采用溶胶-凝胶法, 在不同的焙烧温度下合成锌/铈共掺杂二氧化钛纳米材料。用X射线衍射(XRD)、透射电子显微镜(TEM)、X射线光电子能谱(XPS)和紫外-可见漫反射光谱(DRS)对样品进行了表征。采用抑菌环法, 以大肠杆菌为实验菌种, 对Zn/Ce-TiO2纳米材料抗菌性能进行检测。结果表明: 样品的结晶度和晶相完全取决于焙烧温度的高低; 部分锌进入二氧化钛晶格中, 其余掺杂离子以CeO2和Zn2Ti3O8形式存在; 与暗态下相比, 在可见光激发下, Zn/Ce-TiO2纳米材料具有超强的抗菌性。对锌/铈共掺杂作用机制进行了深入探讨。
Zinc and cerium co-doped titania (Zn/Ce-TiO2) nano-materials were synthesized by a Sol-Gel method at different calcination temperatures. Zn/Ce-TiO2 nano-materials were characterized by X-ray diffraction (XRD), transmission electron microscope (TEM), X-ray photoelectron spectroscope (XPS) and UV-Vis diffuse reflectance spectra (DRS). With the test of bacterial inhibition zone, the antibacterial properties of Zn/Ce-TiO2 nano-materials on
Contamination by microorganisms is of great concern in a variety of areas, such as medical devices, healthcare products, water purification systems, hospitals, dental office equipment, food packaging, food storage, and household sanitation, etc. In order to address the issue of microbial pollution and contamination, researchers have long been engaged in intensive investigations of antibacterial materials[ 1, 2]. TiO2 has received considerable attention in recent years because of its large potential application as an important photocatalytic antibacterial agent. It can decompose organics and kill bacteria under ultraviolet (UV) radiation and has been widely applied to purify air and water and provide self-maintaining clean surfaces because of its high photoreactivity, non-toxicity and inexpensiveness[ 3]. However, TiO2 exhibits less reactivity under the visible light, therefore, its application is restricted in some situations.
To increase the antibacterial activities of TiO2 under the visible light, many studies have succeeded to add silver ions into TiO2 nano-materials. Silver ion is known to have higher antibacterial activity and less toxicity compared with other metal ions[ 4, 5]. Unfortunately, the applications of the silver-doped antibacterial agents are limited because of their discoloration and high cost[ 6]. Therefore, it is important to develop new antibacterial agents with low cost and good antibacterial activity.
It is well established that zinc ions possess some antibacterial effects and color stability with low cost and little toxicity[ 7]. Meanwhile, rare earth ions have been used as antimicrobial agents in medicine for a long time due to the high safety and broad spectrum antibacterial activity. But the applications of the long-acting antibacterial materials doping zinc ions or rare earth ions are limited because of their relatively poor antibacterial activity. Cai, et al[ 8] reported that the zinc ions and cerium ions loaded α-zirconium phosphate showed good antibacterial activity due to the synergistic antibacterial effect of zinc ions and cerium ions.
In this paper, zinc and cerium co-doped TiO2 nano-materials were prepared by a Sol-Gel method, and firstly applied to the fields of antibacterial materials. Materials crystal structures, energy band structures and antibacterial mechanism were studied in detail. This study will contribute researches greatly in pursuit of better technology in antibacterial materials.
TiO2 nano-materials were synthesized by injecting acid solution (consisting of distilled water, ethyl alcohol and acetic acid) into tetrabutyl titanate. The mixture was kept under constant magnetic stirring for 3 h. In order to obtain nanoparticles, the sol was dried for 48 h. Sintering processes were subsequently carried out to obtain desired TiO2 crystalline. The ion doped nanoparticles were synthesized with the same method mentioned above, except for the addition of the corresponding zinc and cerium doping solution into the acid solution.
The X-ray diffraction (XRD, Philips) equipped with a copper target ( λKα1= 0.1541874 nm) was used to identify the formation phase of the obtained samples. The morphology and grain size of the nano-materials were analyzed with TECNAI G2 F20 transmission electron microscope (TEM). The binding energy was identified by X-ray photoelectron spectroscope (XPS) with MgKα radiation (ESCALAB250). Diffuse reflectance spectra (DRS) were measured by using a Shimazu UV-2500 spectrophotometer.
E. coli ( E. coli, ATCC 25922) as the model bacteria, antibacterial properties of Zn-Ce/TiO2 nano-materials were tested by using inhibition zone method under visible light irradiation and in the dark. Observe the materials surrounding bacterial growth, measure transparent antibacterial circle diameter, and then determine antibacterial properties.
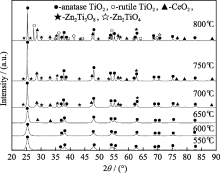
XRD was carried out to investigate the changes patterns TiO2 phase structure after calcination treatment and zinc and cerium co-doping. Fig. 1 shows the effect of calcination temperatures on the phase structure of Zn-Ce/TiO2 nano-materials. It is found that the (101) plane (peak 25.3°) is consistently present at all the calcination temperatures but the FWHM decrease with the increase of temperature. It indicates the crystal becomes larger with increase of calcination temperature. When the nano-material was calcinated at 650℃, Fig. 1 shows some characteristic peaks of CeO2 phase (major peaks: 28.4°, 33.0°) and CeO2 phases intensity increase gradually with the increase of calcination temperature. It is supposed that residual cerium component separated from the anatase phase is crystallized as CeO2 phase. This is because of much lower solubility of cerium in the anatase phase when the calcination temperature increases. As calcination temperature reaches 700℃, the trace of cubic Zn2Ti3O8 phases (major peak:35.4°) form. According to previous report, ZnO reacted with TiO2 to transform into cubic Zn2Ti3O8[ 9]. At the same time, it is reported that Zn2+ cannot substitute for Ti4+ of [TiO4] tetrahedrons in amorphous or [TiO6-] octahedrons in anatase and rutile crystals. At this occasion, Zn2+ mainly located in the discontinuity sites of the amorphous network, enriched around the crystals, or formed ZnO, Zn2Ti3O8 and ZnTiO3 phases. When calcination temperature reaches 800℃, Zn2TiO4 phases (major peaks: 35.4°) appear together with the rutile phases (major peaks: 27.4°). Obviously, the phase transformation from anatase to rutile occurs at 800℃. It also reveals that the doping could retard the transformation from anatase to rutile at elevated temperature.
To investigate the effect of zinc and cerium co-doping on the crystal structure of TiO2, we calculated the lattice parameters of TiO2 nano-materials listed in Table 1. It is clearly seen from Table 1 that the lattice parameters of all nano-materials remain almost unchanged along the a-axis. The c-axis parameters of co-doping TiO2-550 decrease compare with pure TiO2-550. This indicates that the crystal lattices of materials are locally destroyed by Zn and Ce doping. The ionic radii of Ce3+, Ce4+, Zn2+ and Ti4+ are 0.101, 0.087, 0.060 and 0.068 nm, respectively. Unlike the action of Zn2+, Ce3+ and Ce4+ with larger radius remaining in anatase crystals is nearly impossible, therefore, they should be located around anatase crystals[ 9].
| Table 1 Lattice parameters of TiO2 nano-materials |
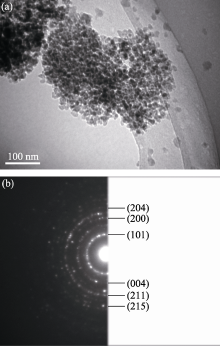
TEM images of Zn-Ce/TiO2-600 are shown in Fig. 2. The morphology, particles sizes and crystallographic lanes have been verified using the TEM study. The TEM analysis has revealed that the grains are round-shaped. And after doping Zn-Ce, the particles sizes become smaller and better dispersion. A corresponding selected area electron diffraction pattern (SADP) shows the Debye rings exhibit a polycrystalline nature of the nano-materials. The Debye rings with d-values corresponding to 0.3512 nm (marked as (101)), 0.2293 nm (marked as (004)), 0.1841 nm (marked as (200)), 0.1626 nm (marked as (211)), 0.1442 nm (marked as (204)), and 0.1297 nm (marked as (215)) are assigned to the TiO2 anatase phases, respectively.
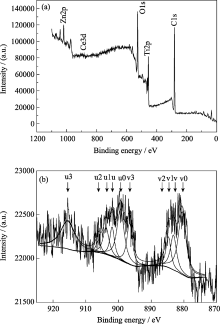
X-ray photoelectron spectroscopy analysis has been applied as an important characteristic method to detect the chemical states of different elements in doped nano-materials. The XPS spectrum of Zn-Ce/TiO2-600 in Fig. 3(a) indicates that the material contains Ti, O, C, Ce and Zn elements. The C element can be ascribed to the residual carbon from precursor.
Figure 3(b) shows the high-resolution XPS spectra of Ce3d. According to the literature [10], the Ce3d spectrum is complex and can be assigned 3d3/2 spin-orbit states (labeled u) and 3d5/2 states (labeled v). The u3/ v3 doublet is ascribed to the primary photoemission from Ce4+-O2. The u/ v and u2/ v2 doublets are shake-down features resulting from the transfer of one or two electrons from a filled O2p orbital to an empty Ce4f orbital. The u1/ v1 doublet is attributed to photoemission from Ce3+. As can be seen from Fig. 3b, it could be assigned to 3d3/2 spin-orbit states containing five main peaks at 899.37 ( u0), 901.91 ( u), 903.75 ( ul), 906.01 ( u2), 915.47 eV ( u3), and 3d5/2 states containing five main peaks at 880.50 ( v0), 883.14 ( v), 884.70 ( vl), 888.49 ( v2), 896.62 eV ( v3). The Ce3d spectrum of Zn-Ce/TiO2-600 nano-material shows a mixture of Ce in 3+/4+ oxidation states giving rise to these five pairs of doublets, indicating the coexistence of Ce3+and Ce4+ in Zn-Ce/TiO2-600 nano-material. Nevertheless, diffraction peaks for Ce2O3 are not found in Fig.1, thereby it indicates that Ce2O3 might be amorphous[ 11].
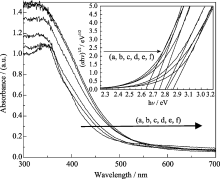
The UV-Vis diffuse reflectance spectrum of Zn-Ce/TiO2 calcinated at different temperatures is illustrated in Fig. 4. It can be clearly observed that the absorption edge positions of all zinc and cerium co-doped materials extend into the visible region. It is clear that the incorporation of co-doped results in a red-shift of the absorption of TiO2 nano-materials. The red shift of the absorption edge implies that the band gap energy decreases, the materials could absorb more photons, and eventually photoactivity of the materials increase. Assuming the materials to be indirect semiconductors, the plot of transformed Kubelka-Munk function vs the energy of light affords band gap energies of 2.63, 2.70 , 2.75, 2.8, 2.82 and 2.86 eV for 550℃, 600℃, 650℃, 700℃, 750℃ and 800℃, respectively (see the inset).
Figure 5 shows the results of inhibition zone Zn/Ce- TiO2 nano-materials. It can be seen from the Fig. 5(a), Zn/Ce-TiO2 nano-materials exhibit strong antibacterial activity under the visible light. With the increase of the calcination temperature, antibacterial activities of Zn/Ce- TiO2 are enhanced. Calcination temperature is 650℃, antibacterial activity of Zn/Ce-TiO2 nano-material is best. Subsequently, antibacterial activities are weakened with increase of temperature. The average inhibition zones are 0, 5, 8.5, 7, 8 and 7 mm for Zn/Ce-TiO2 obtained at 550℃, 600℃, 650℃, 700℃, 750℃ and 800℃, respectively.
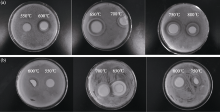 | Fig. 5 Antibacterial results of Zn/Ce co-doped TiO2 at different calcination temperatures(a) Under visible light; (b) In the dark |
In the Fig. 5(b), it also can be seen obscure inhibition zone. It shows Zn/Ce-TiO2 nano-materials have slight antibacterial activity in the dark. But their antibacterial activities are worse in the dark than that under the visible light.
According to the results of antibacterial effect for Zn-Ce/TiO2, we suppose that the antibacterial mechanism of Zn-Ce/TiO2 is as follows:(1) It could be hypothesized that titanium zinc cerium, a semiconductor, would act in a similar manner, and would catalyze the formation of free radicals. Radical oxygen species can inhibit bacterial growth[ 12]. XRD data proves Zn2Ti3O8 phase exists in the materials. Wang, et al[ 13] proposed that the number of Lewis acid sites enhanced in the material surface and OH- increased, due to the cubic Zn2Ti3O8 phase. OH- can capture electron holes pair and transform into free groups (·OH), and cause rupture of the cell membrane.
(2)XPS data shows both Ce3+ and Ce4+ ions reside in the Zn/Ce-TiO2 nano-materials. A mixture of Ce3+ and Ce4+ oxidation states giving rise to a myriad of peaks indicates that the surface of the material is not completely oxidized. It is supposed that the coexistence of Ce3+ and Ce4+ can improve the photocatalytic efficiency through the following ways[ 13]:Ce4++ ecb- → Ce3+
Ce3++ Oads → Ce4++ Oads-
Ce4+ can trap the excited electron in conduction band, inhibiting the electron/hole pair recombination; meanwhile, Ce3+ can offer electron to the oxygen adsorbed on the surface of catalyst, thus the interfacial electron transfer is accelerated.
(3) XRD and XPS data also demonstrate that cerium xide exits in the particles surface. Moon, et al[ 14] proposed the binary oxide catalysts often exhibited higher catalytic activity than what could be directly predicted from the properties of their components. The interface between the two phases could act as a rapid separation site for the photogenerated electrons and holes due to the difference in the energy level of their conduction bands and valence bands. Furthermore, DRS analysis proves absorption edge positions of Zn-Ce/TiO2 materials extend into the visible region. It is also helpful to the photocatalytic reaction under the visible light.
1) Zinc and cerium co-doped TiO2 nano-materials were synthesized successfully by Sol-Gel method under different calcination temperatures. The analyses of XRD and TEM show that the particle sizes of all antibacterial materials are nanoscale. XRD and XPS spectra confirm that the cerium ions and zinc ions exist in the form of CeO2 and Zn2Ti3O8 phase, respectively.
2) The results obtained by the inhibition zone method demonstrate that Zn-Ce/TiO2 antibacterial materials can destroy or inhibit the growth of E. coli. The order of the antibacterial activities of the Zn-Ce/TiO2 materials obtained at different calcination temperatures is 650℃>750℃> 700℃=800℃>600℃>550℃.
3) Zinc and cerium co-doped titania exhibited enhanced antibacterial properties under visible light irradiation. We assume that the zinc and cerium co-doping induce band gap narrowing or Lewis acid in the Zn/Ce-TiO2 surface enhancing and increasing OH- could be responsible for improved antibacterial activity.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|