

石墨烯-ZnIn2S4纳米复合微球的制备及光催化产氢活性
收稿日期: 2014-11-17
修回日期: 2015-01-28
网络出版日期: 2015-06-25
基金资助
国家自然科学基金(51372080);中国博士后基金(2012M51220)
Preparation and Photocatalytic Activity for Hydrogen Evolution of Graphene-ZnIn2S4 Nanocomposite Spheres
Received date: 2014-11-17
Revised date: 2015-01-28
Online published: 2015-06-25
Supported by
National Natural Science Foundation of China (51372080);China Postdoctoral Science Foundation (2012M51220)
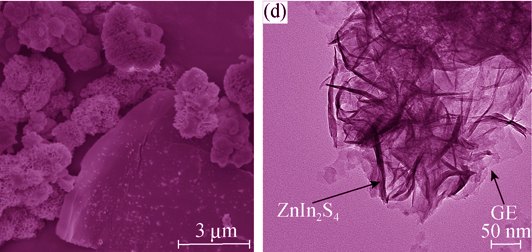
采用光催化还原法制备了石墨烯-ZnIn2S4纳米复合微球。采用XRD、SEM、TEM、FT-IR、XPS和DRS等手段对样品进行表征, 结果表明, 经过光催化还原处理后氧化石墨被还原成石墨烯, ZnIn2S4纳米微球负载在石墨烯表面。光催化产氢的实验结果表明, 当石墨烯含量为2.0wt%、光催化还原时间为24 h时, 石墨烯-ZnIn2S4纳米复合微球在模拟太阳光下产氢量达到1540.8 μmol, 是纯ZnIn2S4纳米微球的9.8倍。增强光催化性能的原因归结为石墨烯在复合光催化剂中起到了电子快速传输作用, 同时还对纳米复合微球光催化产氢反应机理进行了分析讨论。
关键词: 光催化还原法; ZnIn2S4纳米微球; 石墨烯; 产氢
周民杰 , 张娜 , 侯朝辉 . 石墨烯-ZnIn2S4纳米复合微球的制备及光催化产氢活性[J]. 无机材料学报, 2015 , 30(7) : 713 -718 . DOI: 10.15541/jim20140591
Graphene-ZnIn2S4 nanocomposite spheres were prepared via a facile photocatalytic reduction method. The samples were characterized by XRD, SEM, TEM, FT-IR, XPS and DRS. The results showed that the graphene oxide was reduced to graphene and ZnIn2S4 nanospheres were loaded on the surface of graphene sheets through a photocatalytic reduction process. The experimental results for photocatalytic hydrogen evolution of the samples indicated that the amount of evoluted H2 under simulated sunlight irradiation using graphene-ZnIn2S4 nanocomposite spheres was 1540.8 μmol, 9.8 times of the pure ZnIn2S4 nanospheres, under optimal technological condition with the graphene content of 2.0wt% for 24 h. The enhanced performance can be attributed to the graphene which effectively promoted the transfer of photogenerated electrons. Furthermore, a detailed photocatalytic hydrogen evolution mechanism of graphene-ZnIn2S4 nanocomposite spheres was investigated.

| [1] | SHEN S H, GUO P H, ZHAO L, et al.Insights into photoluminescence property and photocatalytic activity of cubic and rhombohedral ZnIn2S4.J. Solid State Chem., 2011, 184(8): 2250-2256. |
| [2] | WEI Q L, MU S, YAN Y, et al.Preparation and surfactant assisted morphology controllable growth of ZnIn2S4.Chinese J. Inorg. Chem., 2010, 26(2): 269-273. |
| [3] | LI C X, LI H H, HAN L J, et al.Ionothermal/hydrothermal synthesis of the ternary metal chalcogenide ZnIn2S4.Mater. Lett., 2011, 65(15/16): 2537-2540. |
| [4] | SHEN S H, ZHAO L, GUAN X J, et al. Improving visible-light photocatalytic activity for hydrogen evolution over ZnIn2S4: a casestudy of alkaline-earth metal doping.J. Phys. Chem. Solids., 2012, 73(1): 79-83. |
| [5] | SHEN S H, CHEN J, WANG X X, et al.Microwave-assisted hydrothermal synthesis of transition-metal doped ZnIn2S4 and its photocatalytic activity for hydrogen evolution under visible light.J. Power Sources, 2011, 196(23): 10112-10119. |
| [6] | SHEN S H, CHEN X B, REN F, et al.Solar light-driven photocatalytic hydrogen evolution over ZnIn2S4 loaded with transition-metal sulfides.Nanoscale Res. Lett., 2011, 6(9): 290-296. |
| [7] | LI Y X, WANG J X. PENG S Q, et al.Photocatalytic hydrogen generation in the presence of glucose over ZnS-coated ZnIn2S4 under visible light irradiation. Int. J. Hydrog. Enenergy, 2010, 35(18): 7116-7126. |
| [8] | MIN S X, LV G X.Preparation of CdS/graphene composites and photocatalytic hydrogen generation from water under visible light irradiation.Acta Phys. Chim. Sin., 2011 27(9): 2178-2184. |
| [9] | YU L H, RUAN H, ZHENG L, et al. A facile solvothermal method to produce ZnS quantum dots-decorated graphene nanosheets with superior photoactivity. Nanotech., 2013, 24(37): 375601-1-11. |
| [10] | ZHANG J, QI L F, RAN J R, et al. Ternary NiS/ZnxCd1-xS/reduced graphene oxide nanocomposites for enhanced solar photocatalytic H2-production activity. Adv. Energy Mater., 2014, 4(10): 1301925-1-6. |
| [11] | GUO D, WANG P, ZHENG Q Y, et al.One-step synthesis of flower-like Bi2WO6-RGO composite photocatalysts.J. Inorg. Mater., 2014, 29(11): 1193-1198. |
| [12] | ZHOU J, TIAN G H, CHEN Y J, et al.In situ controlled growth of ZnIn2S4 nanosheets on reduced graphene oxide for enhanced photocatalytic hydrogen peoduction performance.Chem. Commun., 2013, 49(22): 2237-2239. |
| [13] | LI H F, YU H T, CHEN S, et al.Fabrication of graphene wrapped ZnIn2S4 microspheres heterjunction with enhanced interfacial contact and its improved photocatalytic performance.Dalton Trans., 2014, 43(7): 2888-2894. |
| [14] | ZHOU M J, YAN J H, CUI P.Synthesis and enhanced photocatalytic performance of WO3 nanorods @graphene nanoco, posites.Mater. Lett., 2012, 89(12): 258-261. |
| [15] | ZHU M S, CHEN P L, LIU M H.Graphene oxide enwrapped Ag/AgX(X=Br, Cl) nanocomposite as a highly effcient visible- light plasmonic photocatalyst.ACS Nano, 2011, 5(6): 4529-4536. |
| [16] | ZHANG Q, HE Y Q, CHEN X G, et al.Intercalated structure and photocatalytic properties of TiO2-graphene oxide composite.Acta Phys. Chim. Sin., 2010 26(3): 654-662. |
| [17] | LI Y B, ZHANG H M, LIU P R, et al.Cross-linked g-C3N4/RGO nanocomposites with tunable band structure and enhangced visible light photocatalytic activity.Small, 2013, 9(19): 3336-3344. |
| [18] | CHAUDHARI N S, WARULE S S, KALE B B.Aechitecture of rose and hollow marigold-like ZnIn2S4 flower: structureal, optical and pjotocatalytic study.RSC Adv., 2014, 4(24): 12182-12187. |
| [19] | PATIL1B N, ACHARYA S. A. Preparation of ZnS-graphene nanocomposite and its photocatalytic behavior for dye degradation.Adv. Mat. Lett., 2014, 5(3): 113-116. |
| [20] | LEE E, HONG J Y, KANG H Y. Synthesis of TiO2 nanorod-decorated graphene sheets and their highly efficient photocatalytic activities under visible-light irradiation. J. Hazard. Mater., 2012, 219-220(6): 13-18. |
/
| 〈 |
|
〉 |