Hydroxyapatite (HA) has been widely used as a bioceramic with good biological activity. HA plays an important role in dental and bone regeneration due to its superior biocompatibility and excellent ability to bond bone tissue [1]. However, the crystallinity, morphology, and other physical properties are different between biological apatites and pure HA[2]. In order to modify the solubility and degradation of HA, as well as to add some additional biological and antibacterial properties, many techniques have been employed[3,4]. Recently, introducing extrinsic ions, such as Zn2+[5], Ba2+[6], F-[7,8,9,10], into an apatite structure is the most practicable and effective method to solve these problems.
Sr is proved to be one of the most constituent elements in bones. It has been extensively utilized to endow HA with better performances, such as higher biocompatibility and bioactivity, due to its excellent biocompatibility[11,12]. In vivo and in vitro studies have demonstrated that Sr doping can significantly accelerate differentiation of osteoblast cells and inhibit proliferation of osteoclast cells[13,14]. The stoichiometric component of HA is Ca10(PO4)6(OH)2 and the unit cell of it belongs to P63/m space group which has a C3 symmetry[15]. The Sr ions HA hexagonal structure can be doped into two types of Ca sites [Ca(1) and Ca(2)][16]. Four of the ten Ca sites, named as Ca(1), form channels parallel to the c-axis. Remaining six of the ten Ca sites, denoted as Ca(2), are located as two symmetrical triangles around hydroxyl channels[17,18]. Previous studies found that Sr ions could enter these two types of Ca sites without solubility limitation[19]. It is worth noting that Sr ions shows different site preference during the process of doping. Terra et al.[20] studied the site preference of Sr doping by Rietveld refinement of X-ray diffraction patterns when the doping molar concentrations of Sr are below 1%. Their results showed that the Ca(1) sites are the preferential site for Sr doping. Zeglinski et al.[17] utilized the density functional theory (DFT) method to study the most preferential mode and revealed that the mixed substitution occurred at Ca(1) and Ca(2) sites randomly and Ca(2) sites become the preferential sites with the increase of substitution concentration of Sr ions. Bigi et al.[19] reported a preference for the Ca(2) substitution at or above 10at% of Sr, while Ca(1) was preferred at 5at% of Sr. So far, there is still a controversy regarding the preferred doping sites of Sr ions in the HA crystal structure.
Although there have been many theoretical and experimental studies on Sr doping into HA structure, few studies have combined theory with experimental results. In this study, a hydrothermal method was used to synthesize the pure HA and Sr doped (10at%, 50at%, 100at%) HA nanoparticles. Scanning electron microscopy (SEM), X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), and selected-area electron diffraction (SAED) were employed to provide the morphology, crystallinity, and microstructure of the samples. Ab initio method which is based on quantum mechanics provided the lattice parameters and formation energy according to Sr doping concentrations. The preference sites of Sr ions doping for Ca at 10at% and 50at% concentration were also discussed.
1 Materials and methods
1.1 Preparation of HA and Sr-HA
HA (Ca10(PO4)6(OH)2) and Sr-doped HA (Ca10-xSrx(PO4)6(OH)2, x=0-10) nanoparticles with Sr concentrations at 10at%, 50at%, 100at% (x=1, 5, 10) were prepared by a hydrothermal method. Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O), strontium nitrate (Sr(NO3)2), and diammonium hydrogen phosphate ((NH4)2HPO4) were utilized as Ca, Sr, and P sources, respectively. Preparation procedure of 2 g of 50at% Sr-doped HA was as follows: 1.9 g of Ca(NO3)2·4H2O, 1.703 g of Sr(NO3)2, and 1.275 g of (NH4)2HPO4 were dissolved into 60 mL deionized water. Then, 25% ammonia solution was used to adjust the pH between 10 and 10.5. After stirring the mixture for 5 min, it was injected into a hydrothermal reactor and aged at 150 ℃ for 6 h. The precipitates obtained by centrifugation were cleaned three times with distilled water and anhydrous ethanol. Finally, the products were dried at 70 ℃ for 12 h.
1.2 Characterization of the samples
Lattice parameters (a and c) were quantified from peaks of (002) and (211) in an XRD pattern, respectively, using the standard hexagonal close packed unit cell plane spacing relationship[21]:
where d is the distance between two adjacent planes in the set of Miller indices (hkl).
The degree of crystallinity[22], corresponding to the fraction of crystalline phase present in the examined volume, was evaluated as:
where I300 is the intensity of (300) reflection and V112/300 is the intensity of the hollow between (112) and (300) reflections, which completely disappears in non-crystalline samples.
Composition of individual apatite crystals was examined by X-ray energy dispersive spectrometer (EDS) equipped on SEM. The atomic concentrations of elements (Ca, P, and Sr) in the nanoparticles were further determined by an inductive coupled plasma emission spectrometer (ICP; VISTA-MAX) method.
Surface morphology of the synthesized HA nanoparticles were observed by SEM (HITACHI S-4800, Tokyo, Japan) at an accelerating voltage of 5 kV. The phase analysis was carried out using X-ray powder diffraction instrument (RIGAKU/DMAX). The patterns were recorded using CuKα radiation in the 2θ range of 10-90° at the step size of 0.02° with the scan speed of 8 (°)/min.
The microstructures of nanoparticles were examined by TEM for comparing with XRD results. TEM images were taken using a JEM-2100F microscope at 200 kV. HRTEM images and SAED patterns were also obtained.
1.3 Computational method
The calculations were carried out using the CASTEP module in the Materials Studio software package. The CASTEP code is an ab initio program which can calculate ground state energies of atomic systems accurately based on DFT. The two types of Ca sites [Ca(1) or Ca(2)], with four Ca(1) sites surrounded by nine oxygen atoms and six Ca(2) sites positioning around the OH groups, can be substituted by other doping cations. Based on the unit cell of HA, eight possible Sr-doped HA models were built.
1Sr-Ca(1)-HA model: One Ca(1) was replaced by one Sr atom in pure HA.
4Sr-Ca(1)-1Sr-Ca(2)-HA model: Four Ca(1) were replaced by four Sr atoms and one Ca(2) was replaced by one Sr atom in pure HA.
Similarly, 1Sr-Ca(2)-HA, 3Sr-Ca(1)-2Sr-Ca(2)-HA, 2Sr-Ca(1)-3Sr-Ca(2)-HA, 1Sr-Ca(1)-4Sr-Ca(2)-HA, 5Sr-Ca(2)-HA and 10Sr-HA models were built. The stable models after geometric optimization (pure HA, 1Sr-Ca(1)-HA, 2Sr-Ca(1)-3Sr-Ca(2)-HA and 10Sr-HA) are optimized.
All calculations of structure optimization were conducted by the generalized gradient approximation (GGA) of the Perdew-Burke-Ernzerhof (PBE) exchange-correlation which was based on an energy minimization principle. The electronic ground state was achieved using a conjugate. The Broyden-Fletcher-Goldfarb-Shanno (BFGS) was utilized to relax the atomic structure. The cutoff energy of the plane waves which was set as 380 eV and the Brillouin zone samplings with a k-point grid of 4×4×4 samplings were sufficient to guarantee the convergence of total energy. The convergence criteria of geometric optimization and energy were set to (a) a self-consistent field tolerance of 10-6 eV/atom, (b) an energy tolerance of 10-5 eV/atom, (c) a maximum force tolerance of 0.3 eV/nm, (d) a maximum stress tolerance of 0.05 GPa, and (e) a maximum displacement tolerance of 10-4 nm. The cell sizes and atomic positions were fully relaxed without any constraints in the process of DFT calculation.
To determine the most stable Sr-doped HA models, the formation energies of different substitution models were calculated by DFT method. The formation energy was evaluated by the following equation[23]:
where EHA is the energy of perfect HA and Edoped-HA is the energy of doped-HA. ni denotes the numbers of the corresponding ionic species to be removed from (ni>0) or added (ni<0) to the HA unit cell. The chemical potentials for Ca2+ and Sr2+ are given by μCa and μSr, respectively. In this study, the chemical potentials are approximated by their energies at 0 K.
Table 1 Chemical composition of nanoparticles measured by EDS
| Sample | Ca/mol% | Sr/mol% | P/mol% | n(Ca+Sr)/n(P) | n(Ca)/n(P) | n(Sr)/n(P) | n(Sr)/n(Ca+Sr) |
|---|---|---|---|---|---|---|---|
| HA | 62.02 | 0.03 | 37.95 | - | 1.634 | - | - |
| 10%Sr-HA | 55.98 | 5.64 | 38.38 | 1.605 | 1.459 | 0.147 | 0.092 |
| 50%Sr-HA | 31.85 | 30.59 | 37.56 | 1.662 | 0.847 | 0.814 | 0.490 |
| 100%Sr-HA | 0.05 | 63.85 | 36.10 | - | - | 1.769 | - |
2 Results and discussion
2.1 Chemical composition
Atomic concentrations of Ca, Sr, and P in the nanoparticles were examined by EDS, and the element content was further confirmed by ICP, as listed in Table 1 and Table 2. The results obtained by EDS and ICP were consistent. The ratios of various elements were nearly the same as the raw ratios before the hydrothermal synthesis. The molar ratios of Ca/P in HA and (Ca+Sr)/P in Sr-HA were around 1.67 which was close to the stoichiometric number of pure HA without any significant change after addition of Sr ions, which is similar to previous studies[18,19].
Table 2 Element content of nanoparticles measured by ICP
| Sample | Ca/mol% | Sr/mol% | P/mol% | n(Ca+Sr)/n(P) | n(Ca)/n(P) | n(Sr)/n(P) | n(Sr)/n(Ca+Sr) |
|---|---|---|---|---|---|---|---|
| HA | 62.19 | 0.06 | 37.75 | - | 1.647 | - | - |
| 10%Sr-HA | 56.49 | 5.77 | 37.74 | 1.649 | 1.497 | 0.153 | 0.093 |
| 50%Sr-HA | 31.85 | 30.57 | 37.58 | 1.660 | 10.848 | 0.813 | 0.490 |
| 100%Sr-HA | 0.08 | 62.26 | 37.66 | - | - | 1.653 | - |
2.2 Analysis of XRD patterns
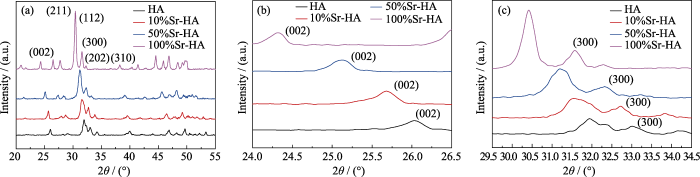
The XRD patterns of particles with different Sr doping concentrations from 0 to 100at% are shown in Fig. 1. The diffraction peaks of (002) and (300) shifted to a low 2θ value with gradual addition of Sr ions, illustrating an increase of lattice parameters (a and c) and d-spacings. This is because radius of Sr ion (0.118 nm) is larger than the radius of Ca ion (0.099 nm). These results were in line with those of previous studies[18, 24-26]. The characteristic diffraction peaks of all XRD patterns were narrow, suggesting good crystallinity of HA and all types of Sr-doped HA nanoparticles. The crystallinity and lattice parameters of all nanoparticles are shown in Table 3. Compared with pure HA, crystallinites of 10%Sr-HA and 50%Sr-HA were slightly lower while 100%Sr-HA exhibited the highest crystallinity, in that doping of Sr ions can inhibit the growth of HA nanoparticles. When doping concentration of Sr ions is low, inhibition effect is more obvious, and the irregular grain size leads to crystallinity decrease. When the doping concentration is high up to 100%, it is more likely to form regular and large-size new grains, leading to crystallinity increase. The result was perfectly consistent with the previous report[23]. Wang et al.[23] reported that the crystallinities of Sr-doped HA nanoparticles decreased steadily with increasing Sr content in the range from 10mol% to 50mol%. When Sr reached 100mol%, the crystallinity increased suddenly[23].
Fig. 1
Fig. 1
(a) XRD patterns of HA and Sr-doped HA nanoparticles with (b) magnified (200) peaks, and (c) (300) peaks
2.3 Morphology by SEM observation
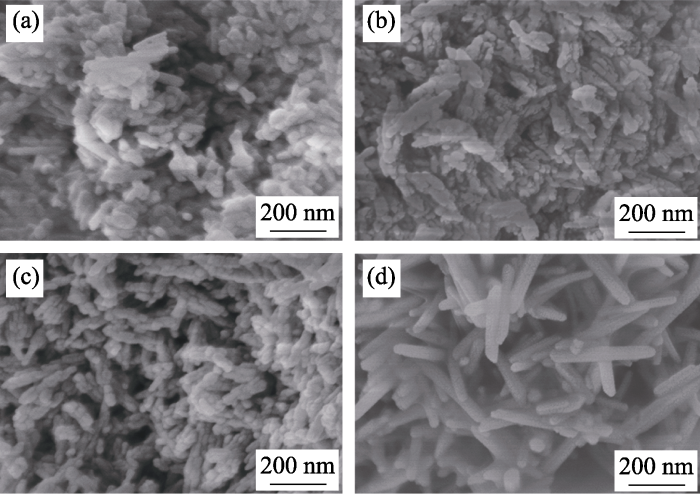
Fig. 2 clearly shows the morphologies of HA and Sr-doped HA nanoparticles. Agglomeration phenomenon occurred in all types of nanoparticles. The pure HA nanoparticles were rod-like. When the doped concentration of Sr increased from 10at% to 50at%, the morphology of Sr-doped HA did not change significantly compared with the pure HA. With the increase of Sr doping concentration, the crystal length increased, and the 100% Sr-HA nanoparticles presented rod-like shape and the largest crystal size. Geng et al.[18] also reported similar morphology changes.
Fig. 2
Fig. 2
SEM images of HA and Sr-doped HA nanoparticles (a) HA; (b) 10%Sr-HA; (c) 50%Sr-HA; (d) 100%Sr-HA
Table 3 Lattice parameters and crystallinity of nanoparticles obtained by XRD
| Sample | Crystallinity | 2θ(002)/ (°) | 2θ(300)/ (°) | a/nm | c/nm |
|---|---|---|---|---|---|
| HA | 53.3% | 26.07 | 33.09 | 0.93679 | 0.68286 |
| 10%Sr-HA | 51.1% | 25.68 | 32.76 | 0.94625 | 0.69326 |
| 50%Sr-HA | 49.4% | 25.12 | 32.34 | 0.95814 | 0.71056 |
| 100%Sr-HA | 73.7% | 24.34 | 31.58 | 0.98058 | 0.73071 |
2.4 Morphology and microstructure by TEM observation
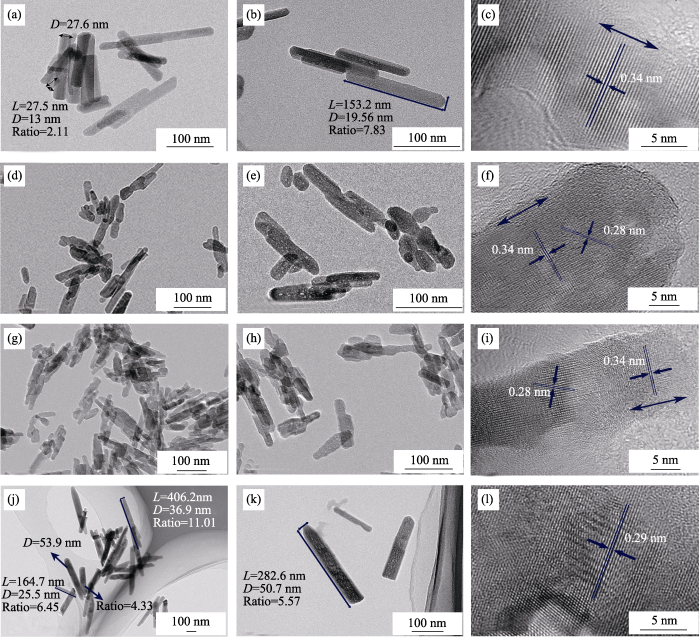
TEM and SAED images of HA and all types of Sr-doped HA nanoparticles are displayed in Fig. 3 and Fig. 4. Fig. 3(a, b) show the morphologies of pure HA nanoparticles. Pure HA displays regular shape and the nanoparticles display rod-like shape. Lengths of most nanoparticles vary from 27.5 to 153.2 nm and the widths of them are between 13 and 27.6 nm. Aspect ratios of crystals are between 2.11 and 7.83. There are no regular morphologies for 10%Sr-HA nanoparticles (Fig. 3(d, e)), which reveals that the crystal structure of HA was distorted because of the doping of Sr ions. As the content of doped Sr increasing to 50%, the irregular crystal size further increased. There was no statistical data about lengths, widths, and aspect ratios of the 10%Sr-HA and 50%Sr-HA nanoparticles due to their irregular shapes. When Sr ions completely replace Ca ions in HA, most of the nanoparticles regain regular shapes as shown in Fig. 3(j, k). The lengths and aspect ratios of the 100%Sr-HA nanoparticles increased significantly compared to those of other three types of nanoparticles, which are consistent with the results obtained by SEM observation. Most of the 100% Sr-HA nanoparticles are between 164.7 and 406.2 nm in length and between 25.5 and 53.9 nm in width. Their aspect ratios varied from 4.33 to 11.01. Morphologies of all types of nanoparticles are consistent with the previous studies[19,24].
HRTEM images corresponding to each type of nanoparticle (Fig. 3(c, f, i, l)) were used to analyze the crystal plane d-spacing and crystallinity changes of nanoparticles. Pure HA or Sr-doped HA nanoparticles exhibited good crystallinity, as proved by XRD. In pure HA nanoparticles, most of the observed crystal planes were (002) which had a d-spacing of 0.34 nm, and was perpendicular to c axis, in consistent with previous study[25]. In the nanoparticles doped with 10at% and 50at% Sr, crystal planes with d-spacings of 0.28 and 0.34 nm can be found. When the doping concentration of Sr is 100%, only 0.28 nm crystal plane d-spacing can be observed. SAED patterns (Fig. 4) corresponding to all types of nanoparticles exhibit polycrystalline rings, which is consistent with previous study[18]. These results also demonstrated that all types of nanoparticles had good crystallinity, in consistent with XRD and HRTEM results.
Fig. 3
(a, b) TEM images of HA; (c) HRTEM image of HA; (d, e) TEM images of 10%Sr-HA; (f) HRTEM image of 10%Sr-HA; (g, h) TEM images of 50%Sr-HA; (i) HRTEM image of 50%Sr-HA; (j, k) TEM images of 100%Sr-HA; (l) HRTEM image of 100%Sr-HA. Scale bars
Fig. 4
Fig. 4
SAED patterns of samples with different Sr concentrations (a) HA; (b) 10%Sr-HA; (c) 50%Sr-HA; (d) 100%Sr-HA
2.5 DFT results
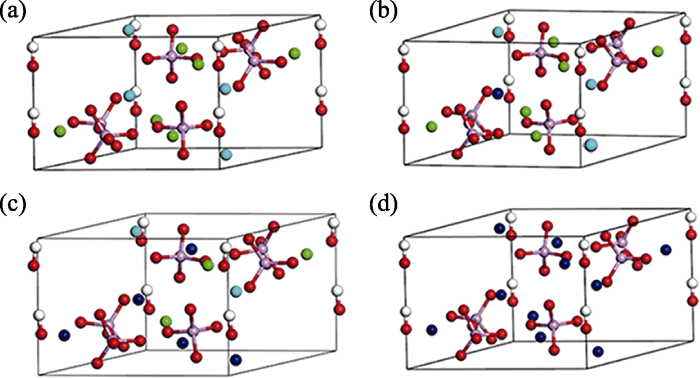
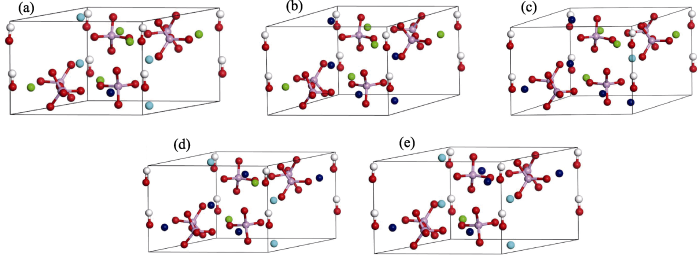
The unit cells of nine Sr-doped HA models after structure optimization are shown in Fig. 5 and Fig. S1. The calculation results obtained by the DFT method are shown in Table 4. To explore the effect of Sr ions doping into HA crystal structures, the lattice parameters of pure HA unit cell were calculated for comparison. Simulation results well confirmed the accuracy of the experimental ones.
Fig. 5
Fig. 5
Unit cell of (a) HA, (b) 1Sr-Ca(1)-HA, (c) 2Sr-Ca(1)- 3Sr-Ca(2)-HA, (d) 10Sr-HA after structural optimization Calcium (1): light blue; Calcium (2): green; Strontium: dark blue; Phosphorus: pink; Oxygen: red; Hydrogen: white
The lattice parameters (a-c) obtained by the simulation method were slightly larger than those provided by the experiment method, as shown in Table 4. Lattice parameters (both a and c) of all types of Sr-doped HA unit cells distinctly increased with increasing Sr concentrations, no matter which sites [Ca(1) or Ca(2)] were substituted by Sr ions. For 10%Sr-HA models, the lattice parameters a, b, and c of 1Sr-Ca(2)-HA and 1Sr-Ca(1)-HA models were larger than those of pure HA model. For 50%Sr-HA and 100%Sr-HA models, the results were roughly the same as that of the 10%Sr-HA model and in line with the previous studies[19,23].
Table 4 Lattice parameters and formation energy calculated by DFT method and lattice parameters (a & c) obtained from experiments
| Method | Model | a/nm | b/nm | c/nm | V/nm3 | Formation energy/eV |
|---|---|---|---|---|---|---|
| DFT | HA | 0.9642 | 0.9642 | 0.6853 | 5517 | |
| 1Sr-1Ca(1)-HA | 0.9695 | 0.9695 | 0.6910 | 5595 | -0.307 | |
| 1Sr-1Ca(2)-HA | 0.9717 | 0.9727 | 0.6897 | 5603 | -0.250 | |
| 4Sr-4Ca(1)-1Sr-1Ca(2)-HA | 0.9724 | 0.9744 | 0.7219 | 5879 | -0.404 | |
| 3Sr-3Ca(1)-2Sr-2Ca(2)-HA | 0.9765 | 0.9799 | 0.7159 | 5897 | -0.346 | |
| 2Sr-2Ca(1)-3Sr-3Ca(2)-HA | 0.9778 | 0.9841 | 0.7117 | 5891 | -0.557 | |
| 1Sr-1Ca(1)-4Sr-4Ca(2)-HA | 0.9788 | 0.9869 | 0.7108 | 5894 | -0.369 | |
| 5Sr-5Ca(2)-HA | 0.9809 | 0.9861 | 0.7113 | 5906 | -0.259 | |
| 10Sr-HA | 0.9922 | 0.9922 | 0.7361 | 6274 | -0.321 | |
| Experiment | HA | 0.9368 | - | 0.6829 | - | - |
| 10%Sr-HA | 0.9463 | - | 0.6933 | - | - | |
| 50%Sr-HA | 0.9581 | - | 0.7106 | - | - | |
| 100%Sr-HA | 0.9806 | - | 0.7307 | - | - |
Negative formation energy of all types of Sr-doped HA models suggested that Sr doping enhanced the stability of the apatite crystal structure. For 10at% Sr-HA models, results of formation energy showed that 1Sr-Ca(1)-HA model was more stable than 1Sr-Ca(2)-HA model. At 10at% doping concentration, Sr2+ was more likely to replace Ca2+ ion at Ca(1) position. Therefore, Ca(1) site was the preferential site for Sr doping at 10%Sr concentration, which was in line with the previous report[27]. For 50%Sr-HA models, 2Sr-Ca(1)-3Sr-Ca(2)-HA model was the most stable model owing to its lowest negative formation energy, which was consistent with the previous study[23].
3 Conclusions
In this work, HA, 10%Sr-HA, 50%Sr-HA, and 100%Sr-HA were prepared by a hydrothermal synthesis method. The conclusions can be drawn as follows:
1) All HA and Sr-doped HA nanoparticles have good crystallinity and show polycrystalline properties. Crystallinity of 10%Sr-HA and 50%Sr-HA are lower than that of pure HA. When Sr concentration reaches 100at%, the crystallinity becomes the highest.
2) Lattice parameters a and c of HA unit cell increases with increasing concentration of Sr ions. Stabilities of the Sr-HA nanoparticles enhance with the doping of Sr ions due to their negative formation energy. The 2Sr-2Ca(1)- 3Sr-3Ca(2)-HA model is more stable than the others because of its minimum formation energy.
Supporting materlals:
Supporting materlals related to this article can be found at
Strontium Doped Hydroxyapatite Nanoparticles: Synthesis, Characterization and Simulation
ZHOU Zihang1, WANG Qun2, GE Xiang3, LI Zhaoyang1
(1. School of Materials Science and Engineering, Tianjin University, Tianjin 300350, China; 2. College of Life Science and Biotechnology, MianYang Teachers’ College, Mianyang 621006, China; 3. Key Laboratory of Mechanism Theory and Equipment Design of Ministry of Education, School of Mechanical Engineering, Tianjin University, Tianjin 300354, China)
Fig. S1
Fig. S1
The unit cell of (a) 1Sr-Ca(2)-HA, (b) 4Sr-Ca(1)-1Sr-Ca(2)-HA, (c) 3Sr-Ca(1)-2Sr-Ca(2)-HA, (d) 1Sr-Ca(1)-4Sr-Ca(2)-HA, (e) 5Sr-Ca(2)-HA after structural optimization. Color codes: Calcium (1): light blue, Calcium (2): green, Strontium: dark blue, Phosphorus: pink, Oxygen: red, Hydrogen: white
参考文献
Synthesis methods for nanosized hydroxyapatite with diverse structure
DOI:10.1016/j.actbio.2013.04.012
URL
PMID:23583646
[本文引用: 1]

Hydroxyapatite (HAp) is the major mineral constituent of vertebrate bones and teeth. It has been well documented that HAp nanoparticles can significantly increase the biocompatibility and bioactivity of man-made biomaterials. Over the past decade, HAp nanoparticles have therefore increasingly been in demand, and extensive efforts have been devoted to develop many synthetic routes, involving both scientifically and economically new features. Several investigations have also been made to determine how critical properties of HAp can be effectively controlled by varying the processing parameters. With such a wide variety of methods for the preparation of HAp nanoparticles, choosing a specific procedure to synthesize a well-defined powder can be laborious; accordingly, in the present review, we have summarized all the available information on the preparation methodologies of HAp, and highlighted the inherent advantages and disadvantages involved in each method. This article is focused on nanosized HAp, although recent articles on microsized particles, especially those assembled from nanoparticles and/or nanocrystals, have also been reviewed for comparison. We have also provided several scientific figures and discussed a number of critical issues and challenges which require further research and development.
Biomimetic Mg-and Mg, CO3-substituted hydroxyapatites: synthesis characterization and in vitro behaviour
DOI:10.1016/j.jeurceramsoc.2005.06.040 URL [本文引用: 1]
Ca/P ratio effects on the degradation of hydroxyapatite in vitro
DOI:10.1002/jbm.a.10538
URL
PMID:14566803
[本文引用: 1]

Phase purity is a well-recognized but not well-understood variable affecting the biological integration of hydroxyapatite (HA)-based biomaterials. Minor amounts of specific, relevant impurities--calcium oxide (CaO) and tricalcium phosphate (TCP)--may often be present either as deliberate additions or as a result of decomposition during sintering. We investigated the influence of these two impurities in terms of their effects on surface morphology, weight loss/gain, and microstructural-level degradation. Phase purity variations were deliberately introduced into an otherwise-standardized HA matrix--the parent HA grain size and bulk density were relatively constant--produced using identical fabrication conditions. Stability varied markedly during exposure to mildly acidic, neutral, and pH 7.4 phosphate-buffered saline. Equivalent molar variations in the Ca/P ratio (1.62 vs 1.72) on either side of the stoichiometric ratio produce relatively small volumetric amounts of CaO (1.6 vol%) versus TCP (27 vol%) in HA. However, the relatively small amounts of CaO render the bulk more susceptible to degradation and more likely to have negative effects on a biological milieu. Interestingly, the presence of CaO is also a potent nucleating agent for the precipitation of new surface phases and detectable weight gain. The TCP-containing ceramic, in contrast, paradoxically exhibited slightly greater resistance to degradation than HA.
Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells
DOI:10.1016/j.actbio.2008.07.023
URL
[本文引用: 1]

Abstract
Nano-hydroxyapatite (nano-HAP) may be a better candidate for an apatite substitute of bone in biomedical applications than micro-sized hydroxyapatite (m-HAP). However, size control is always difficult when synthesizing well-defined nano-HAP particles. In this study, nano-HAP particles with diameters of ∼20 nm (np20) and ∼80 nm (np80) were synthesized and characterized. The size effects of these nano-HAPs and m-HAP were studied on human osteoblast-like MG-63 cells in vitro. Our results demonstrate that both cell proliferation and cell apoptosis are related to the size of the HAP particles. Np20 has the best effect on promotion of cell growth and inhibition of cell apoptosis. This work provides an interesting view of the role of nano-HAPs as ideal biomedical materials in future clinical applications.
et al. Zinc-substituted hydroxyapatite: a biomaterial with enhanced bioactivity and antibacterial properties
DOI:10.1007/s10856-012-4817-x
URL
PMID:23160913
[本文引用: 1]

Hydroxyapatite (HA) is a synthetic biomaterial and has been found to promote new bone formation when implanted in a bone defect site. However, its use is often limited due to its slow osteointegration rate and low antibacterial activity, particularly where HA has to be used for long term biomedical applications. This work will describe the synthesis and detailed characterization of zinc-substituted HA (ZnHA) as an alternative biomaterial to HA. ZnHA containing 1.6 wt% Zn was synthesized via a co-precipitation reaction between calcium hydroxide, orthophosphoric acid and zinc nitrate hexahydrate. Single-phase ZnHA particles with a rod-like morphology measuring ~50 nm in length and ~15 nm in width, were obtained and characterized using transmission electron microscopy and X-ray diffraction. The substitution of Zn into HA resulted in a decrease in both the a- and c-axes of the unit cell parameters, thereby causing the HA crystal structure to alter. In vitro cell culture work showed that ZnHA possessed enhanced bioactivity since an increase in the growth of human adipose-derived mesenchymal stem cells along with the bone cell differentiation markers, were observed. In addition, antibacterial work demonstrated that ZnHA exhibited antimicrobial capability since there was a significant decrease in the number of viable Staphylococcus aureus bacteria after in contact with ZnHA.
Barium hydroxyapatite nanoparticles synthesized by citric acid Sol-Gel combustion method
DOI:10.1016/j.materresbull.2005.04.033 URL [本文引用: 1]
Antibacterial coatings of fluoridated hydroxyapatite for percutaneous implants
DOI:10.1002/jbm.a.32862
URL
PMID:20725973
[本文引用: 1]

Percutaneous orthopedic and dental implants require not only good adhesion with bone but also the ability to attach and form seals with connective tissues and the skin. To solve the skin-seal problem of such implants, an electrochemical deposition method was used to modify the surfaces of metallic implants to improve their antibacterial ability and skin seals around them. A dense and uniform fluoridated calcium phosphate coating with a thickness of about 200 nm was deposited on an acid-etched pure titanium substrate by controlling the current density and reaction duration of the electrochemical process. The as-deposited amorphous fluoridated calcium phosphate transformed to fluoridated hydroxyapatite (FHA) after heat treatment at 600 degrees C in a water vapor environment for 3 h. Both single crystal diffraction patterns and high-resolution transmission electron microscope (HRTEM) images confirmed the phase of the fluoridated calcium phosphate after the heat treatment. The antibacterial activities of FHA coatings were tested against Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), and Porphyromonas gingivalis (P. gingivalis) with the film attachment method. The antibacterial activity of FHA coating is much higher than that of pure hydroxyapatite (HA) coating and acid-etched pure titanium surface. The promising features of FHA coating make it suitable for orthopedic and dental applications.
Integrity and zeta potential of fluoridated hydroxyapatite nanothick coatings for biomedical applications
DOI:10.1016/j.jmbbm.2011.03.013
URL
[本文引用: 1]

Fluoridated hydroxyapatite (FHA) coatings exhibit great potential for applications on implants which require good bioactivity and high antibacterial activity. This work is a comparative study of chemical stability, adhesive strength and zeta potential of a nanothick (about 200 nm) FHA coating which is densely and uniformly deposited on a titanium substrate electrochemically. The chemical stability of the nanothick coatings was evaluated using a dissolution test in a simulated physiological solution. The dissolution tests indicate that the fluorine-containing calcium phosphate (CaP) coating with an appropriate heat treatment is chemically stable in a physiological environment, even more so than its HA counterpart. The adhesive strength of FHA coatings was evaluated by the critical load obtained from nanoscratching tests. The critical load which caused coating failure in scratching was determined by a method which correlated the scratching morphology with a sudden change in friction coefficient. The adhesive strength of a nanothick FHA coating was 147% larger than that of HA coating with the same thickness level. The fluorine addition does not have a significant effect on the characteristics of the negative zeta potential of apatite coatings. The solid integrity of this nanothick FHA coating makes it an excellent candidate for biomedical applications. (C) 2011 Elsevier Ltd.
Surfactant-free electrochemical synthesis of fluoridated hydroxyapatite nanorods for biomedical applications
DOI:10.1016/j.ceramint.2019.05.292 URL [本文引用: 1]
In vitro bioactivity, physical and mechanical properties of carbonated-fluoroapatite during mechanochemical synthesis
DOI:10.1016/j.ceramint.2018.08.184 URL [本文引用: 1]
Modification of titanium surface via Ag-, Sr-and Si-containing micro-arc calcium phosphate coating
DOI:10.1016/j.bioactmat.2019.07.001
URL
PMID:31406950
[本文引用: 1]

The current research is devoted to the study of the modification of the titanium implants by the micro-arc oxidation with bioactive calcium phosphate coatings containing Ag or Sr and Si elements. The coatings' microstructure, phase composition, morphology, physicochemical and biological properties were examined by scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDX) and X-ray diffraction (XRD). Ag-containing and Sr-Si-incorporated coatings were formed in alkaline and acid electrolytes, respectively. The formation of the coatings occurred at different ranges of the applied voltages, which led to the significant difference in the coatings properties. The trace elements Ag, Sr and Si participated intensively in the plasma-chemical reactions of the micro-arc coatings formation. Ag-containing coatings demonstrated strong antibacterial effect against Staphylococcus aureus Acapital TE, Cyrilliccapital ES, Cyrilliccapital ES, Cyrillic 6538-P. MTT in vitro test with 3T3-L1 fibroblasts showed no cytotoxicity appearance on Sr-Si-incorporated coatings.
Combined effect of strontium and zoledronate on hydroxyapatite structure and bone cell response
DOI:10.1016/j.biomaterials.2014.03.053
URL
[本文引用: 1]

The influence of the simultaneous presence of the two inhibitors of bone degradation, strontium and zoledronate, on the direct synthesis of hydroxyapatite was explored in the range of Sr concentration up to 50 atom% at two different bisphosphonate concentrations (ZOL7 and ZOL14). The results of structural analysis indicated that HA can be obtained as a single crystalline phase up to a Sr concentration in solution of 20 and 10 atom% within the ZOL7 and ZOL14 series respectively. Both Sr substitution and ZOL incorporation affect the length of the coherently scattering crystalline domains and the dimensions of HA nanocrystals. At greater Sr content, XRD full profile fitting data indicate that zoledronate provokes the segregation of Sr in two crystalline apatitic phases, at different strontium content. Co-cultures of osteoblast-like MG63 cells and human osteoclast show that ZOL displays a greater inhibitory influence than Sr on osteoclast proliferation and activity. On the other hand, the results obtained on osteoblast surnatant and on gene expression indicate that Sr exerts a greater promotion on osteoblast proliferation and differentiation. The co-presence of Sr and ZOL has a combined effect on the differentiation markers, so that HA containing about 4 wt% ZOL and 4 Sr atom%, and even more HA containing about 4 wt% ZOL and 8 Sr atom%, result the best compromise for osteoblast promotion and osteoclast inhibition. (C) 2014 Elsevier Ltd.
Strontium promotes osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway
DOI:10.1159/000204105 URL [本文引用: 1]
Regulation of osteoblast proliferation and differentiation by interrod spacing of Sr-HA nanorods on microporous titania coatings
DOI:10.1021/am401339n
URL
PMID:23668394
[本文引用: 1]

Strontium-doped hydroxyapatite (Ca9Sr1(PO4)6(OH)2, Sr1-HA) nanorods with different lateral spacing (e.g., interrod spacing) values (67.3 +/- 3.8, 95.7 +/- 4.2, and 136.8 +/- 8.7 nm) and nanogranulates were grown on microarc-oxidized microporous TiO2, respectively, to form multilayer coatings. The coatings reveal two kinds of micro/nanoscaled hierarchical surfaces with a similar microscale roughness, e.g., nanogranulated 2D pattern and nanorod-shaped 3D pattern in nanotopography. When hFOB1.19 cells are employed, the proliferation and differentiation of osteoblasts on the coatings were evaluated by examining MTT assay, expressions of osteogenesis-related genes [alkaline phosphatase (ALP), runt-related transcription factor 2, osterix, osteopontin (OPN), osteocalcin (OCN), and collagen I (Col-I)], ALP activity, contents of intracellular Ca(2+), Col-I, OPN, and OCN, extracellular collagen secretion, and extracellular matrix mineralization. The results reveal that the proliferation and differentiation of osteoblasts can be directly regulated by the interrod spacing of the Sr1-HA nanorods, which are significantly enhanced on the nanorod-shaped 3D patterns with interrod spacing smaller than 96 nm and more pronounced with decreasing the interrod spacing but inhibited on the nanorods with spacing larger than 96 nm compared to the nanogranulated 2D pattern. The difference in the cellular activity is found to be related with the intracellular Ca(2+) concentrations, which are regulated by variation of the surface topology of Sr1-HA crystals. Our work provides insight to the surface structural design of a biomedical implant favoring osteointegration.
A high-resolution 43Ca solid-state NMR study of the calcium sites of hydroxyapatite
DOI:10.1021/ja710557t URL PMID:18237175 [本文引用: 1]
A resilient and flexible chitosan/silk cryogel incorporated Ag/Sr co-doped nanoscale hydroxyapatite for osteoinductivity and antibacterial properties
DOI:10.1039/c8tb01672k
URL
PMID:32254744
[本文引用: 1]

Cryogels from natural biopolymers, especially chitosan (CS) and silk fibroin (SF), have attracted significant attention for bone tissue engineering due to their good biocompatibility and tissue affinity. However, they are still not widely applied in clinics because of their poor mechanical properties, and lack of osteoinductivity and antibacterial properties. Here, by integrating a physico-chemical hybrid-crosslinking strategy and a freeze-drying method, the authors have prepared a resilient and flexible chitosan/silk cryogel with Ag and Sr co-doped hydroxyapatite (AgSrHA). The cryogel exhibits super resilience and flexibility, which guarantees the mechanical strength required for bone repair. Furthermore, the cryogel possesses long-term effective antibacterial properties and osteoinductivity due to the slow release of Ag and Sr ions. The physico-chemical hybrid-crosslinking points were introduced into a CS/SF network by treating with a mixture of alkaline and polyethylene glycol glycidyl ether (PEGDE) at 60 degrees C, which endowed the cryogel with super resilience and flexibility. Nanoscale AgSrHA was incorporated into the CS/SF network to further enhance the mechanical properties of the cryogel. In addition, Ag and Sr were doped into the HA crystal lattice, which not only prevents cytotoxicity by avoiding the ion burst release behavior, but also can achieve antibacterial and osteoinductive properties for a long period of time. In short, the AgSrHA/CS/SF cryogel with excellent mechanical properties and long-term effective dual-biofunction of antibacterial properties and osteoinductivity would be an ideal candidate for successful bone repair in clinics.
Unravelling the specific site preference in doping of calcium hydroxyapatite with strontium from ab initio investigations and Rietveld analyses
DOI:10.1039/c2cp23163h
URL
PMID:22307038
[本文引用: 2]

Strontium can be substituted into the calcium sublattice of hydroxyapatite without a solubility limit. However, recent ab initio simulations carried out at 0 K report endothermic nature of this process. There is also striking discrepancy between experimentally observed preference of Sr doping at Ca-II sites and the first principles calculations, which indicate that a Ca-I site is preferred energetically for the Sr substitution. In this paper we combine insights from Density Functional Theory simulations and regular configurational entropy calculations to determine the site preference of Sr doping in the range of 0-100 at% at finite temperatures. In addition, samples of Sr-HA are synthesized and refinement of the relevant structural information provides benchmark information on the experimental unit cell parameters of Sr-HA. We find that the contribution of the entropy of mixing can efficiently overcome the endothermic excess energy at a temperature typical of the calcining step in the synthesis route of hydroxyapatite (700-950 degrees C). We observe that the most preferential substitution pattern is mixed substitution of Sr regardless of the concentration. For a wet chemical method, carried out at a moderate temperature (90 degrees C), the mixed doping is still slightly favourable at higher Sr-concentrations, except the range at 20% Sr, where Site II substitution is not restricted energetically and equally possible as the mixed doping. We observe a close correspondence between our theoretical results and available experimental data. Hence it should be possible to apply this theory to other divalent dopants in HA, such as Zn(2+), Mg(2+), Pb(2+), Cu(2+), Ba(2+), Cd(2+) etc.
Synthesis, characterization and the formation mechanism of magnesium-and strontium-substituted hydroxyapatite
DOI:10.1039/c4tb02148g
URL
PMID:32262848
[本文引用: 5]

Magnesium (Mg) and strontium (Sr) have been widely used in the field of implanted devices because of their excellent bioactivity. However, the local high ion concentration caused by the implant affects the growth of hydroxyapatite (Ca10(PO4)6(OH)2, HA), which is the main inorganic component of bone and teeth. Many studies have investigated the effect of Mg(2+) and Sr(2+) on the growth of HA, but no systematic research has been conducted to compare these two ions in terms of the growth of HA. In this study, the substitution of a series of Sr- and Mg-substituted HA was conducted through a conventional hydrothermal method. Comprehensive characterization techniques, including X-ray diffraction, inductive coupled plasma, field emission scanning electron microscopy, transmission electron microscopy, selected-area electron diffraction, thermo gravimetric-differential scanning calorimetry, and Fourier transform infrared spectroscopy, were used to examine the effects of Sr(2+) and Mg(2+) on the phase, morphology, crystallinity, chemical composition, thermal stability, and lattice parameters of HA. The results indicated that Mg ions partially substituted for calcium (Ca) ions in the apatite structure, thus decreasing the lattice parameters, partially adsorbing on the apatite surface that formed the amorphous phase, and inhibiting the crystal growth. By contrast, Sr ions fully substituted for Ca ions and increased the lattice parameters. Both Mg and Sr ions affected the morphology of HA. Crystallinity decreased with the addition of Mg ions (transition from the crystal to amorphous phase was between 30% and 40% Mg), but it was not affected by Sr ions. Thermostability decreased with the addition of Mg (a total weight loss from 8.06 wt% for 10% Mg to 25.81 wt% for 50% Mg), but it had no significant changes in the Sr-substituted samples.
Strontium-substituted hydroxyapatite nanocrystals
DOI:10.1016/j.ica.2006.07.074 URL [本文引用: 5]
The structure of strontium-doped hydroxyapatite: an experimental and theoretical study
DOI:10.1039/b802841a
URL
PMID:19283275
[本文引用: 1]

First-principles modeling combined with experimental methods were used to study hydroxyapatite in which Sr2+ is substituted for Ca2+. Detailed analyses of cation-oxygen bond distributions, cation-cation distances, and site 1-oxygen polyhedron twist angles were made in order to provide an atomic-scale interpretation of the observed structural modifications. Density functional theory periodic band-structure calculations indicate that the Ca2+ to Sr2+ substitution induces strong local distortion on the hydroxyapatite lattice: the nearest neighbor Sr-O bond structures in both cationic sites are comparable to pure SrHA, while Sr induces more distortion at site 2 than site 1. Infrared vibrational spectroscopy (FTIR) and extended X-ray absorption fine structure (EXAFS) analysis suggest increasing lattice disorder and loss of OH with increasing Sr content. Rietveld refinement of synchrotron X-ray diffraction patterns shows a preference for the Ca1 site at Sr concentrations below 1 at.%. The ideal statistical occupancy ratio Sr2/Sr1=1.5 is achieved for approximately 5 at.%; for higher Sr concentrations occupation of the Ca2 site is progressively preferred.
Osteoblast response to hydroxyapatite doped with divalent and trivalent cations
DOI:10.1016/j.biomaterials.2003.09.001
URL
[本文引用: 1]

Abstract
The present in vitro study doped hydroxyapatite (HA) with various metal cations (Mg2+, Zn2+, La3+, Y3+, In3+, and Bi3+) in an attempt to enhance properties of HA pertinent to orthopedic and dental applications. X-ray diffraction material characterization indicated that the metal cations may have substituted for calcium in the HA crystal structure and that all of the doped HA formulations were single-phase and crystalline. Scanning electron microscopy analysis revealed a variety of grain sizes, depending on the dopant utilized. Energy-dispersive spectroscopy confirmed that the dopants added during synthesis were present and that all of the HA formulations synthesized were within the defined range of HA phase in the CaO–P2O5–H2O system. Lastly, Bi-doped HA had a slower dissolution rate than either undoped HA or HA doped with other cations when exposed to simulated physiological conditions for 21 days. In terms of cell function, results provided the first evidence that osteoblasts, bone-forming cells, adhered and differentiated (as measured by alkaline phosphatase synthesis) in response to HA doped with trivalent cations (specifically, La3+, Y3+, In3+, Bi3+) at earlier time points than either HA doped with divalent cations (Mg2+, Zn2+) or undoped HA. Of the dopants examined, Bi3+ most enhanced osteoblast long-term calcium-containing mineral deposition. For these reasons, this study revealed for the first time the potential benefits of doping HA with Bi3+ according to criteria critical for bone prosthetic clinical success.
Densification behaviour and mechanisms of synthetic hydroxyapatites
DOI:10.1016/S0955-2219(00)00154-0 URL [本文引用: 1]
Experimental and simulation studies of strontium/fluoride-codoped hydroxyapatite nanoparticles with osteogenic and antibacterial activities
DOI:10.1016/j.colsurfb.2019.110359 URL [本文引用: 6]
Preparation and property of strontium-substituted hydroxyapatite
DOI:10.3724/SP.J.1077.2011.00049
URL
[本文引用: 2]

The biological HA in the body differs from that of pure HA in stoichiometry,composition, physical properties and mechanical properties, because this HA isparticularly prone to ion substitution. As a cation that can substitute forcalcium in the structure of hydroxyapatite, strontium provokes an increasinginterest because of its beneficial effect on bone formation, and prevention ofbone resorption. Here, strontium-substituted HA powders were prepared by thehydrothermal method at 180℃ for 8 h using Ca(NO3)2·4H2O, (NH4)2HPO4and Sr(NO3)2 as reagents. X-ray diffraction, Fouriertransform infrared spectroscope, transmission electron microscope, energy dispersiveX-ray, and thermogravimetric-differential thermal analysis were employed toinvestigate the crystalline phase, chemical composition, morphology, and thermalstability of the Sr-HA. And the cytotoxicity of Sr-HA was analyzed through MTT assay. The results showthat Sr is incorporated into the HA crystal structure and the crystal grainsize of the Sr-HA decreases as the Sr content (under 50wt%) is increased.Moreover, the HA thermal stability decreases. However, there’s no apparentdifference between the pure hydroxyapatite and all of the Sr-HA groups in cytotoxicity,which may have good biocompatibility.
Chemical composition, crystal size and lattice structural changes after incorporation of strontium into biomimetic apatite
DOI:10.1016/j.biomaterials.2006.11.001
URL
[本文引用: 1]

Abstract
Recently, strontium (Sr) as ranelate compound has become increasingly popular in the treatment of osteoporosis. However, the lattice structure of bone crystal after Sr incorporation is yet to be extensively reported. In this study, we synthesized strontium-substituted hydroxyapatite (Sr-HA) with different Sr content (0.3%, 1.5% and 15% Sr-HA in mole ratio) to simulate bone crystals incorporated with Sr. The changes in chemical composition and lattice structure of apetite after synthetic incorporation of Sr were evaluated to gain insight into bone crystal changes after incorporation of Sr. X-ray diffraction (XRD) patterns revealed that 0.3% and 1.5% Sr-HA exhibited single phase spectrum, which was similar to that of HA. However, 15% Sr-HA induced the incorporation of HPO42− and more CO32−, the crystallinity reduced dramatically. Transmission electron microscopy (TEM) images showed that the crystal length and width of 0.3% and 1.5% Sr-HA increased slightly. Meanwhile, the length and width distribution were broadened and the aspect ratio decreased from 10.68±4.00 to 7.28±2.80. The crystal size and crystallinity of 15% Sr-HA dropped rapidly, which may suggest that the fundamental crystal structure is changed. The findings from this work indicate that current clinical dosage which usually results in Sr incorporation of below 1.5% may not change chemical composition and lattice structure of bone, while it will broaden the bone crystal size distribution and strengthen the bone.
Experimental and simulation studies of strontium/zinc-codoped hydroxyapatite porous scaffolds with excellent osteoinductivity and antibacterial activity
DOI:10.1016/j.apsusc.2018.08.068 URL [本文引用: 1]
Strontium substitution in bioactive calcium phosphates: a first-principles study
DOI:10.1021/jp808713m
URL
PMID:19243110
[本文引用: 1]

First-principles calculations are performed to investigate atomic and electronic structures of Sr(2+) ions substituting for Ca(2+) in octacalcium phosphate (OCP). The defect formation energies are evaluated from total energies of supercells and ionic chemical potentials of Sr(2+) and Ca(2+) determined under the chemical equilibrium with aqueous solution saturated with hydroxyapatite (HAp). The defect formation energy depends on the solution pH and the substitutional Ca sites in OCP, and the estimated equilibrium concentrations of Sr(2+) in OCP and HAp are in reasonable agreement with previous experimental results obtained in physiological conditions. It is also found that Sr(2+) ions can be more favorably substituted in OCP than in HAp. It is thought, therefore, that Sr(2+) plays its role to promote bone formation by being incorporated into the metastable OCP phase occurring during HAp nucleation.