众多二元金属复合氧化物中, Bi2Mn4O10既具有锰酸盐的高质量比容量, 又兼备铋基材料的高体积比容量的特性, 是一种很有前景的新型负极材料; 另外, Bi2Mn4O10的理论比容量高达873 mAh/g[16,17]。目前, Bi2Mn4O10的制备方法主要为湿式球磨法[18]和溶胶-凝胶法。湿式球磨法操作简单、易于产业化, 适用于二元过渡金属氧化物的制备, 但所制备的产物存在粒度分布不均匀、颗粒团聚严重等不足, 对材料的电化学性能有一定影响。如本课题组[17]曾 通过机械球磨法制备了Bi2Mn4O10负极材料, 在0.2C下经过50次电化学循环后放电比容量为402.3 mAh/g, 但容量保持率仅为64.2%。溶胶-凝胶法是一种常用的微米、纳米级材料制备方法, 具有工艺、设备简单, 合成相纯度高、热处理温度低等优点。Zhang等[16]采用溶胶-凝胶法制备Bi2Mn4O10, 并辅以球磨法掺杂科琴黑, 合成了Bi2Mn4O10/C负极材料, 在120 mAh/g的电流密度下经300次循环后容量保持率高达100%。聚丙烯酰胺凝胶法是在溶液成胶过程中, 丙烯酰胺聚合成高分子三维网络结构的凝胶, 经过干燥、烧结固化后可得到形貌均一、比表面积大、粒度小的纳米材料, 有助于改善材料的电化学性能[19,20]。基于此, 本研究拟以硝酸铋为铋源, 乙酸锰为锰源, 采用聚丙烯酰胺凝胶法制备Bi2Mn4O10负极材料, 重点考察丙烯酰胺与总金属离子摩尔比、葡萄糖浓度以及热处理温度对Bi2Mn4O10形貌、物相和电化学性能的影响。
1 实验方法
1.1 实验试剂
本实验所用试剂主要有五水硝酸铋(Bi(NO3)3·5H2O)、醋酸锰(Mn(CH3COO)2·4H2O)、浓硝酸、柠檬酸、葡萄糖、丙烯酰胺(C3H5NO)、N,N’-亚甲基双丙烯酰胺(C7H10N2O2)、氨水和去离子水, 所有试剂都是分析纯级别(Analytical grade, AR), 不经二次提纯直接使用。
1.2 材料制备
Bi2Mn4O10材料的制备: 首先配制浓度为3 mol/L的稀硝酸溶液, 然后根据Bi2Mn4O10的化学计量比, 按摩尔比Mn/Bi=2/1称取Bi(NO3)3·5H2O和Mn(CH3COO)2·4H2O。在搅拌中先将Bi(NO3)3·5H2O加入预配制的稀硝酸溶液中, 再加入Mn(CH3COO)2·4H2O, 控制金属离子的摩尔浓度为0.15 mol/L。待金属离子充分溶解后, 向溶液中加入与金属锂离子摩尔比为1.5 : 1的柠檬酸, 继续搅拌至充分溶解; 然后向溶液中滴加氨水, 并控制溶液pH在2~3范围内, 再加入一定量葡萄糖。充分溶解后, 继续向溶液中加入与金属离子为一定摩尔比例的丙烯酰胺, 随后加入N,N’-亚甲基双丙烯酰胺, 其比例为w(丙烯酰胺)/w(N,N’-亚甲基双丙烯酰胺)=5/1, 全部溶解后, 超声振荡15 min。超声振荡完毕后, 将其转移至358 K的水浴锅内持续搅拌, 形成凝胶。接着将胶体转移至393 K的干燥箱内干燥24 h之后, 将干凝胶取出并研细, 放在马弗炉中, 先在673 K下预烧结, 最后在特定温度下退火10 h得到Bi2Mn4O10颗粒。
Bi2Mn4O10/ECP-N复合材料的制备: 将科琴黑(纳米级)放入通有NH3气氛的管式炉内, 气体流速为150 mL/min, 并于1073 K下热处理10 h后得到掺有N元素的ECP-N复合材料。然后, 根据特定质量比将ECP-N复合材料以及制备的Bi2Mn4O10颗粒放入玛瑙球磨罐内球磨, 转速为400 r/min, 球磨8 h后得到科琴黑-N复合材料前驱体。再将前驱体置于573 K温度下热处理3 h, 得到Bi2Mn4O10/ECP-N复合材料。
1.3 材料表征
采用Rigaku-TTRIII(Cu Ka, λ=0.154056 nm)型X射线衍射仪(XRD)、扫描电镜(SEM, JSM-6360LV)和透射电镜(TEM, JEOL-2010)分析样品的物相组成和形貌结构; 采用型号为QU ADRASORB EVO的自动氮吸附比表面分析仪测试Bi2Mn4O10粉末的比表面积和孔径分布; 采用英国马尔文公司, 型号为JL-1177的激光粒度分析仪分析Bi2Mn4O10粉末的粒度分布。
1.4 电池组装与性能测试
将制备好的Bi2Mn4O10粉末、导电炭黑(乙炔黑)和粘结剂(聚偏氟乙烯, PVDF)按质量比7 : 2 : 1混合, 放入玛瑙研钵中研磨均匀后, 滴加适量的N-甲基吡咯烷酮调节浆料的粘稠度, 利用刮刀将浆料均匀地涂覆在2 μm厚的铜箔上, 放入60 ℃的鼓风干燥箱中干燥12 h, 再用冲片机冲成直径为12 mm的圆形极片。极片称重后置于60 ℃真空干燥箱中干燥6 h以上。以金属锂片为对电极, 1 mol/L LiPF6的碳酸乙烯酯+碳酸二甲酯+碳酸甲乙酯体积比(1 : 1 : 1)溶液为电解液, 在无水、充满高纯氩气的手套箱中组装CR2025扣式电池。采用电化学工作站(CHI, 760e)进行循环伏安(CV)测试。采用LAND电池测试系统进行恒电流充放电测试, 充放电电压范围为0.05~3.00 V。
2 结果与讨论
2.1 不同制备条件对Bi2Mn4O10的形貌、物相及电化学性能的影响
2.1.1 丙烯酰胺与总金属离子摩尔比的影响
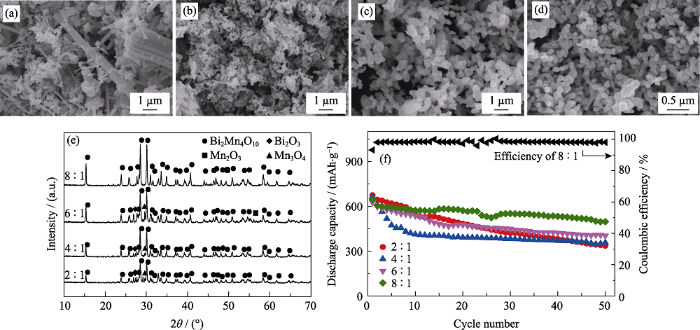
固定葡萄糖浓度为1.11 mol/L, 热处理温度为873 K, 丙烯酰胺与N,N’-亚甲基双丙烯酰胺的质量比为5 : 1, 考察不同丙烯酰胺与总金属离子摩尔比对Bi2Mn4O10形貌的影响, SEM照片如图1(a~d)所示。由图可知, 当丙烯酰胺与总金属离子摩尔比等于2 : 1时, 所得产物为棒状、粒状, 无规则; 当丙烯酰胺与总金属离子摩尔比为4 : 1时, 产物呈现类球形粒子的团聚体, 形貌基本一致; 当丙烯酰胺与总金属离子的摩尔比为6 : 1~8 : 1时, 所得到的产物呈类球形, 分散性好。这是因为不断加入丙烯酰胺与N,N’-亚甲基双丙烯酰胺可形成3D网络结构, 此时溶液中的金属盐良好地分散在由这些密集网 络结构所形成的狭小空间内, 形成了众多的微反应器[21]。溶液中金属离子与柠檬酸形成的金属络合物分散在这些微反应器中, 显著降低了热处理过程中分解产物的团聚程度, 从而得到粒度均一、分散性良好的Bi2Mn4O10颗粒[19,20,21]。此外, 材料颗粒尺寸越小、粒度越均匀、分散性越好, 意味着Li+在该电极材料内部的扩散路径越短, 有更多的活性材料参与到电化学反应过程中, 储锂能力也得到大幅度提升[22]。
图1
图1
不同丙烯酰胺与总金属离子摩尔比下所得产物的SEM照片((a) 2 : 1, (b) 4 : 1, (c) 6 : 1, (d) 8 : 1), (e) XRD图谱, (f)作为负极材料在0.2C时的比容量循环性能以及摩尔比为8 : 1时产品的库伦效率曲线(0.1C活化3圈, 1C=800 mA/g)
Fig. 1
SEM images ((a) 2 : 1, (b) 4 : 1, (c) 6 : 1, (d)8 : 1), (e) XRD patterns and (f) cycling performance at 0.2C of the products obtained with different molar ratios of acrylamide to total metal ions, and Coulombic efficiency of the product with molar ratio of acrylamide to total metal ions of 8 : 1 (after 3 cycles at 0.1C, 1C=800 mA/g)
Glucose concentrations: 1.11 mol/L, heat treatment temperature: 873 K, weight ratio of acrylamide to N,N’-methylene bisacrylamide: 5 : 1
固定其他条件, 改变丙烯酰胺与总金属离子的摩尔比, 考察所得到的Bi2Mn4O10负极材料在0.2C时的比容量循环性能, 结果如图1(f)所示。由图可知, 当丙烯酰胺与金属离子摩尔比分别为2 : 1、4 : 1、6 : 1、8 : 1时, 所得到的负极材料在循环50圈后, 电池保持的比容量分别为335.8、356.7、403.4以及496.8 mAh/g, 表明纯相Bi2Mn4O10负极材料具有比较好的电化学性能。此外, 用该条件下得到的纯相Bi2Mn4O10负极材料组装Bi2Mn4O10/Li+扣式锂离子电池, 其第一圈库伦效率即可达到93.21%, 第二圈及之后库伦效率上升到98%以上, 这是因为高的丙烯酰胺与总金属离子浓度比(丙烯酰胺与金属离子摩尔比为8 : 1且葡萄糖浓度为1.11 mol/L时, 溶胶已经饱和, 继续加入丙烯酰胺不会再溶解)可以形成密集的网络结构, 有效减少了热处理过程中粉末的团聚, 有利于获得分散性好的Bi2Mn4O10负极材料, 增加材料与电解液接触机会, 从而提高电化学反应速率和库伦效率[19,20]。此外, 良好的分散性也有利于缓解Bi2Mn4O10脱嵌锂过程中的体积膨胀, 提高Bi2Mn4O10/Li+扣式锂离子电池的循环容量[23]。
2.1.2 葡萄糖浓度的影响
图2
图2
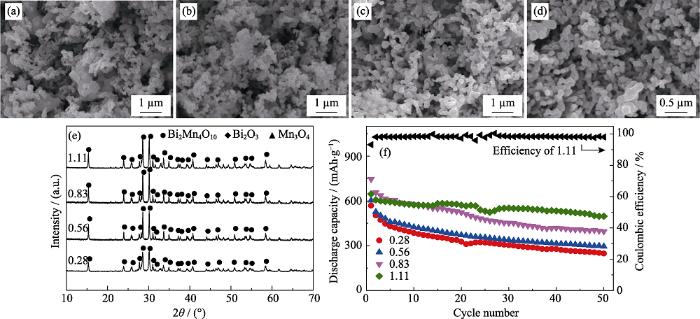
不同葡萄糖浓度下所得产物的SEM照片((a) 0.28 mol/L, (b) 0.56 mol/L, (c)0.83 mol/L, (d) 1.11 mol/L), (e) XRD图谱, (f) 作为负极材料在0.2C时的比容量循环性能以及葡萄糖浓度为1.11 mol/L时产品的库伦效率曲线(0.1C活化3圈, 1C=800 mA/g)
Fig. 2
SEM images ((a) 0.28 mol/L, (b) 0.56 mol/L, (c) 0.83 mol/L, (d) 1.11 mol/L), (e) XRD patterns and (f) cycling performance at 0.2C of the products obtained with different glucose concentrations, and Coulombic efficiency of the product with 1.11 mol/L glucose (after three cycles at 0.1C, 1C=800 mA/g)
Molar ratio of acrylamide to total metal ions: 8 : 1; heat treatment temperature: 873 K; weight ratio of acrylamide to N,N’-methylene bisacrylamide of 5 : 1
图2(f)为固定其他条件, 改变葡萄糖浓度, 所得到的Bi2Mn4O10负极材料在0.2C时的比容量循环性能。由图可知, 随葡萄糖浓度增加, 所得Bi2Mn4O10负极材料循环50圈后的比容量明显增大, 葡萄糖浓度为0.28 mol/L时比容量仅为247.5 mAh/g。当葡萄糖浓度为1.11 mol/L时(此时葡萄糖在溶胶中已经饱和)达到最高(496.8 mAh/g), 这可能是由于增加葡萄糖浓度, 在热处理过程中葡萄糖碳化容易形成大量的碳骨架, 这样热分解时凝胶的坍塌变少, 热分解产物颗粒之间黏连程度降低, 导致Bi2Mn4O10颗粒分散性增强, 使材料与电解液的接触更充分, 锂离子迁移速率加快, 储锂性能更佳。
2.1.3 热处理温度的影响
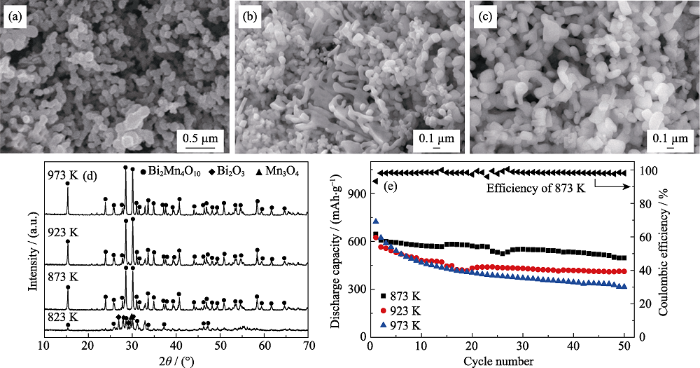
固定丙烯酰胺与总金属离子的摩尔比为8 : 1, 葡萄糖浓度为1.11 mol/L, 丙烯酰胺与N,N’-亚甲基双丙烯酰胺的质量比为5 : 1的条件下, 热处理温度对Bi2Mn4O10负极材料形貌的影响, SEM照片如 图3(a~c)所示。由图可知, 当热处理温度873 K时, 其颗粒分布较为均匀、团聚少; 继续升高热处理温度至923和973 K后, Bi2Mn4O10的纳米颗粒逐渐长大, 颗粒间相互粘连、团聚严重, 分散性变差。这是因为当热处理温度高于873 K时已形成的Bi2Mn4O10颗粒再次长大, 导致颗粒团聚在一起。
图3
图3
不同热处理温度下所得产物SEM照片((a) 873 K, (b) 923 K, (c) 973 K), (d) XRD图谱, (e)作为负极材料在0.2C时的比容量循环性能以及873 K时产品的库伦效率曲线(0.1C活化3圈, 1C=800 mA/g)
Fig. 3
SEM images ((a) 873 K, (b) 923 K, (c) 973 K), (d) XRD patterns and (e) cycling performance at 0.2C of the products obtained with different heat treatment temperatures, and Coulombic efficiency of the product with heat-treatment temperature of 873 K (after three cycles at 0.1C, 1C=800 mA/g)
Molar ratio of acrylamide to total metal ions: 8 : 1, glucose concentrations of 1.11 mol/L, weight ratio of acrylamide to N,N’-methylene bisacrylamide: 5 : 1
图3(d)为不同热处理温度下所得产物对Bi2Mn4O10的物相的影响。由图可知, 当热处理温度为823 K时, 产物为Bi2Mn4O10、Bi2O3和Mn3O4的混合物; 随着热处理温度的升高, 其它杂相峰随之消失, 当热处理温度超过873 K时, 所得产物的XRD图谱中的衍射峰与Bi2Mn4O10的标准卡片JCPDS#27-0048基本一致, 说明此时所得Bi2Mn4O10的物相较纯。当热处理温度继续升高时, Bi2Mn4O10特征峰随之增强, 所得产物的结晶度也更好。结合图3(a~d)可知, 高的热处理温度有利于获得单一物相的Bi2Mn4O10粉末, 但热处理温度太高则导致Bi2Mn4O10粉末团聚加剧。
2.2 优化工艺条件下所得Bi2Mn4O10的表征及电化学性能
图4
图4
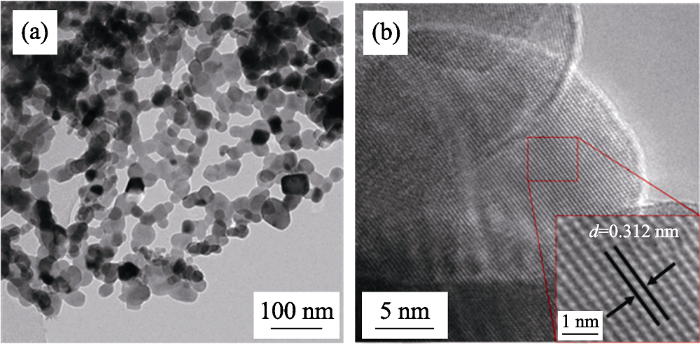
在优化工艺条件下制备的Bi2Mn4O10的(a)TEM和(b) HRTEM照片
Fig. 4
(a) TEM and (b) HRTEM images of Bi2Mn4O10 obtained at optimized conditions
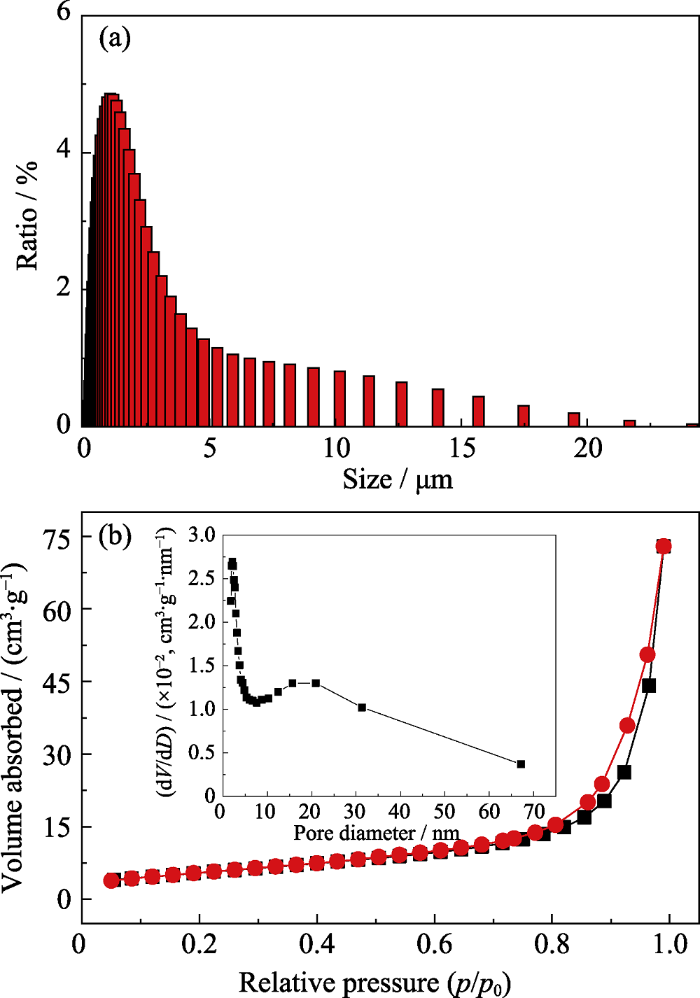
图5为优化工艺条件下制备的Bi2Mn4O10颗粒粒度分布柱状图, N2吸脱附曲线以及孔径分布曲线。由图5(a)粒度分布柱状图可知, Bi2Mn4O10颗粒的粒度分布均匀, 颗粒的D50为1.128 μm。由图5(b) N2吸脱附曲线以及孔径分布曲线可知, N2吸脱附曲线为典型的IV型滞留环, 表明制备的Bi2Mn4O10颗粒呈现明显的介孔结构, 平均孔径为22.22 nm。BET测试结果表明该材料的比表面积为20.3096 m2/g, 总孔体积为0.1128 cm3/g。大的比表面积和多孔结构不仅能促进电解液的渗透, 提供足够的表面/界面, 促进电荷转移和缩短离子扩散的路径长度, 而且有利于控制活性物质易团聚的特性, 缓解连续循环期间Bi2Mn4O10的体积膨胀, 提高Bi2Mn4O10负极材料的循环性能[25]。
图5
图5
在优化工艺条件下制备的Bi2Mn4O10的(a)粒度分布柱状图和(b)氮气吸脱附曲线以及孔径分布图
Fig. 5
(a) Particle size distribution and (b) N2 adsorption and desorption curves of Bi2Mn4O10 obtained at optimized conditions with inset in (b) showing the pore size distribution curve
图6
图6
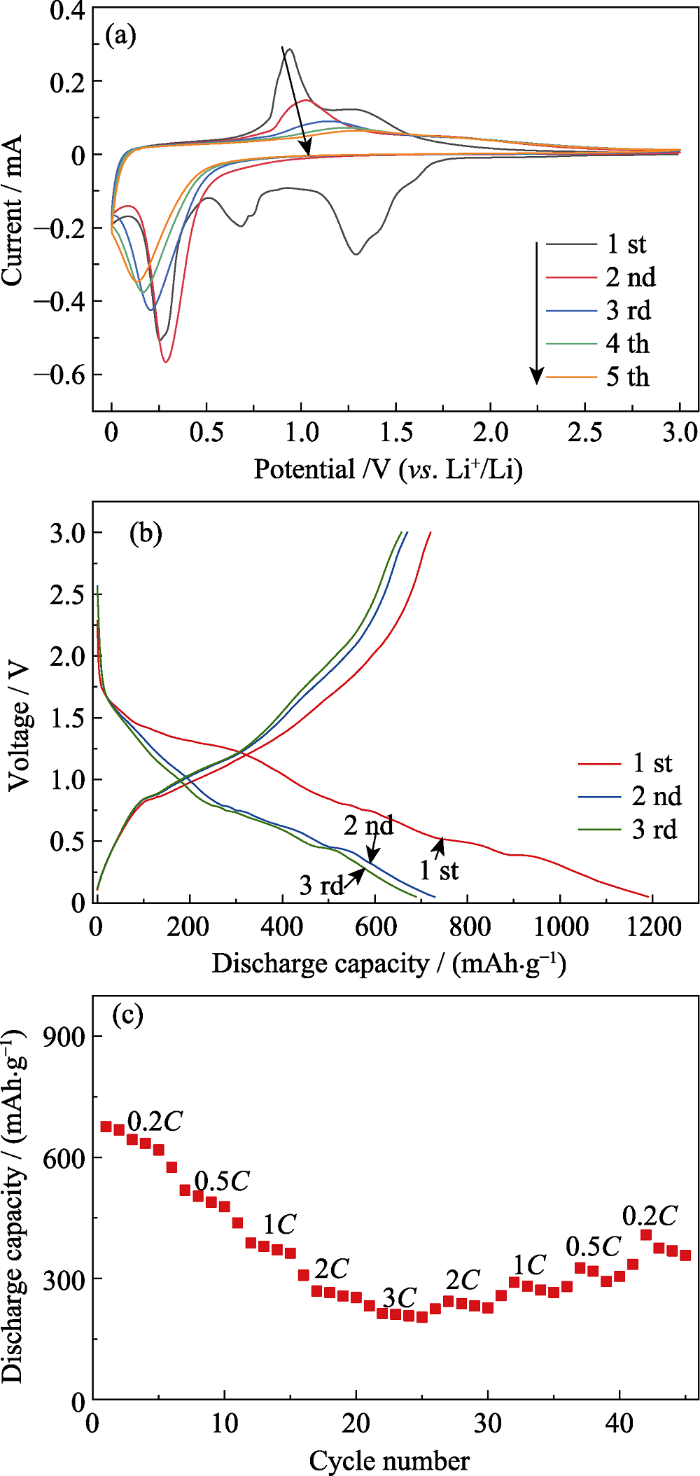
优化工艺条件下制备的Bi2Mn4O10的(a)循环伏安曲线, (b)在0.1C时的电压-比容量曲线和(c)倍率性能图
Fig. 6
(a) CV curves, (b) voltage-specific capacity curves at 0.1C and (c) rate performance curve of Bi2Mn4O10 obtained at optimized conditions
图6(b~c)为优化工艺条件下制备的Bi2Mn4O10负极材料在0.1C时的电压-比容量曲线和倍率性能图。由图6(b)可知, 该负极材料首圈充电比容量与放电比容量分别为720.5和1190.4 mAh/g, 这是由于首圈放电过程形成固体电解质界面(SEI)膜, 导致Bi2Mn4O10负极材料首圈放电比容量超过其理论比容量(873 mAh/g)[25]。此外, 由图可知, Bi2Mn4O10负极材料首圈库仑效率为60.52%, 但在随后的第二圈和第三圈充放电过程中其库仑效率升高到91.9%和95.41%。由图6(c)可知, 在充放电倍率为0.2C、0.5C、1C、2C和3C时, 该Bi2Mn4O10负极材料的比容量分别为675.4、575.3、437.5、308和232 mAh/g, 表现出良好的倍率性能。
3 结论
1)在丙烯酰胺含量与总金属离子摩尔比为8 : 1, 葡萄糖浓度为1.11 mol/L, 热处理温度为873 K条件下, 可获得高纯度、类球型、分散性良好的Bi2Mn4O10粉末, 该Bi2Mn4O10粉末的粒度分布D50=1.128 μm, 比表面积为20.31 m2/g, 平均孔径为22.22 nm。
2)最优工艺条件下所制备的Bi2Mn4O10负极材料在0.2C倍率下循环50圈后的比容量达496.8 mAh/g。在0.1C下首圈库仑效率为60.52%, 第二圈和第三圈充放电过程中Bi2Mn4O10的库仑效率可以升高到91.9%和95.41%。在3C倍率下仍可以获得232 mAh/g的比容量。
参考文献
The Li-ion rechargeable battery: a perspective
Each cell of a battery stores electrical energy as chemical energy in two electrodes, a reductant (anode) and an oxidant (cathode), separated by an electrolyte that transfers the ionic component of the chemical reaction inside the cell and forces the electronic component outside the battery. The output on discharge is an external electronic current I at a voltage V for a time Deltat. The chemical reaction of a rechargeable battery must be reversible on the application of a charging I and V. Critical parameters of a rechargeable battery are safety, density of energy that can be stored at a specific power input and retrieved at a specific power output, cycle and shelf life, storage efficiency, and cost of fabrication. Conventional ambient-temperature rechargeable batteries have solid electrodes and a liquid electrolyte. The positive electrode (cathode) consists of a host framework into which the mobile (working) cation is inserted reversibly over a finite solid-solution range. The solid-solution range, which is reduced at higher current by the rate of transfer of the working ion across electrode/electrolyte interfaces and within a host, limits the amount of charge per electrode formula unit that can be transferred over the time Deltat = Deltat(I). Moreover, the difference between energies of the LUMO and the HOMO of the electrolyte, i.e., electrolyte window, determines the maximum voltage for a long shelf and cycle life. The maximum stable voltage with an aqueous electrolyte is 1.5 V; the Li-ion rechargeable battery uses an organic electrolyte with a larger window, which increase the density of stored energy for a given Deltat. Anode or cathode electrochemical potentials outside the electrolyte window can increase V, but they require formation of a passivating surface layer that must be permeable to Li(+) and capable of adapting rapidly to the changing electrode surface area as the electrode changes volume during cycling. A passivating surface layer adds to the impedance of the Li(+) transfer across the electrode/electrolyte interface and lowers the cycle life of a battery cell. Moreover, formation of a passivation layer on the anode robs Li from the cathode irreversibly on an initial charge, further lowering the reversible Deltat. These problems plus the cost of quality control of manufacturing plague development of Li-ion rechargeable batteries that can compete with the internal combustion engine for powering electric cars and that can provide the needed low-cost storage of electrical energy generated by renewable wind and/or solar energy. Chemists are contributing to incremental improvements of the conventional strategy by investigating and controlling electrode passivation layers, improving the rate of Li(+) transfer across electrode/electrolyte interfaces, identifying electrolytes with larger windows while retaining a Li(+) conductivity sigma(Li) > 10(-3) S cm(-1), synthesizing electrode morphologies that reduce the size of the active particles while pinning them on current collectors of large surface area accessible by the electrolyte, lowering the cost of cell fabrication, designing displacement-reaction anodes of higher capacity that allow a safe, fast charge, and designing alternative cathode hosts. However, new strategies are needed for batteries that go beyond powering hand-held devices, such as using electrode hosts with two-electron redox centers; replacing the cathode hosts by materials that undergo displacement reactions (e.g. sulfur) by liquid cathodes that may contain flow-through redox molecules, or by catalysts for air cathodes; and developing a Li(+) solid electrolyte separator membrane that allows an organic and aqueous liquid electrolyte on the anode and cathode sides, respectively. Opportunities exist for the chemist to bring together oxide and polymer or graphene chemistry in imaginative morphologies.
A fiber-shaped solar cell showing a record power conversion efficiency of 10%
Noble-metal-free heterostructure for efficient hydrogen evolution in visible region: molybdenum nitride/ultrathin graphitic carbon nitride
Recent advances in Mn-based oxides as anode materials for lithium ion batteries
The development of new electrode materials for lithium-ion batteries (LIBs) is of great interest because available electrode materials may not meet the high-energy demands for electronic devices, especially the demands for good cyclic and rate performance. Mn-based oxides have received substantial attention as promising anode materials for LIBs due to their high theoretical specific capacities, low charge potential vs. Li/Li+, environmental benignity and natural abundance. Herein, the preparation of Mn-based oxide nanomaterials with various nanostructures and chemical compositions along with their applications as negative electrodes for LIBs are reviewed. The review covers MnO, Mn3O4, Mn2O3, MnO2, CoMn2O4, ZnMn2O4 and their carbonaceous composite/oxide supports with different morphologies and compositions. The aim of this review is to provide an in-depth and rational understanding of the relationships among the chemical compositions, morphologies and electrochemical properties of Mn-based anode materials and, to understand how electrochemical performance can be improved using materials engineering strategies. Special attention has been paid to the discussion of challenges in the practical applications of Mn-based oxides in LIB full cells.
Challenges in the development of advanced Li-ion batteries: a review
Hierarchical porous ZnMn2O4 hollow nanotubes with enhanced lithium storage toward lithium-ion batteries
Construction of a novel ZnCo2O4/Bi2O3 heterojunction photocatalyst with enhanced visible light photocatalytic activity
Exploration and crystal phase engineering from bismuthinite ore to visible-light responsive photocatalyst of Bi2O3
Novel synthesis method of sheet-like agglomerates beta-Bi2O3 with high photocatalytic activity
Sheet-like agglomerates β-Bi2O3 were synthesized via an extraction-precipitation stripping-decomposition method using a leaching solution of bismuthinite as raw materials in acidic chloride media. Phase form of as-prepared Bi2O3 was confirmed by X-ray diffraction (XRD) and its morphology was observed by scanning electron microscope (SEM) and transmission electron microscope (TEM). Moreover, photocatalytic activity of the β-Bi2O3 was evaluated by measuring the degradation of Rhodamine B (RhB) under visible light irradiation. The results showed that the powders consisted of nanoflakes. The β-Bi2O3 exhibited a smaller band gap energy and larger absorbance edge than α-Bi2O3 and reported β-Bi2O3. In addition, 99.23% of the RhB was degraded under 4 h visible light irradiation and β-Bi2O3 was a fairly stable photocatalyst under the experimental conditions.
Nanostructured conversion-type anode materials for advanced lithium-ion batteries
Metal oxides and oxysalts as anode materials for Li ion batteries
Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions
Despite the imminent commercial introduction of Li-ion batteries in electric drive vehicles and their proposed use as enablers of smart grids based on renewable energy technologies, an intensive quest for new electrode materials that bring about improvements in energy density, cycle life, cost, and safety is still underway. This Progress Report highlights the recent developments and the future prospects of the use of phases that react through conversion reactions as both positive and negative electrode materials in Li-ion batteries. By moving beyond classical intercalation reactions, a variety of low cost compounds with gravimetric specific capacities that are two-to-five times larger than those attained with currently used materials, such as graphite and LiCoO(2), can be achieved. Nonetheless, several factors currently handicap the applicability of electrode materials entailing conversion reactions. These factors, together with the scientific breakthroughs that are necessary to fully assess the practicality of this concept, are reviewed in this report.
Double-shelled CoMn2O4 hollow microcubes as high-capacity anodes for lithium-ion batteries
Formation of ZnMn2O4 ball-in-ball hollow microspheres as a high-performance anode for lithium-ion batteries
Novel ZnMn(2)O(4) ball-in-ball hollow microspheres are fabricated by a facile two-step method involving the solution synthesis of ZnMn-glycolate hollow microspheres and subsequent thermal annealing in air. When evaluated as an anode material for lithium-ion batteries, these ZnMn(2)O(4) ball-in-ball hollow microspheres show significantly enhanced electrochemical performance with high capacity, excellent cycling stability and good rate capability.
High electrochemical performance of monodisperse NiCo2O4 mesoporous microspheres as an anode material for Li-ion batteries
Binary metal oxides have been regarded as ideal and potential anode materials, which can ameliorate and offset the electrochemical performance of the single metal oxides, such as reversible capacity, structural stability and electronic conductivity. In this work, monodisperse NiCo(2)O(4) mesoporous microspheres are fabricated by a facile solvothermal method followed by pyrolysis of the Ni(0.33)Co(0.67)CO(3) precursor. The Brunauer-Emmett-Teller (BET) surface area of NiCo(2)O(4) mesoporous microspheres is determined to be about 40.58 m(2) g(-1) with dominant pore diameter of 14.5 nm and narrow size distribution of 10-20 nm. Our as-prepared NiCo(2)O(4) products were evaluated as the anode material for the lithium-ion-battery (LIB) application. It is demonstrated that the special structural features of the NiCo(2)O(4) microspheres including uniformity of the surface texture, the integrity and porosity exert significant effect on the electrochemical performances. The discharge capacity of NiCo(2)O(4) microspheres could reach 1198 mA h g(-1) after 30 discharge-charge cycles at a current density of 200 mA g(-1). More importantly, when the current density increased to 800 mA.g(-1), it can render reversible capacity of 705 mA h g(-1) even after 500 cycles, indicating its potential applications for next-generation high power lithium ion batteries (LIBs). The superior battery performance is mainly attributed to the unique micro/nanostructure composed of interconnected NiCo(2)O(4) nanocrystals, which provides good electrolyte diffusion and large electrode-electrolyte contact area, and meanwhile reduces volume change during charge/discharge process. The strategy is simple but very effective, and because of its versatility, it could be extended to other high-capacity metal oxide anode materials for LIBs.
Bi2Mn4O10: a new mullite-type anode material for lithium-ion batteries
The low specific capacity of graphite limits the further increase of the energy density of lithium-ion batteries and their widespread applications. Exploring new anode materials is the key issue. Herein, a new mullite-type compound Bi2Mn4O10 is designed and synthesized. The Bi2Mn4O10/C composite delivers a high reversible specific capacity of 846 mA h g-1 (more than twice that of graphite), and exhibits a high capacity retention of 100% after 300 cycles at 600 mA g-1, which is reported for the first time. The high specific capacity originates from the combination of the conversion reaction and alloying-dealloying reaction, which has been confirmed by the ex situ XRD, IR, SEM and TEM studies. In addition, the unique nanocomposite generated during the charge-discharge process provides excellent cycling stability. This work proves that Bi2Mn4O10/C is a potential anode material for advanced lithium-ion batteries.
Synthesis of Bi2Mn4O10 nanoparticles and its anode properties for LIB
Preparation and characterization of ultrafine Bi2Mn4O10 powders
Preparation of LaxSr1-xMO3 nanopowders by polyacrylamide Sol-Gel method
Synthesis of BiFeO3 nanoparticles by a polyacrylamide gel route
A new polyacrylamide gel route was used to prepare BiFeO3 nanoparticles. In this route, the solution containing the required cations was gelled by using acrylamide; during the gelation process, acrylamide was polymerized to form a polymer network, which provided a structural framework for the growth of particles. It is demonstrated that highpurity BiFeO3 powders can be prepared using ethylenediaminetetraacetic acid (EDTA) as the chelating agent. On the other hand, it is found that adding an appropriate amount of glucose to the precursor solution can effectively suppress the gel shrinkage during drying, and consequently lead to a significant improvement of powder quality. Scanning electron microscope (SEM) observation reveals that the asprepared BiFeO3 powder has a uniform particle size, and the particles are almost spherical without any agglomeration or adhesion. Differential scanning calorimetry (DSC) analysis reveals that the product has an antiferromagnetic phase transition at about 370℃ and a ferroelectric phase transition at about 830℃. Ferroelectric and magnetic hysteresis loop measurements show that the product exhibits clear ferroelectric property and weak ferromagnetism.
Synthesis and properties of intermediate-temperature solid electrolyte La0.9Sr0.1Ga0.8Mg0.2O3-δ from polyacrylamidesol-gelprecursor
Multilayered Si nanoparticle/ reduced graphene oxide hybrid as a high-performance lithium-ion battery anode
Multilayered Si/RGO anode nanostructures, featuring alternating Si nanoparticle (NP) and RGO layers, good mechanical stability, and high electrical conductivity, allow Si NPs to easily expand between RGO layers, thereby leading to high reversible capacity up to 2300 mAh g(-1) at 0.05 C (120 mA g(-1)) and 87% capacity retention (up to 630 mAh g(-1)) at 10 C after 152 cycles.
Well-dispersed bi-component-active CoO/CoFe2O4 nanocomposites with tunable performances as anode materials for lithium-ion batteries
CoO/CoFe2O4 nanocomposites, derived from scalably prepared CoFe-layered double hydroxide (CoFe-LDH) single-resource precursors, exhibit tunable cycle performances and rate capabilities, which are supported by the homogenous dispersion of bi-component active CoO and CoFe2O4 phases.
Effect of glucose on the perflormance of Li1.2Ni0.13Co0.13Mn0.54O2 synthesized by Sol-Gel method
The cathode material Li1.2Ni0.13Co0.13Mn0.54O2 was synthesized by sol-gel method modified by glucose as carbon source. The structure, morphology and electrochemical performances of the as-prepared sample was studied by methods of XRD, SEM, EDS, BET, Laser Particle Size Analysis, cyclic voltammetry, galvanostatic charge-discharge and AC impedance. Test results showed that the distribution of particles became uniform and the sizes became smaller for the modification by glucose. The D50 decreased from 11.56 to 9.94 μm. The specific surface area nearly doubled. The initial discharge specific capacity at 0.2C reached 183.4 mAh·g-1 and 211.6 mAh·g-1 after been activated by 0.05C for blank and compared groups, respectively. The capacity at 2C retained 62.2% and 77.6% of that at 0.2C for the two samples, respectively. After 50 cycles at 1C, the discharge specific capacities retained 133.3 mAh·g-1 and 173.6 mAh·g-1, and the capacity retention rates were 95.1% and 100% for the two samples, respectively. The initial irreversible capacity loss was reduced for the modification by glucose. The rate performance and cycle stability were obviously improved. The impedance of charge transfer and Warburg, and dispersion effect of the electric double layer were decreased. The crystal structure of the sample stayed unchanged.
Enhanced electrochemical properties of graphene-wrapped ZnMn2O4 nanorods for lithium-ion batteries
Thermally reduced graphene oxide (rGO)-wrapped ZnMn2O4 nanorods have been successfully fabricated via a facile bottom-up approach. Characterization results show that porous ZnMn2O4 nanorods are uniformly wrapped by ultrathin rGO sheets. The unique structure of this rGO-ZnMn2O4 composite could facilitate both ion and electron diffusion, thus providing suitable characteristics of an anode material for high performance lithium-ion batteries. Specifically, the conductive rGO sheets could act as an efficient buffer to relax the volume changes from Li+ insertion/extraction, and enable the structural and interfacial stabilization of ZnMn2O4 crystals. As a consequence, a high and stable reversible capacity (707 mA h g(-1) at 100 mA g(-1) over 50 cycles) and an excellent rate capability (440 mA h g(-1) at 2000 mA g(-1)) are achieved with this composite material.
Bismuth oxide: a new lithium-ion battery anode
Bismuth oxide directly grown on nickel foam (p-Bi2O3/Ni) was prepared by a facile polymer-assisted solution approach and was used directly as a lithium-ion battery anode for the first time. The Bi2O3 particles were covered with thin carbon layers, forming network-like sheets on the surface of the Ni foam. The binder-free p-Bi2O3/Ni shows superior electrochemical properties with a capacity of 668 mA h g(-1) at a current density of 800 mA g(-1), which is much higher than that of commercial Bi2O3 powder (c-Bi2O3) and Bi2O3 powder prepared by the polymer-assisted solution method (p-Bi2O3). The good performance of p-Bi2O3/Ni can be attributed to higher volumetric utilization efficiency, better connection of active materials to the current collector, and shorter lithium ion diffusion path.
Enhanced electrochemical performances of Bi2O3/rGO nanocomposite via chemical bonding as anode materials for lithium ion batteries
Bismuth oxide/reduced graphene oxide (termed Bi2O3@rGO) nanocomposite has been facilely prepared by a solvothermal method via introducing chemical bonding that has been demonstrated by Raman and X-ray photoelectron spectroscopy spectra. Tremendous single-crystal Bi2O3 nanoparticles with an average size of approximately 5 nm are anchored and uniformly dispersed on rGO sheets. Such a nanostructure results in enhanced electrochemical reversibility and cycling stability of Bi2O3@rGO composite materials as anodes for lithium ion batteries in comparison with agglomerated bare Bi2O3 nanoparticles. The Bi2O3@rGO anode material can deliver a high initial capacity of approximately 900 mAh/g at 0.1C and shows excellent rate capability of approximately 270 mAh/g at 10C rates (1C = 600 mA/g). After 100 electrochemical cycles at 1C, the Bi2O3@rGO anode material retains a capacity of 347.3 mAh/g with corresponding capacity retention of 79%, which is significantly better than that of bare Bi2O3 material. The lithium ion diffusion coefficient during lithiation-delithiation of Bi2O3@rGO nanocomposite has been evaluated to be around approximately 10(-15)-10(-16) cm(2)/S. This work demonstrates the effects of chemical bonding between Bi2O3 nanoparticles and rGO substrate on enhanced electrochemical performances of Bi2O3@rGO nanocomposite, which can be used as a promising anode alterative for superior lithium ion batteries.
Self-assembled lamellar alpha-molybdenum trioxide as high performing anode material for lithium-ion batteries
Graphene-wrapped MnO2-graphene nanoribbons as anode materials for high-performance lithium ion batteries
A facile and cost-effective approach for the fabrication of a hierarchical nanocomposite material of graphene-wrapped MnO2 -graphene nanoribbons (GMG) is developed. The resulting composite has a high specific capacity and an excellent cycling stability owing to the synergistic combination of the electrically conductive graphene, graphene nanoribbons, and MnO2 .