

MoS2修饰TiO2纳米管阵列光电化学性能研究
收稿日期: 2016-03-01
修回日期: 2016-06-13
网络出版日期: 2016-10-25
基金资助
国家自然科学基金(21476262);青岛市科技发展计划(14-2-4-108-jch);中央高校基本科研业务费(15CX05032A, 14CX05038A)
Photoelectrochemical Properties of MoS2 Modified TiO2 Nanotube Arrays
Received date: 2016-03-01
Revised date: 2016-06-13
Online published: 2016-10-25
Supported by
National Natural Science Foundation of China (21476262);Technology Project of Qingdao (14-2-4-108-jch);Fundamental Research Funds for the Central Universities (15CX05032A, 14CX05038A)
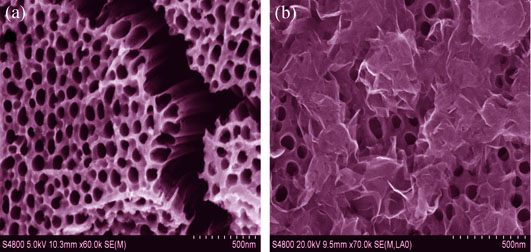
通过阳极氧化法在乙二醇电解液中制备TiO2纳米管阵列, 以钼酸钠和硫脲作为钼源和硫源, 并添加半胱氨酸为辅助剂, 水热法制备纳米花状二硫化钼修饰的TiO2纳米管阵列。用X射线衍射仪、扫描电子显微镜、能谱仪和拉曼光谱对复合材料的晶型、形貌、物相等进行分析, 通过电化学工作站测试复合材料的线性扫描伏安曲线、电化学阻抗谱和莫特-肖特基曲线。结果表明: MoS2/TiO2复合材料形貌比较规整均匀, MoS2纳米花尺寸约为200 nm; MoS2与TiO2复合有利于形成异质结, 促进光生电子和空穴的分离; 当钼酸钠浓度为0.8 mmol/L时制备的复合材料光化学能转化率为纯氧化钛的2.89倍, 达到了1.65%, 而且复合材料的电荷转移电阻降低了约50%, 光生载流子浓度提高了24倍, 达到了3.38×1023 cm-3, 具有非常优异的光电化学性能。
于濂清 , 黄承兴 , 张亚萍 , 董开拓 , 郝兰众 . MoS2修饰TiO2纳米管阵列光电化学性能研究[J]. 无机材料学报, 2016 , 31(11) : 1237 -1241 . DOI: 10.15541/jim20160107
Well aligned TiO2 nanotube arrays were grown by anodization method on Ti foils in ethylene glycol electrolyte. Flowers-like molybdenum disulfide composed with nanoflakes was synthesized by hydrothermal process on TiO2 nanotube arrays, using sodium molybdate, thiourea and L-Cysteine as reactants. The obtained samples were analyzed by X-ray diffraction (XRD) and scanning electron microscope (SEM) equipped with energy dispersive X-ray detector. Linear sweeps voltammetry, electrochemical impedance spectroscopy and Mott-Schottky plots of samples were analyzed by electrochemical workstation. The results showed that a flowers-like MoS2 with diameter of c.a. 200 nm was synthesized, forming a heterojunction between TiO2 nanotube arrays and MoS2, which can effectively promote the separation of photogenerated charges, reduce the electron-hole recombination. Optimum concentration of sodium molybdate was obtained at 0.8 mmol/L, its corresponding photoconversion efficiency reached 1.65%, which was 2.89 times as high as that of pure TiO2, and the charge transfer resistance decreased approximately 50%. The MoS2/TiO2 composite exhibited high carrier density of 3.38×1023 cm-3, which was about 24 times higher than that of pure TiO2 nanotube arrays.

| [1] | LI H, XIA Z B, CHEN J Q, et al. Constructing ternary CdS/reduced graphene oxide/TiO2 nanotube arrays hybrids for enhanced visible-light-driven photoelectrochemical and photocatalytic activity. Applied Catalysis B: Environmental, 2015, 168-169: 105-113. |
| [2] | YU L Q, ZHI Q Q, HUANG C X, et al.Photocatalytic properties of TiO2 porous network film.Journal of Nanoscience & Nanotechnology, 2015, 15(9): 6576-6581. |
| [3] | YU L Q, ZHANG Y P, ZHI Q Q, et al.Enhanced photoelectrochemical and sensing performance of novel TiO2 arrays to H2O2 detection.Sensors and Actuators B: Chemical, 2015, 211: 111-115. |
| [4] | YU L Q, LIU R S, ZHANG Y P, et al.Photoelectrochemical property of Fe-N modified titania nanotube array films. Journal of Optoelectronics and Advanced Materials, 2014, 16(5/6): 519-523. |
| [5] | GONG D, GRIMES C A, VARGHESE O K, et al.Titanium oxide nanotube arrays prepared by anodic oxidation.Journal of Materials Research, 2001, 16(12): 3331-3334. |
| [6] | MACAK J M, TSUCHIYA H, GHICOV A, et al.TiO2 nanotubes: self-organized electrochemical formation, properties and applications.Current Opinion in Solid State & Materials Science, 2007, 11(1/2): 3-18. |
| [7] | XIANG Q J, YU J, JARONIEC M.Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles.Journal of the American Chemical Society, 2012, 134(15): 6575-6578. |
| [8] | ZHANG L M, LIU K H, WONG A B, et al.Three-dimensional spirals of atomic layered MoS2.Nano Letters, 2014, 14(11): 6418-6423. |
| [9] | FURCHI M M, POLYUSHKIN D K, POSPISCHIL A, et al.Mechanisms of photoconductivity in atomically thin MoS2. Nano Letters, 2014, 14(11): 6165-6170. |
| [10] | XIA X H, ZHAO X J, YE W C, et al.Highly porous Ag-Ag2S/MoS2 with additional active sites synthesized by chemical etching method for enhanced electrocatalytic hydrogen evolution.Electrochimica Acta, 2014, 142: 173-181. |
| [11] | GAO W Y, WANG M Q, RAN C X, et al.Facile one-pot synthesis of MoS2 quantum dots-graphene-TiO2 composites for highly enhanced photocatalytic properties.Chemical Communications, 2015, 51(9): 1709-1712. |
| [12] | MAO M L, MEI L, GUO D, et al.High electrochemical performance based on the TiO2 nanobelt@few-layered MoS2 structure for lithium-ion batteries. Nanoscale, 2014, 6(21): 12350-12353. |
| [13] | WU W Z, WANG L, LI Y K, et al.Piezoelectricity of single-atomic-layer MoS2 for energy conversion and piezotronics. Nature, 2014, 514(7523): 470-476. |
| [14] | DU T, WANG N, CHEN H J, et al.TiO2-based solar cells sensitized by chemical-bath-deposited few-layer MoS2.Journal of Power Sources, 2015, 275: 943-949. |
| [15] | LI Y G, WANG H L, XIE L M, et al.MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution Reaction.Journal of the American Chemical Society, 2011, 133(19): 7296-7299. |
| [16] | CHEN Y, SONG B H, TANG X S, et al.Ultrasmall Fe3O4 nanoparticle/MoS2 nanosheet composites with superior performances for lithium ion batteries.Small, 2014, 10(8): 1536-1543. |
| [17] | ZHANG L M, LIU C, WONG A B, et al.MoS2-wrapped silicon nanowires for photoelectrochemical water reduction.Nano Research, 2015, 8(1): 281-287. |
| [18] | XIONG F Q, WEI X, ZHENG X, et al.Fabrication of multilayered TiO2 nanotube arrays and separable nanotube segments.Journal of Materials Chemistry A, 2014, 2(13): 4510-4513. |
| [19] | ZHU X Q, YANG C, XIAO F, et al.Synthesis of nano-TiO2-decorated MoS2 nanosheets for lithium ion batteries. New Journal of Chemistry, 2015, 39(1): 683-688. |
| [20] | MIGNUZZI S, POLLARD A J, BONINI N, et al.Effect of disorder on Raman scattering of single-layer MoS2.Physical Review B, 2015, 91(19): 195411-195417. |
| [21] | LIN T Z, KANG B T, JEON M H, et al.Controlled layer-by-layer etching of MoS2.ACS Applied Materials & Interfaces, 2015, 7(29): 15892-15897. |
| [22] | ZHAO Y F, YANG Z Y, ZHANG Y X, et al.Cu2O decorated with cocatalyst MoS2 for solar hydrogen production with enhanced efficiency under visible light.Journal of Physical Chemistry C, 2014, 118(26): 14238-14245. |
| [23] | YU L Q, DONG K T, ZHANG Y P, et al.Tuned n/n or n/p heterojunctions for reduced graphene oxide and titania nanosheets and their electrochemical properties.Materials Chemistry and Physics, 2014, 148(3): 803-809. |
| [24] | JOHN S E, MOHAPATRA S K, MISRA M.Double-wall anodic titania nanotube arrays for water photooxidation.Langmuir, 2009, 25(14): 8240-8247. |
| [25] | DONG K T, YU L Q, ZHANG Y P, et al.Green synthesis of sulfur/graphene nanocomposite and photocatalytic performance.Science of Advanced Materials, 2014, 6(8): 1828-1835. |
| [26] | ZHANG Y P, ZHANG A Y, YU L Q, et al.Photoelectrochemical properties of AgX(Cl,Br)-TiO2 heterojunction nanocomposites.Journal of Inorganic Materials, 2016, 31(3): 269-273. |
/
| 〈 |
|
〉 |