nm) that shows only very low thermal quenching (>95% relative to the quantum efficiency at 200 degrees C), a prototype phosphor-converted LED (pc-LED), employing Sr[LiAl3N4]:Eu2+ as the red-emitting component, already shows an increase of 14% in luminous efficacy compared with a commercially available high colour rendering index (CRI) LED, together with an excellent colour rendition (R(a)8 = 91, R9 = 57). Therefore, we predict great potential for industrial applications in high-power white pc-LEDs.
Toward new phosphors for application in illumination-grade white pc-LEDs: the nitridomagnesosilicates Ca[Mg3SiN4]:Ce 3+, Sr[Mg3SiN4]:Eu 2+, and Eu[Mg3SiN4]
The isotypic compounds M[Mg3SiN4] (M = Ca,Sr,Eu) have been synthesized by solid-state reactions in sealed tantalum ampules or in a radio-frequency furnace. The nitridomagnesosilicates crystallize in space group I4(1)/a (No. 88). Crystal structures were solved and refined from single-crystal X-ray diffraction data (Z = 16, Ca[Mg3SiN4]:Ce3+, a = 11.424(2), c = 13.445(3) angstrom, R1 = 0.040, wR2 = 0.106; Sr[Mg3SiN4]:Eu2+, a = 11.495(2), c = 13.512(3) angstrom, R1 = 0.036, wR2 = 0.102; Eu[Mg3SiN4], a = 11.511(4), c = 13.552(4) angstrom, R1 = 0.016, wR2 = 0.039). The nitridomagnesosilicates are isotypic to Na[Li3SiO4], containing a condensed tetrahedra network with a high degree of condensation (i.e., atomic ratio (Mg,Si):N) kappa = 1. The crystal structures were confirmed by Rietveld refinement, lattice energy (MAPLE) calculations, and further investigated by Si-29-MAS NMR. Ce3+-doped samples of Ca[Mg3SiN4] show yellow emission (lambda(max) = 530 and 585 nm, fwhm similar to 3900 cm(-1) (similar to 130 nm)), while Sr[Mg3SiN4]:Eu2+ exhibits red luminescence (lambda(max) = 615 nm) with the most narrow red emission of Eu2+-phosphors reported in the literature so far (fwhm similar to 1170 cm(-1) (similar to 43 nm)). According to this outstanding narrow red emission, originating from parity allowed 4f(6)5d(1) -> 4f(7) transition in Eu2+, Sr[Mg3SiN4]:Eu2+ may point the way to the next generation red phosphor materials for application in illumination-grade white pc-LEDs.
Photoluminescence spectra and modeling analyses of Mn 4+-activated fluoride phosphors: a review
Mn4+-activated red and deep red-emitting phosphors
K2SiF6:Mn 4+ as a red phosphor for displays and warm-white LEDs: a review of properties and perspectives
Efficient Mn(IV) emission in fluorine coordination
Research progress and development trend of fluoride phosphor for white LED
K2MnF6 as a precursor for saturated red fluoride phosphors: the struggle for structural stability
Research progress and application prospects of transition metal Mn 4+-activated luminescent materials
Recent advances in Mn 4+-doped fluoride narrow-band red-emitting phosphors
New Narrow Band Red Phosphors for White Light Emitting Diodes
On the absorption spectra of complex ions II
Spin-forbidden transitions in the spectra of transition metal ions and nephelauxetic effect
On the Mn 4+ R-line emission intensity and its tunability in solids
Intense deep-red zero phonon line emission of Mn 4+ in double perovskite La4Ti3O12
Phosphors that emit in the deep-red spectral region are critical for plant cultivation light-emitting diodes. Herein, ultrabroadband deep-red luminescence of Mn(4+) in La4Ti3O12 was studied, which showed intense zero phonon line emission. The double-perovskite structural La4Ti3O12 simultaneously contains two Ti(4+) sites forming slightly- and highly-distorted TiO6 octahedra, respectively. The influence of octahedral distortion on the Mn(4+) emission energy in the two distinct Ti(4+) sites was studied both experimentally and theoretically. The spectral measurements indicated that Mn(4+) in La4Ti3O12 showed intense zero phonon line emission (ZPL) at deep-red 710-740 nm under excitation of 400 nm charging the O(2-)--> Mn(4+) charge transfer transition. The splitting of the ZPL of the Mn(4+ 2)Eg-->(4)A2g transition as well as the intensity of ZPL relative to the vibronic phonon sideband emissions were found to be greatly influenced by the degree of octahedral distortion. The crystal-field strength and Racah parameters of Mn(4+) in each Ti(4+) site were also estimated. The Mn(4+ 2)Eg-->(4)A2g luminescence exhibited severe thermal quenching, which was explained by the low-lying (4)T2g level and charge-transfer state.
A highly-distorted octahedron with a C2v group symmetry inducing an ultra-intense zero phonon line in Mn 4+-activated oxyfluoride Na2WO2F4
Excitation power dependent optical temperature behaviors in Mn 4+ doped oxyfluoride Na2WO2F4
A Mn(4+) doped Na2WO2F4 phosphor was synthesized through a two-step wet chemical method. The relationship between crystal structure and luminescence properties is discussed and unusual strong intense zero phonon lines (ZPLs) have been found in a distorted octahedral environment. The power dependent luminescence spectra exhibit the existence of down conversion luminescence intensity saturation under a high pumping power limit. The fluorescence intensity ratios of anti-Stokes bands to the ZPL and Stokes bands reveal an obvious temperature dependent relationship based on thermal de-population from the low states to the upper states of an intrinsic Mn(4+ 2)Eg --> (4)A2g transition. The temperature dependent emission intensity of Mn(4+) is investigated by changing the excitation power, and an optical temperature sensitivity as high as 0.00658 K(-1) is achieved at 193 K with the intensity ratio of anti-Stokes bands to the ZPL under 488 nm excitation by a Xenon lamp. This work presents a new method to realize optical thermometry at low temperature by controlling the intensity ratio of the anti-Stokes bands to the ZPL.
Luminescence, energy transfer and optical thermometry of a novel narrow red emitting phosphor: Cs2WO2F4:Mn 4+
Novel Mn 4+-activated oxyfluoride Cs2NbOF5:Mn 4+ red phosphor for warm white light- emitting diodes
A novel Cs2NbOF5:Mn 4+ oxyfluoride red phosphor for light-emitting diode devices
A novel red phosphor of Mn 4+ ion-doped oxyfluoroniobate BaNbOF5 for warm WLED applications
Photoluminescence properties of layered perovskite-type strontium scandium oxyfluoride activated with Mn 4+
In this research, we have found that layered perovskite titanate Sr2TiO4 doped with Mn(4+) exhibits photoluminescence even at room temperature despite no luminescence from Mn(4+)-doped SrTiO3 with a three-dimensional bulky perovskite structure. The relative position of t2g orbital of Mn to the valence band is a key factor for appearance of Mn(4+)-emission in Sr2TiO4:Mn. This result suggested usefulness of layered perovskite-type materials as hosts for Mn(4+)-activated phosphors than the bulky perovskite-type materials. Our investigation into photoluminescence of Mn(4+)-doped layered perovskite compounds has revealed that strontium scandium oxyfluoride Sr2ScO3F activated with Mn(4+) exhibits Mn(4+)-emission with a peak at 697 nm under excitation at 300-600 nm and its emission intensity is much stronger than that of Sr2TiO4:Mn. The internal and external quantum yields of Sr2ScO3F:Mn were determined to be 50.5 and 43.5% under excitation at 345 nm, respectively.
Synthesis, luminescence properties of a novel oxyfluoride red phosphor BaTiOF4:Mn 4+ for LED backlighting
A computation study of site occupancy in the commercial Mg28Ge7.55O32F15.04:Mn 4+ phosphor
Luminescence properties of a non-rare-earth doped oxyfluoride LiAl4O6F:Mn 4+ red phosphor for solid-state lighting
Synthesis and luminescence properties of Cs2NbOF5 and Cs2NbOCl5 with isolated [NbOX5] -2 (X=F -, Cl -) octahedra
Synthesis and luminescence properties of barium niobium oxide fluoride (BaNbOF5) with isolated [NbOF5] 2- octahedra
Anion and cation defect structure in magnesium fluorogermanate