

硅藻土原位生长Nb2O5纳米棒及表面Cr(VI)吸附转化行为研究
收稿日期: 2017-06-21
修回日期: 2017-09-28
网络出版日期: 2018-04-26
基金资助
国家重点研发计划项目(2017YFB0310804);北京市自然科学基金(2172011)
In-situ Growth of Nb2O5 Nanorods on Diatomite and Highly Effective Removal of Cr(VI)
Received date: 2017-06-21
Revised date: 2017-09-28
Online published: 2018-04-26
Supported by
Project of the National Science and Technology of China (2017YFB0310804);The Beijing Natural Science Foundation (2172011)
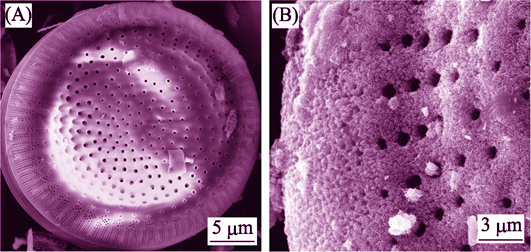
以活化铌酸为铌源, 草酸铵为沉积剂, 十二烷基苯磺酸钠为模板剂, 采用水热法在硅藻土表面原位生长Nb2O5纳米棒。采用SEM、TEM、XRD、BET、FT-IR和XPS等分析方法对样品进行表征, 反应14 h后, Nb2O5纳米棒长度为500~700 nm, 直径为25~35 nm; 硅藻土原位生长Nb2O5纳米棒样品比表面积为157 m2/g。研究了样品对Cr(VI)的吸附与光还原行为, 可见光条件下对Cr(VI)吸附量可达220 mg/g; 紫外光条件下, 可将表面吸附的Cr(VI)转变为Cr(III), 样品经过5次循环使用后, 对Cr(VI)(100 mg/L)降解率仍能保持在93%左右。样品可对重金属污染废水中Cr(VI)进行吸附与毒性降解一体化去除。
杜玉成 , 王学凯 , 侯瑞琴 , 吴俊书 , 张时豪 , 祁超 . 硅藻土原位生长Nb2O5纳米棒及表面Cr(VI)吸附转化行为研究[J]. 无机材料学报, 2018 , 33(5) : 557 -564 . DOI: 10.15541/jim20170308
Nb2O5 nanorods decorated diatomite were synthesized via a hydrothermal method by using niobic acid, ammonium oxalate and sodium dodecyl benzene sulfonate (SDBS). The as-prepared samples were characterized by SEM, TEM, XRD, BET, and FT-IR techniques. The Nb2O5 nanorod was obtained with length of 500~700 nm and diameter of 25~35 nm. BET test showed that specific surface area of Nb2O5 nanorods decorated diatomite reached 157 m2/g which show a high adsorbance tendence. Under visible light irradiation, the adsorption capacity for Cr (VI) could be 220 mg/g. With the assistance of UV-light photoreduction, the maximum removal capacity for Cr (VI) could be 340 mg/g, and the absorbed Cr(VI) was transformed to Cr(III). After photodegradation for five times, the degradation rate of Cr(VI) (100 mg/L) were still at about 93%. Nb2O5 nanorods/diatomite could adsorb Cr(VI) from sewage effectively and in the meantime accomplish the toxicity degradation, showing a promising treatment of Cr(VI) containing waste water.

Key words: diatomite; Nb2O5 nanorod; Cr(VI); adsorption; toxicity degradation
| [1] | METIN G, DUYGU V, AYSE M.Removal of trivalent chromium from water using low-cost natural diatomite.J. Hazard. Mater., 2008, 160(2/3): 318-323. |
| [2] | JIANG BO, XIN SHUAI-SHUAI, GAO LI,et al. Dramatically enhanced aerobic Cr(VI) reduction with scrap zero-valent aluminum induced by oxalate. Chemical Engineering Journal, 2016, 308: 588-596. |
| [3] | HANS R, SENANAYAKE G, DHARMASIRI L C S,et al. A preliminary batch study of sorption kinetics of Cr(VI) ions from aqueous solutions by a magnetic ion exchange (MIEX®;) resin and determination of film/pore diffusivity. Hydrometallurgy, 2016, 164: 208-218. |
| [4] | FU XIAO-FEI, YANG HAN-PEI, LU GUANG-HUA,et al. Improved performance of surface functionalized TiO2/activated carbon for adsorption-photocatalytic reduction of Cr(VI) in aqueous solution. Materials Science in Semiconductor Processing, 2015, 39: 362-370. |
| [5] | DINDA D, KUMAR S S.Sulfuric acid doped poly diaminopyridine/ graphene composite to remove high concentration of toxic Cr(VI). Journal of Hazardous Materials, 2015, 291: 93-101. |
| [6] | GUAN XIAO-HONG, DU JUAN-SHAN, MENG XIAO-GUANG,et al. Application of titanium dioxide in arsenic removal from water: a review. Journal of Hazardous Materials, 2012, 215-216(10): 1-16. |
| [7] | LI HUI, LI WEI, ZHANG YAN-JUN,et al. Chrysanthemum-like a-FeOOH microspheres produced by a simple green method and their outstanding ability in heavy metal ion removal . J. Mater. Chem., 2011, 21: 7878-7881. |
| [8] | MISHRA S, BHARAGAVA R N.Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. Journal of Environmental Science & Health Part C Environmental Carcinogenesis & Ecotoxicology Reviews, 2016, 34(1): 1-32. |
| [9] | YAO HUA, GUO LAN, JIANG BING-HUA,et al. Oxidative stress and chromium(VI) carcinogenesis. Journal of Environmental Pathology Toxicology & Oncology Official Organ of the International Society for Environmental Toxicology & Cancer, 2008, 27(2): 77-88. |
| [10] | WISE S S, HOLMES A L, SR J P W. Particulate and soluble hexavalent chromium are cytotoxic and genotoxic to human lung epithelial cells. Mutation Research/genetic Toxicology & Environmental Mutagenesis, 2006, 610(1/2): 2-7. |
| [11] | MYROSLAV SPRYNSKYY.The separation of uranium ions by natural and modified diatomite from aqueous solution. Hazard. Mater. , 2010, 181: 700-707. |
| [12] | LI CONG-JU, LI YONG-JIAN, WANG JIAO-NA,et al. PA6@FexOy nanofibrous membrane preparation and its strong Cr (VI)- removal performance. Chemical Engineering Journal, 2013, 220: 294-301. |
| [13] | LOPES O F, PARIS E C, RIBERIRO C.Synthesis of Nb2O5, nanoparticles through the oxidant peroxide method applied to organic pollutant photodegradation: a mechanistic study. Applied Catalysis B Environmental, 2014, 144(2): 800-808. |
| [14] | ZHAO YUN, ELEY CLIVE, HU JING-PING,et al. Shape- dependent acidity and photocatalytic activity of Nb2O5 nanocrystals with an active TT (001) surface. Angew. Chem. Int. Ed., 2012, 51(16): 3846-3849. |
| [14] | ZHAO YUN, ELEY CLIVE, HU JING-PING,et al. Shape- dependent acidity and photocatalytic activity of Nb2O5 nanocrystals with an active TT (001) surface. Angew. Chem. Int. Ed., 2012, 51(16): 3846-3849. |
| [15] | GAO BAO-JIAO, JIANG PENG-FEI, AN FU-QIANG,et al. Studies on the surface modification of diatomite with polyethyleneimine and trapping effect of the modified diatomite for phenol. Applied Surface Science, 2005, 250(1-4): 273-279. |
| [16] | ONOZATO T, KATASE T, YAMAMOTO A,et al. Optoelectronic properties of valence-state-controlled amorphous niobium oxide. Journal of Physics Condensed Matter An Institute of Physics Journal, 2016, 28(25): 1-8. |
| [17] | HUO QI-SHENG, MARGOLESE DAVID I, CIESLA U LRIKE,et al. Organization of organic molecules with inorganic molecular species into nanocomposite biphase arrays. Chemistry of Materials, 1994, 6(8): 1176-1191. |
| [18] | YAN CHENG-LIN, XUE DONG-FENG.Formation of Nb2O5 nanotube arrays through phase transformation. Advanced Materials, 2010, 20(5): 1055-1058. |
| [19] | DU YU-CHENG, YAN JING, MENG QI,et al. Fabrication and excellent conductive performance of antimony-doped tin oxide- coated diatomite with porous structure. Materials Chemistry & Physics, 2012, 133(2/3): 907-912. |
| [20] | SHENG GUO-DONG, WANG SUO-WEI, HU JUN,et al. Adsorption of Pb(II) on diatomite as affected via aqueous solution chemistry and temperature. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2009, 339(1/2/3): 159-166. |
| [21] | KHRAISHEH M A, AL-GHOUTI M A, ALLEN S J,et al. Effect of OH and silanol groups in the removal of dyes from aqueous solution using diatomite. Water Research, 2005, 39(5): 922-932. |
| [22] | HADJAR H, HAMDI B, JABER M,et al. Elaboration and characterization of new mesoporous materials from diatomite and charcoal. Microporous and Mesoporous Materials, 2007, 107(3): 219-226. |
| [23] | CHENG YUE-HONG, JIANG HENG, GONG HONG,et al. Synthesis and characterization of niobic acid. Industrial Catalysis, 2011,19(1): 50-52. |
| [24] | JULIEN C M, MASSOT M.Spectroscopic studies of the structural transitions in positive electrodes for lithium batteries. Physical Chemistry Chemical Physics, 2002, 4(17): 4226-4235. |
/
| 〈 |
|
〉 |