

Fe, N掺杂二维多孔碳双功能催化剂及锌-空气电池中的应用
收稿日期: 2018-06-11
修回日期: 2018-08-18
网络出版日期: 2018-12-17
基金资助
四川省科学技术厅项目 (2017JY0088);香港城市大学项目 (9610372);深圳市科技创新委员会项目(JCYJ20170818103435068) Science & Technology Department of Sichuan Province (2017JY0088);City University of Hong Kong (9610372);Science Technology and Innovation Committee of Shenzhen Municipality (JCYJ20170818103435068)
Fe, N Doped 2D Porous Carbon Bifunctional Catalyst for Zinc-air Battery
Received date: 2018-06-11
Revised date: 2018-08-18
Online published: 2018-12-17
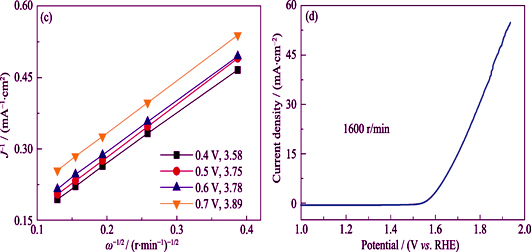
在石墨烯表面负载金属有机框架材料ZIF-8, 同时在金属有机框架材料表面分散Fe-2,2-Bipy螯合物, 通过高温煅烧分解制备了Fe, N 掺杂多孔碳催化剂材料。采用SEM, XRD, XPS对制备的催化剂材料进行了形貌、结构以及成分分析。采用旋转圆盘电极, CV曲线, LSV曲线对Fe, N掺杂多孔碳催化剂材料的氧还原(ORR)以及析氧(OER)电催化性能进行了分析。并且将Fe, N 掺杂多孔碳催化剂应用于锌-空气电池。结果表明, 所制备的Fe, N掺杂多孔碳催化剂材料显示出均匀的二维结构形貌, Fe元素含量为1.32%。催化剂在0.1 mol/L KOH溶液中半波电位为0.83 V, 在1 mol/L KOH溶液中, 10 mA/cm2电流密度下过电势为420 mV。将催化剂应用于锌-空气电池, 锌-空气电池功率密度达到245 mV/cm2, 并且表现出优异的循环稳定性。
马龙涛 , 支春义 . Fe, N掺杂二维多孔碳双功能催化剂及锌-空气电池中的应用[J]. 无机材料学报, 2019 , 34(1) : 103 -108 . DOI: 10.15541/jim20180260
Fe, N doped 2D porous carbon catalyst was synthesized by pyrolysizing the precursor, ZIF-8, on graphene. Meanwhile, Fe-2,2-bipy were coordinated on ZIF-8. The catalyst was analyzed by SEM, XRD, and XPS for morphology, structure and component. The ORR and OER performance of the Fe, N doped 2D porous carbon catalyst were characterized by RDE, CV curves and LSV curves. It was found that the Fe, N doped 2 D porous carbon catalyst shows uniform 2D structure and that the content of Fe element is 1.32%. The catalyst shows 0.83 V half-wave potentials for oxygen reduction reaction (ORR) in 0.1 mol/L KOH solution and 420 mV over-potential for oxygen evolution reaction (OER) at 10 mA/cm2 in 1 mol/L KOH solution. Then, a zinc-air battery was assembled using as-synthesized catalyst. The power density of zinc-air battery is up to 245 mV/cm2. Furthermore, it shows superior cycling stability.

Key words: Fe; N doped; 2 D porous materials; bifuncational catalyst; zinc-air battery
| [1] | XU Y, ZHANG Y, GUO Z, et al.Flexible, stretchable, and rechargeable fiber-shaped zinc-air battery based on cross-stacked carbon nanotube sheets. Angew. Chem. Int. Ed., 2015, 54(51): 15390-15394. |
| [2] | LI G, WANG X, FU J, et al.Pomegranate-inspired design of highly active and durable bifunctional electrocatalysts for rechargeable metal-air batteries. Angew. Chem. Int. Ed., 2016, 55(16): 4977-4982. |
| [3] | FU J, LEE D, HASSAN F, et al.Flexible high-energy polymer- electrolyte-based rechargeable zinc-air batteries. Adv. Mater., 2015, 27(37): 5617-5622. |
| [4] | ABBASI H, SALEHI S, GHORBANI R, et al.Design and manufacturing of a micro zinc-air fuel cell for mobile applications. Iranica [J]. Energy Environ., 2013, 4(2): 110-115. |
| [5] | WEI L, KARAHAN H, ZHAI S, et al. Amorphous bimetallic oxide- graphene hybrids as bifunctional oxygen electrocatalysts for rechargeable Zn-air batteries. Adv. Mater., 2017, 29(38): 1701410- 1-10. |
| [6] | ZHANG J, SUN B, ZHAO Y, et al.Modified tetrathiafulvalene as an organic conductor for improving performances of Li-O2 batteries. Angew. Chem. Int. Ed., 2017, 56(29): 8505-8509. |
| [7] | LIU B, XU W, YAN P, et al. Stabilization of Li metal anode in DMSO-based electrolytes via optimization of salt-solvent coordination for Li-O2 batteries. Adv. Energy Mater., 2017, 7(14): 1602605-1-10. |
| [8] | HOU Y, HUANG T, WEN Z, et al. Metal-organic framework- derived nitrogen-doped core-shell-structured porous Fe/Fe3C@C nanoboxes supported on graphene sheets for efficient oxygen reduction reactions. Adv. Energy Mater., 2014, 4(11): 1400337-1-8. |
| [9] | NI B, OUYANG C, XU X, et al. Modifying commercial carbon with trace amounts of ZIF to prepare derivatives with superior ORR activities. Adv. Mater., 2017, 29(27): 1701354-1-7. |
| [10] | LIU J, ZHU D, GUO C, et al. Design strategies toward advanced MOF-derived electrocatalysts for energy-conversion reactions. Adv. Energy Mater., 2017, 7(23): 1700518-1-26. |
| [11] | YE L, CHAI G, WEN Z. Zn-MOF-74 derived N-doped mesoporous carbon as pH-universal electrocatalyst for oxygen reduction reaction. Adv. Funct. Mater., 2017, 27(14): 1606190-1-8. |
| [12] | ZHONG H, WANG J, ZHANG Y, et al.ZIF-8 derived graphene- based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew. Chem. Int. Ed., 2014, 53(51): 14235-14239. |
| [13] | CHEN X, LIU B, ZHONG C, et al. Ultrathin Co3O4 layers with large contact area on carbon fibers as high-performance electrode for flexible zinc-air battery integrated with flexible display. Adv. Energy Mater., 2017, 7(18): 1700779-1-11. |
| [14] | CHEN S, CHENG J, MA L, et al.Light-weight 3D Co-N-doped hollow carbon spheres as efficient electrocatalysts for rechargeable zinc-air batteries. Nanoscale, 2018, 10(22): 10412-10419. |
| [15] | MA L, CHEN S, PEI Z, et al.Single-site active iron-based bifunctional oxygen catalyst for a compressible and rechargeable zinc-air battery. ACS Nano, 2018, 12(2): 1949-1958. |
| [16] | ZAGAI J, KOPER M.Reactivity descriptors for the activity of molecular MN4 catalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed., 2016, 55(47): 14510-14521. |
| [17] | ZHONG H, WANG J, ZHANG Y, et al.ZIF-8 derived graphene- based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew. Chem. Int. Ed., 2014, 53(51): 14235-14239. |
| [18] | SU C, CHENG H, LI W, et al. Atomic modulation of FeCo-nitrogen-carbon bifunctional oxygen electrodes for rechargeable and flexible all-solid-state zinc-air battery. Adv. Energy Mater., 2017, 7(13): 1602420-1-12. |
| [19] | LIU K, ZHONG H, MENG F, et al.Recent advances in metal-nitrogen-carbon catalysts for electrochemical water splitting. Mater. Chem. Front., 2017, 1(11): 2155-2173. |
| [20] | ZHU Y, ZHANG B, LIU X, et al.Unravelling the structure of electrocatalytically active Fe-N complexes in carbon for the oxygen reduction reaction. Angew. Chem. Int. Ed., 2014, 53(40): 10673-10677. |
| [21] | ZITOLO A, GOELLNER V, ARMEL V, et al.Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater., 2015, 14(9): 937-945. |
| [22] | SHEN H, EDUARDO G, MA J, et al.Synergistic effects between atomically dispersed Fe-N-C and C-S-C for the oxygen reduction reaction in acidic media. Angew. Chem. Int. Ed., 2017, 129(44): 13988-13992. |
| [23] | MA L, FAN H, FU K, et al.Metal-organic framework/layered carbon nitride nano-sandwiches for superior asymmetric supercapacitor. Chemistry Select, 2016, 1(13): 3730-3738. |
| [24] | MA L, FAN H, WANG J, et al.Water-assisted ions in situ intercalation for porous polymeric graphitic carbon nitride nanosheets with superior photocatalytic hydrogen evolution performance. Appl. Catal. B: Environ., 2016, 190: 93-102. |
| [25] | MA L, FAN H, FU K, et al.Protonation of g carbon nitride (g-C3N4) for an electrostatically self-assembling carbon@g-C3N4 core-shell nanostructure toward high hydrogen evolution. ACS Sustain. Chem. Eng., 2017, 5(8): 7093-7103. |
| [26] | FERRERO G, PREUSS K, MARINOVIC A, et al.Fe-N-doped carbon capsules with outstanding electrochemical performance and stability for the oxygen reduction reaction in both acid and alkaline conditions. ACS Nano, 2016, 10(6): 5922-5932. |
| [27] | JIANG W, GU L, LI L, et al.Understanding the high activity of Fe-N-C electrocatalysts in oxygen reduction: Fe/Fe3C nanoparticles boost the activity of Fe-N-C. [J]. Am. Chem. Soc., 2016, 138(10): 3570-3578. |
| [28] | MALKO D, KUCERNAK A, LOPES T.In situ electrochemical quantification of active sites in Fe-N/C non-precious metal catalysts. Nat. Commun., 2016, 7: 13285-13292. |
/
| 〈 |
|
〉 |