

聚烯丙基氯化铵调控下多孔羟基磷灰石微球的合成及作为药物载体的应用研究
收稿日期: 2017-01-18
修回日期: 2017-03-13
网络出版日期: 2017-10-20
基金资助
国家自然科学基金(21571004);安徽省高等学校自然科学基金重点项目(KJ2015A084);National Natural Science Foundation of China (21571004);Natural Science Foundation of the Anhui Higher Education Institutions of China (KJ2015A084)
Porous Hydroxyapatite Microspheres Prepared by Using Poly (Allylamine Hydrochloride) and Its Application in Drug Delivery
Received date: 2017-01-18
Revised date: 2017-03-13
Online published: 2017-10-20
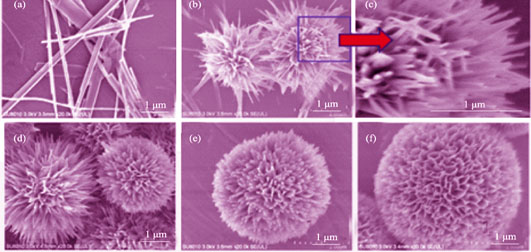
选用聚烯丙基氯化铵(PAH)作为晶体生长调节剂, 在水热条件下成功制备了多孔羟基磷灰石(Hydroxyapatite, HAP)中空微球。详细研究了反应时间和添加剂浓度等因素的影响: 150℃水热反应12 h, 控制PAH 浓度0.3~0.5 g/L, 可合成尺寸均匀、孔径密集的HAP中空微球。微球生长经历早期前驱体微结构、异相成核、相转化等不同阶段, 聚合物在各阶段都起到重要的调节作用。以典型的布洛芬(ibuprofen, IBU)作为模型药物, 研究微球的药物负载和脱附能力。结果显示: 多孔微球具有良好的药物负载和释放能力, 吸附量较好, 可达到413.65 mg/g。且药物具有较好的pH响应释放行为, 可作为pH敏感靶向药物载体应用到生物医学等领域。
马芳 , 崔名芳 , 朱建华 , 李雅丽 . 聚烯丙基氯化铵调控下多孔羟基磷灰石微球的合成及作为药物载体的应用研究[J]. 无机材料学报, 2017 , 32(11) : 1215 -1222 . DOI: 10.15541/jim20170041
Porous and hollow hydroxyapatite (HAP) microspheres were synthesized successfully in hydrothermal method utilizing poly(allylamine hydrochloride) (PAH) as the crystal growth regulator. Effects of reaction time and concentration of the polymer on the growth of final products were investigated. Uniform microspheres with dense pores can be synthesized by controlling the PAH concentration (0.3-0.5 g/L) after 12 h hydrothermal reaction at 150℃. Formation of hollow microspheres includes different stages of early precursor microstructures, heterogeneous nucleation and phase transformations. At different stages, the cationic polyelectrolyte PAH plays an important role in regulating growth of hollow microspheres. Ibuprofen (IBU) was chosen as a typical model drug to study the drug loading and the desorption ability. The results show that porous and hollow microspheres have relatively high drug loading capacity (413.65 mg/g). Drug release of the microspheres is favorably pH-responsive, which may have close relationship with the surface properties of HAP nanorods. Date from this study suggest that the porous microspheres will have potential application as the targeted-drug carrier in the biomedicine field.

| [1] | DOROZHKIN S V.Calcium orthophosphates in nature, biology and medicine.Materials, 2009, 2(2): 399-498. |
| [2] | PALMER L C, NEWCOMB C J, KALTZ S R,et al. Biomimetic systems for hydroxyapatite mineralization in inspired by bone and enamel. Chemical Reviews, 2009, 108(6): 4754-4783. |
| [3] | WANG K W, ZHU Y J, CHEN X Y,et al. Flower-like hierarchically nanostructured hydroxyapatite hollow spheres: facile preparation and application in anticancer drug cellular delivery. Chemistry-An Asian Journal, 2010, 5(12): 2477-2482. |
| [4] | TANG Q L, ZHU Y J, WU J,et al. Calcium phosphate drug nanocarriers with ultrahigh and adjustable drug-loading capacity one-step synthesis, in situ drug loading and prolonged drug release. Nanomedicine Nanotechnology Biology & Medicine, 2011, 7(4): 428-434. |
| [5] | DEVILLE S, SAIZ E, TOMSIA A P.Freeze casting of hydroxyapatite scaffolds for bone tissue engineering.Biomaterials, 2006, 27(32): 5480-5489. |
| [6] | WANG C, WANG Y, MENG H Y,et al. Research progress regarding nanohydroxyapatite and its composite biomaterials in bone defect repair. International Journal of Polymeric Materials and Polymeric Biomaterials, 2016, 65(12): 601-610. |
| [7] | ZHANG G D, CHEN J D, YANG S,et al. Preparation of amino-acid-regulated hydroxyapatite particles by hydrothermal method. Materials Letters, 2011, 65(3): 572-574. |
| [8] | DING G J, ZHU Y J, QI C,et al. Amorphous calcium phosphate nanowires prepared using beta-glycerophosphate disodium salt as an organic phosphate source by a microwave-assisted hydrothermal method and adsorption of heavy metals in water treatment. RSC Advances, 2015, 5(50): 40154-40162. |
| [9] | LU B Q, ZHU Y J, CHEN F,et al. Solvothermal transformation of a calcium oleate precursor into large-sized highly ordered arrays of ultralong hydroxyapatite microtubes. Chemistry, 2014, 20(23): 7116-7121. |
| [10] | LIU D M, YANG Q, TROCZYNSKI T,et al. Structural evolution of Sol-Gel derived hydroxylapatite. Biomaterials, 2002, 23(7): 1679-1687. |
| [11] | KIM W, SAITO F.Sonochemical synthesis of hydroxyapatite from H3PO4 solution with Ca(OH)2.Ultrasonics Sonochemistry, 2001, 8(2): 85-88. |
| [12] | WANG K W, ZHU Y J, CHEN F,et al. Microwave-assisted synthesis of hydroxyapatite hollow microspheres in aqueous solution. Materials Letters, 2011, 65(15): 2361-2363. |
| [13] | LIN K L, CHEN L, LIU P Y,et al. Hollow magnetic hydroxyapatite microspheres with hierarchically mesoporous microstructure for pH-responsive drug delivery. CrystEngComm, 2013, 15(15): 2999-3008. |
| [14] | MA L, ZHU J H, HUANG L.Rapid synthesis of hydroxyapatite nanorods at low temperature controlled by sodium alginate.Journal of Inorganic Materials, 2015, 30(3): 311-317. |
| [15] | ZHAO H, ZHU Y D, SUN J,et al. Synthesis of hollow hydroxyapatite nanospheres by the control of nucleation and growth in a two phase system. Chemical Communications, 2014, 50(83): 12519-12522. |
| [16] | WU Y J, TSENG Y H, CHAN J C C. Morphology control of fluorapatite crystallites by citrate ions. Crystal Growth & Design. 2010, 10(10): 4240. |
| [17] | LEE G S, PARK J H, SHIN U S,et al. Direct deposited porous scaffolds of calcium phosphate cement with alginate for drug delivery and bone tissue engineering. Acta Biomaterialia, 2011, 7(8): 3178-3186. |
| [18] | CANTAERT B, KIM Y Y, LUDWIG H,et al. Think positive: phase separation enables a positively charged additive to induce dramatic changes in calcium carbonate morphology. Advanced Functional Materials, 2012, 22(5): 907-915. |
| [19] | HIROFUMI D, EITARO M, SATOSHI M.Fabrication of hollow poly-allylamine hydrochloride/poly-sodium styrene sulfonate microcapsules from microbubble templates.Soft Matter, 2010, 6(9): 1892-1897. |
| [20] | SUZUKI O, KAMAKURA S, KATAGIRI T,et al. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials, 2006, 27(13): 2671-2681. |
| [21] | DING H C, PAN H H, XU X R,et al. Toward a detailed understanding of magnesium ions on hydroxyapatite crystallization inhibition. Crystal Growth & Design, 2014, 14(2): 763-769. |
| [22] | ZHAN J, TSENG Y, CHAN J C C,et al. Biomimetic formation of hydroxyapatite nanorods by a single-crystal-to-single-crystal transformation. Advanced Functional Materials, 2005, 15(12): 2005-2010. |
| [23] | LIN K, CHANG J, ZHU Y,et al. A facile one-step surfactant-free and low-temperature hydrothermal method to prepare uniform 3D structured carbonated apatite flowers. Crystal Growth & Design, 2009, 9(1): 177-178. |
| [24] | ZHANG Y J, LU J J, WANG J Q,et al. Synthesis of nanorod and needle-like hydroxyapatite crystal and role of pH adjustment. Journal of Crystal Growth, 2009, 311(23/24): 4740-4746. |
| [25] | REN F, LENG Y, DING Y,et al. Hydrothermal growth of biomimetic carbonated apatite nanoparticles with tunable size, morphology and ultrastructure. CrystEngComm, 2013, 15(11): 2137-2146. |
| [26] | VISWANATH B, RAVISHANKAR N.Controlled synthesis of plate-shaped hydroxyapatite and implications for the morphology of the apatite phase in bone.Biomaterials, 2008, 29(36): 4855-4863. |
| [27] | ZHANG X J, LIN D Y, YAN X H,et al. Evolution of the magnesium incorporated amorphous calcium phosphate to nano- crystallized hydroxyapatite in alkaline solution. Journal of Crystal Growth, 2011, 336(1): 60-66. |
| [28] | GORDON L M, COHEN M J, MACRENARIS K W,et al. Amorphous intergranular phases control the properties of rodent tooth enamel. Science, 2015, 347(6223): 726-750. |
| [29] | CHEN F, ZHU Y J, ZHAO X Y,et al. Solvothermal synthesis of oriented hydroxyapatite nanorod/nanosheet arrays using creatine phosphate as phosphorus source. CrystEngComm, 2013, 15(22): 4527-4531. |
| [30] | QI C, ZHU Y J, LU B Q,et al. Hydroxyapatite hierarchically nanostructured porous hollow microspheres: rapid, sustainable microwave-hydrothermal synthesis by using creatine phosphate as an organic phosphorus source and application in drug delivery and protein adsorption. Chemistry, 2013, 19(17): 5332-5341. |
| [31] | VALLET-REGÍ, GONZÁLEZ-CALBET J M. Calcium phosphates as substitution of bone tissues.Progress in Solid State Chemistry, 2004, 32(1): 1-31. |
/
| 〈 |
|
〉 |